Variation in the Ovine Glycogen Synthase Kinase 3 Beta-Interaction Protein Gene (GSKIP) Affects Carcass and Growth Traits in Romney Sheep
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sheep Studied and Data Gleaned
2.2. The PCR Amplification of Ovine GSKIP
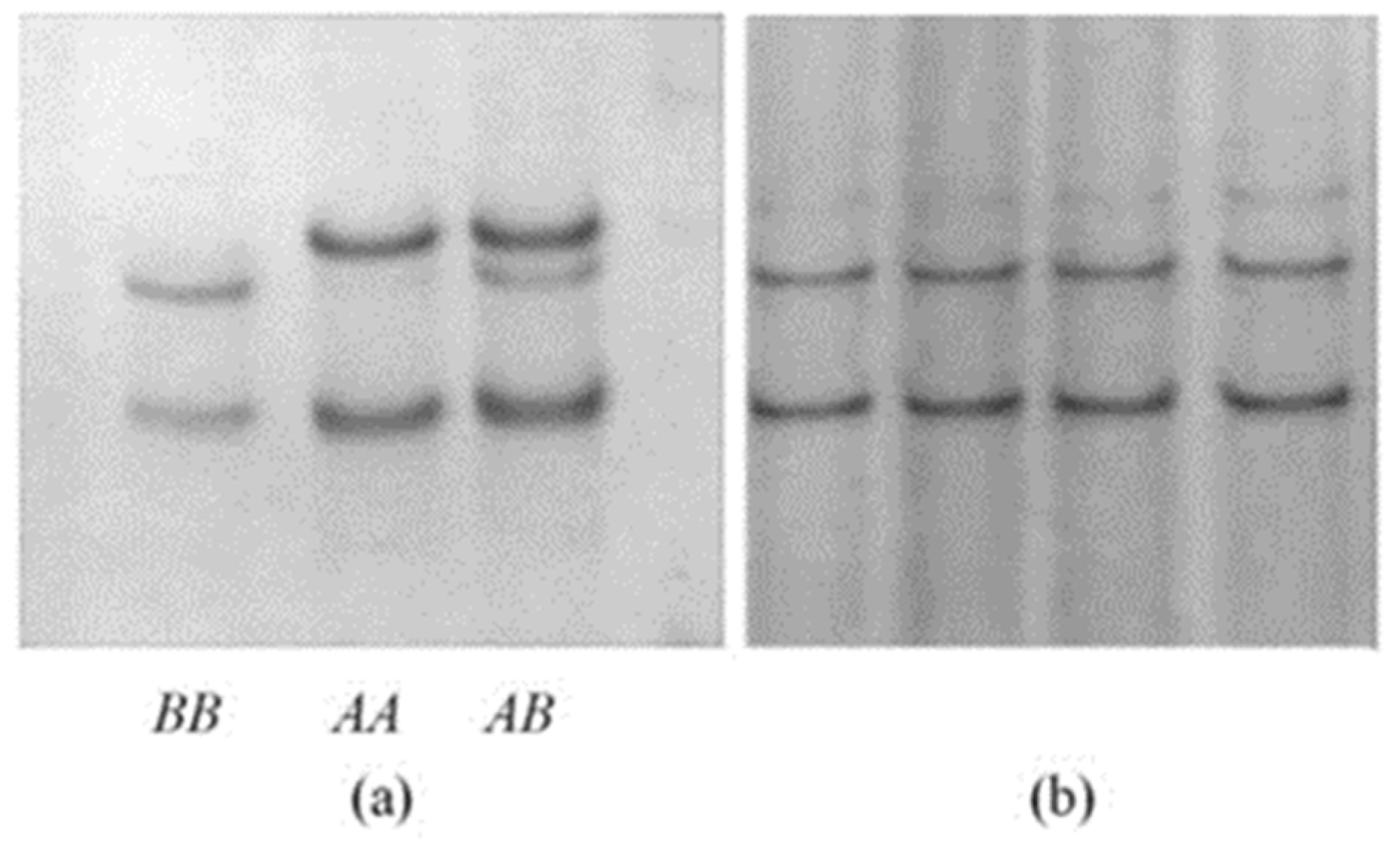
2.3. Screening for Variation in Ovine GSKIP
2.4. Sequencing and Analysis of Ovine GSKIP Variants
2.5. Statistical Analyses
3. Results
3.1. Ovine GSKIP Variation
3.2. Frequencies of the Ovine GSKIP Variants in the NZ Romney Sheep
3.3. Effect of Ovine GSKIP Variants on Carcass and Growth Traits
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cohen, P.; Frame, S. The renaissance of GSK3. Mol. Cell Biol. 2001, 2, 769–776. [Google Scholar] [CrossRef]
- Manning, B.D.; Cantley, L.C. AKT/PKB signaling: Navigating downstream. Cell 2007, 129, 1261–1274. [Google Scholar] [CrossRef] [PubMed]
- Luo, J. Glycogen synthase kinase 3beta (GSK3beta) in tumorigenesis and cancer chemotherapy. Cancer Lett. 2009, 273, 194–200. [Google Scholar] [CrossRef]
- Jain, S.; Ghanghas, P.; Rana, C.; Sanyal, S.N. Role of GSK-3β in Regulation of Canonical Wnt/β-catenin Signaling and PI3-K/Akt Oncogenic Pathway in Colon Cancer. Cancer Investig. 2017, 35, 473–483. [Google Scholar] [CrossRef]
- Jope, R.S.; Yuskaitis, C.J.; Beurel, E. Glycogen Synthase Kinase-3 (GSK3): Inflammation, Diseases, and Therapeutics. Neurochem. Res. 2007, 32, 577–595. [Google Scholar] [CrossRef]
- Woodgett, J.R. Physiological roles of glycogen synthase kinase-3: Potential as a therapeutic target for diabetes and other disorders. Curr. Drug Targets Immune Endocr. Metab. Disord. 2003, 3, 281–290. [Google Scholar] [CrossRef]
- Anakwe, K.; Robson, L.; Hadley, J.; Buxton, P.; Church, V.; Allen, S.; Hartmann, C.; Harfe, B.; Nohno, T.; Brown, A.M.C.; et al. Wnt signalling regulates myogenic differentiation in the developing avian wing. Development 2003, 130, 3503–3514. [Google Scholar] [CrossRef]
- Tee, J.M.; van Rooijen, C.; Boonen, R.; Zivkovic, D. Regulation of Slow and Fast Muscle Myofibrillogenesis by Wnt/b-Catenin and Myostatin Signaling. PLoS ONE 2009, 4, e5880. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.S.; Nowsheen, S.; Wang, T.; Thotala, D.K.; Xia, F. Glycogen synthase kinase 3{beta} inhibition enhances repair of DNA double-strand breaks in irradiated hippocampal neurons. Neuro Oncol. 2011, 13, 459–470. [Google Scholar] [CrossRef]
- Chou, C.-H.; Yang, M.-C.; Hsiao, B.-X.; Wang, Y.-H.; Liu, H.-F.; Chiou, S.-J.; Chuang, Y.-C.; Yang, C.-N.; Lieu, A.-S.; Loh, J.-K.; et al. The origin of GSKIP, a multifaceted regulatory factor in the mammalian Wnt pathway. Biochim. Biophys. Acta (BBA) Bioenerg. 2018, 1865, 1046–1059. [Google Scholar] [CrossRef]
