Improving Stereotaxic Neurosurgery Techniques and Procedures Greatly Reduces the Number of Rats Used per Experimental Group—A Practice Report
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Implementation of Aseptic Techniques
2.1.1. Environment and Materials
2.1.2. Preparation of the Surgeon
2.1.3. Preparation of the Animal
2.2. Anesthesia and Presurgical Analgesia
2.3. Stereotaxic Surgery Procedure
2.4. Monitoring Vital Signs during Surgery
2.5. Post-Surgical Procedure
2.6. Pharmacological Experiments
2.7. Post-Mortem Examination of Animals
2.8. Statistics
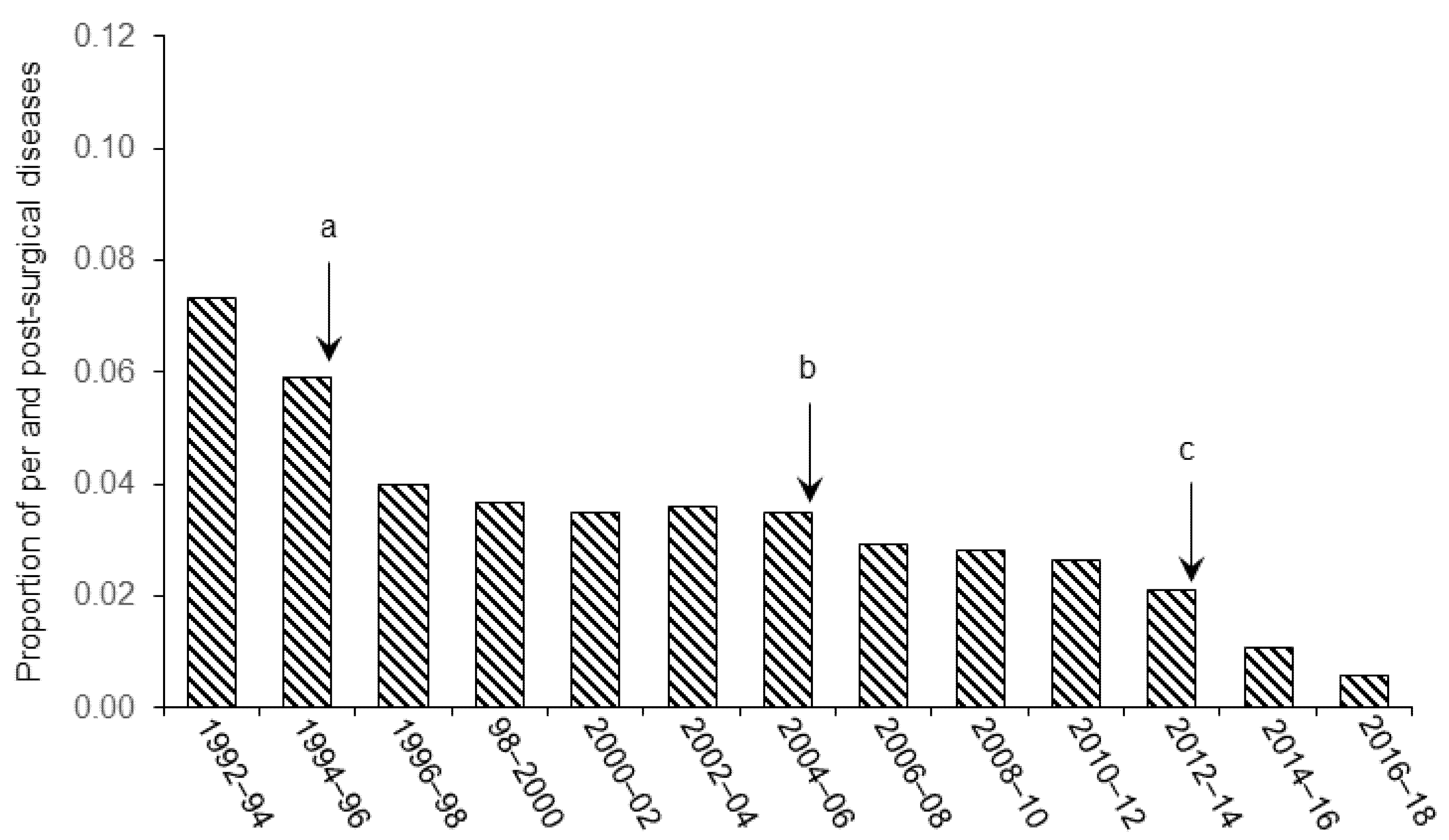
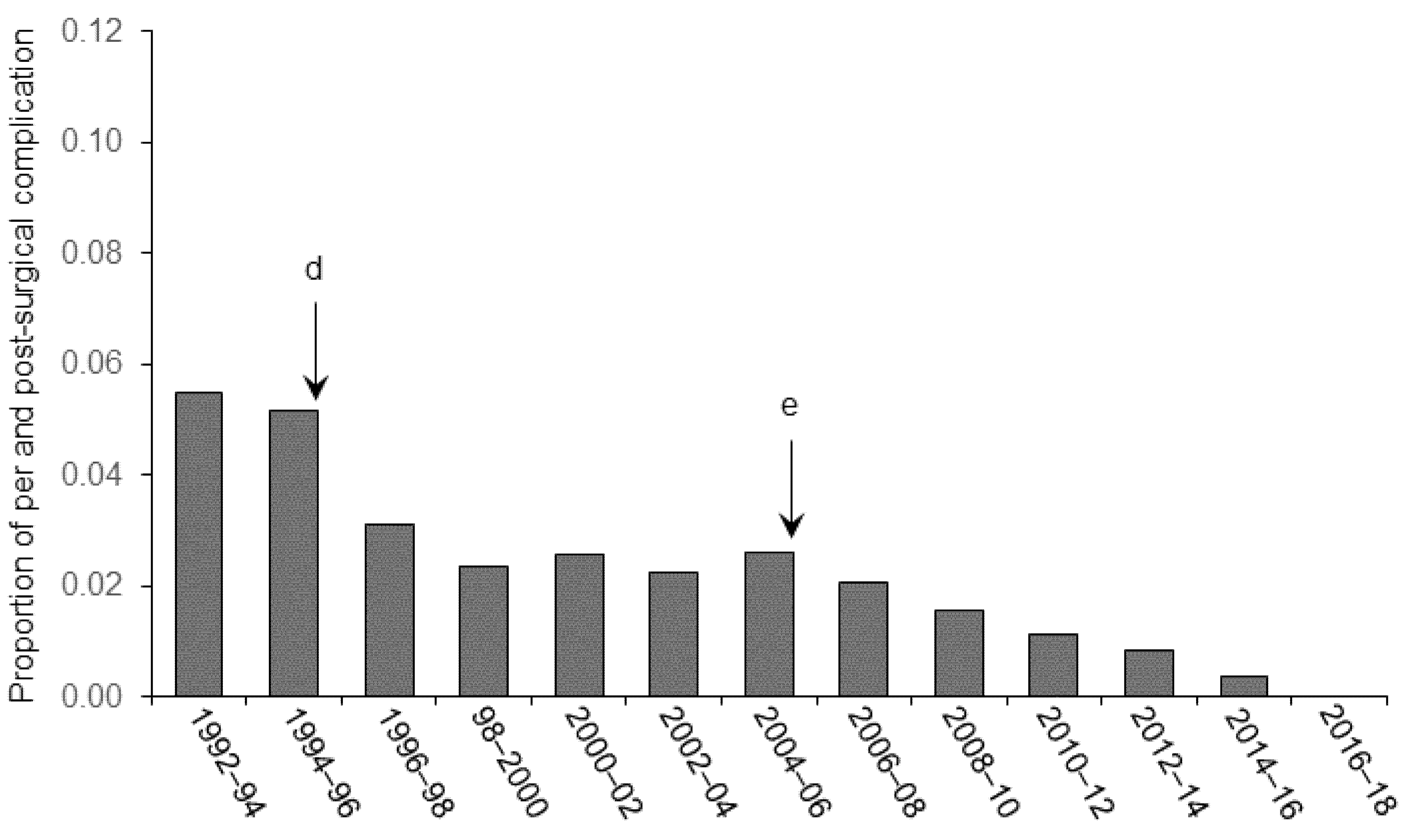
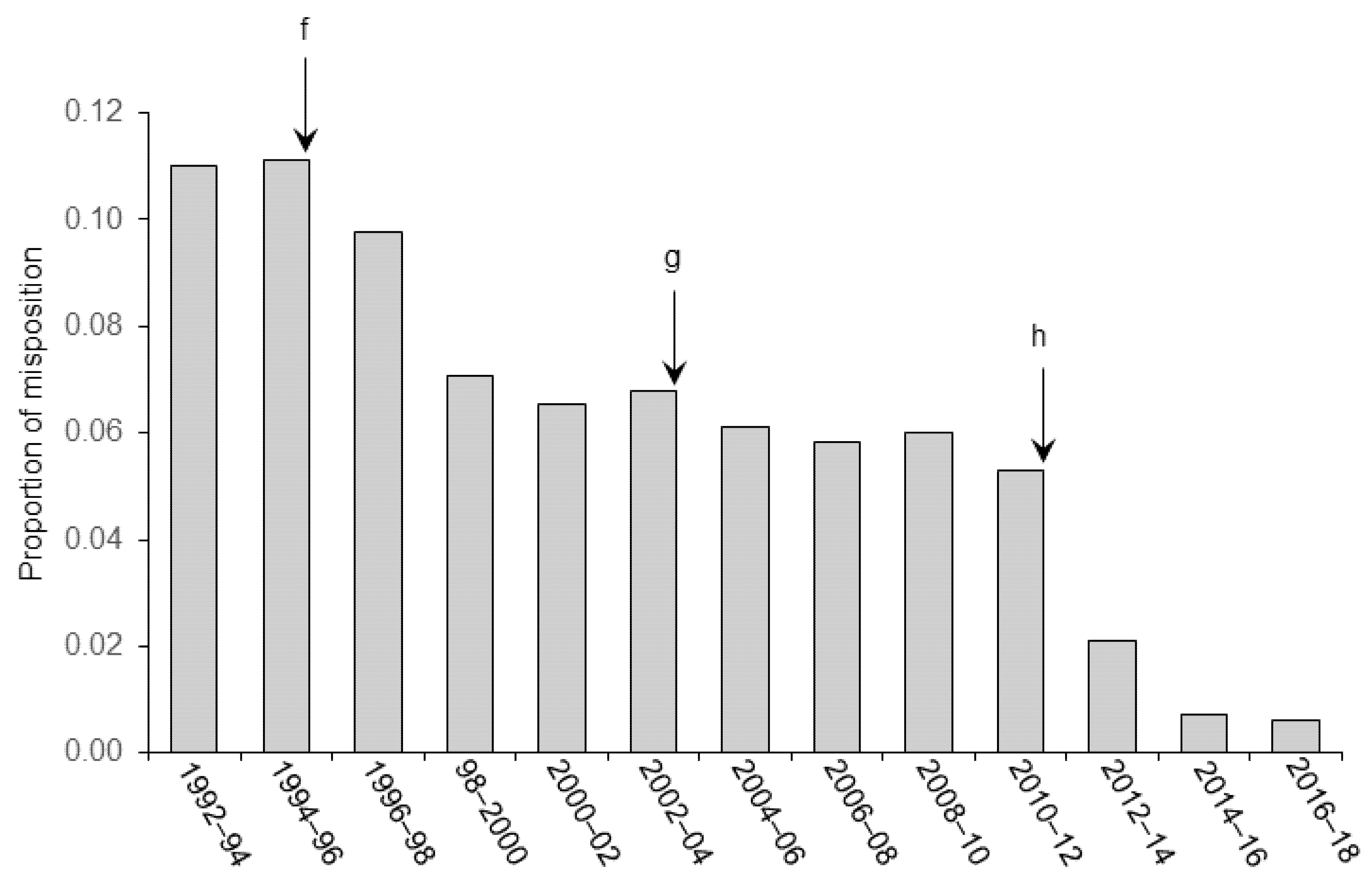
3. Results and Discussion
3.1. Pain and Distress
3.2. Surgical Asepsis
3.3. Stereoaxic Coordinates and Angle of Approach during Surgery
3.4. Further Refinement Perspectives
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes. Official Journal of the European Union, Strasbourg. Available online: http://data.europa.