- Dema, A.; Schroeter, M.F.; Perets, E.; Skroblin, P.; Moutty, M.C.; Deak, V.A.; Birchmeier, W.; Klussmann, E. The A-Kinase Anchoring Protein (AKAP) Glycogen Synthase Kinase 3beta Interaction Protein (GSKIP) Regulates beta-Catenin through Its Interactions with Both Protein Kinase A (PKA) and GSK3beta. J. Biol. Chem. 2016, 291, 19618–19630. [Google Scholar] [CrossRef]
- Lin, C.C.; Chou, C.H.; Howng, S.L.; Hsu, C.Y.; Hwang, C.C.; Wang, C.; Hsu, C.M.; Hong, Y.R. GSKIP, an inhibitor of GSK3beta, mediates the N-cadherin/beta-catenin pool in the differentiation of SH-SY5Y cells. Cell Biochem. 2009, 108, 1325–1336. [Google Scholar] [CrossRef] [PubMed]
- Loh, J.-K.; Lin, C.-C.; Yang, M.-C.; Chou, C.-H.; Chen, W.-S.; Hong, M.-C.; Cho, C.-L.; Hsu, C.-M.; Cheng, J.-T.; Chou, A.-K.; et al. GSKIP- and GSK3-mediated anchoring strengthens cAMP/PKA/Drp1 axis signaling in the regulation of mitochondrial elongation. Biochim. Biophys. Acta (BBA) Bioenerg. 2015, 1853, 1796–1807. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Deak, V.A. Knockout of A-kinase Anchoring Protein GSKIP in Mice Causes Perinatal Lethality. Ph.D. Thesis, Pharmazie der Freien University, Berlin, Germany, 2015. [Google Scholar]
- Pegliasco, J.; Hirsch, P.; Marzac, C.; Isnard, F.; Meniane, J.-C.; Deswarte, C.; Pellet, P.; Lemaitre, C.; Leroy, G.; Moraes, G.R.; et al. Germline ATG2B/GSKIP-containing 14q32 duplication predisposes to early clonal hematopoiesis leading to myeloid neoplasms. Leukemia 2021, 1–12. [Google Scholar] [CrossRef]
- Chou, H.Y.; Howng, S.L.; Cheng, T.S.; Hsiao, Y.L.; Lieu, A.S.; Loh, J.K.; Hwang, S.L.; Lin, C.C.; Hsu, C.M.; Wang, C.; et al. GSKIP is homologous to the Axin GSK3-beta interaction domain and functions as a negative regulator of GSK3-beta. Biochemistry 2006, 45, 11379–11389. [Google Scholar] [CrossRef]
- Zhao, F.; Zhou, H.; Li, S.; Fang, Q.; Luo, Y.; Hickford, J.G.H. Growth and carcass trait association with variation in the somatostatin receptor 1 (SSTR1) gene in New Zealand Romney sheep. N. Z. J. Agric. Res. 2017, 61, 1–10. [Google Scholar] [CrossRef]
- Hopkins, D.L.; Safari, E.; Thompson, J.M.; Smith, C.R. Video image analysis in the Australian meat industry—precision and accuracy of predicting lean meat yield in lamb carcasses. Meat Sci. 2004, 67, 269–274. [Google Scholar] [CrossRef]
- Zhou, H.T.; Hickford, J.G.H.; Fang, Q.A. A two-step procedure for extracting genomic DNA from dried blood spots on filter paper for polymerase chain reaction amplification. Anal. Biochem. 2006, 354, 159–161. [Google Scholar] [CrossRef]
- Byun, S.O.; Fang, Q.A.; Zhou, H.T.; Hickford, J.G.H. An effective method for silver-staining DNA in large numbers of poly-acrylamide gels. Anal. Biochem. 2009, 385, 174–175. [Google Scholar] [CrossRef] [PubMed]
- Oldham, C.M.; Thompson, A.N.; Ferguson, M.B.; Gordon, D.J.; Kearney, G.A.; Paganoni, B.L. The birthweight and survival of Merino lambs can be predicated form the profile of liveweight change of their mothers during pregnancy. Anim. Prod. Sci. 2011, 51, 776–783. [Google Scholar] [CrossRef]
- Nowak, R.; Poindron, P. From birth to colostrum: Early steps leading to lamb survival. Reprod. Nutr. Dev. 2006, 46, 431–446. [Google Scholar] [CrossRef] [PubMed]
| Trait Ⅰ | Mean ± SE Ⅱ | p | ||
|---|---|---|---|---|
| AA | AB | BB | ||
| (n = 377) | (n = 332) | (n = 122) | ||
| Birth weight (kg) | 5.9 ± 0.06 a | 5.7 ± 0.06 b | 5.7 ± 0.09 b | 0.023 |
| Weaning weight (kg) | 33.6 ± 0.46 | 33.9 ± 0.46 | 34.2 ± 0.56 | 0.537 |
| Growth rate to weaning (g/d) | 319.9 ± 4.35 | 320.8 ± 4.42 | 323.9 ± 5.79 | 0.799 |
| (n = 236) | (n = 224) | (n = 77) | ||
| HCW (kg) | 17.2 ± 0.22 b | 17.6 ± 0.22 a | 18.0 ± 0.29 a | 0.012 |
| V-GR (mm) | 7.7 ± 0.31 b | 8.3 ± 0.30 a | 8.5 ± 0.39 a | 0.016 |
| Shoulder yield (%) | 17.3 ± 0.10 | 17.4 ± 0.10 | 17.3 ± 0.13 | 0.116 |
| Loin yield (%) | 14.9 ± 0.10 | 14.9 ± 0.10 | 14.9 ± 0.13 | 0.852 |
| Leg yield (%) | 22.2 ± 0.13 | 22.2 ± 0.13 | 22.0 ± 0.17 | 0.276 |
| Total yield (%) | 54.4 ± 0.27 | 54.5 ± 0.26 | 54.2 ± 0.34 | 0.546 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, F.; Zhou, H.; Li, S.; An, Q.; Fang, Q.; Luo, Y.; Hickford, J.G.H. Variation in the Ovine Glycogen Synthase Kinase 3 Beta-Interaction Protein Gene (GSKIP) Affects Carcass and Growth Traits in Romney Sheep. Animals 2021, 11, 2690. https://doi.org/10.3390/ani11092690
Zhao F, Zhou H, Li S, An Q, Fang Q, Luo Y, Hickford JGH. Variation in the Ovine Glycogen Synthase Kinase 3 Beta-Interaction Protein Gene (GSKIP) Affects Carcass and Growth Traits in Romney Sheep. Animals. 2021; 11(9):2690. https://doi.org/10.3390/ani11092690
Chicago/Turabian StyleZhao, Fangfang, Huitong Zhou, Shaobin Li, Qingming An, Qian Fang, Yuzhu Luo, and Jon G. H. Hickford. 2021. "Variation in the Ovine Glycogen Synthase Kinase 3 Beta-Interaction Protein Gene (GSKIP) Affects Carcass and Growth Traits in Romney Sheep" Animals 11, no. 9: 2690. https://doi.org/10.3390/ani11092690
APA StyleZhao, F., Zhou, H., Li, S., An, Q., Fang, Q., Luo, Y., & Hickford, J. G. H. (2021). Variation in the Ovine Glycogen Synthase Kinase 3 Beta-Interaction Protein Gene (GSKIP) Affects Carcass and Growth Traits in Romney Sheep. Animals, 11(9), 2690. https://doi.org/10.3390/ani11092690







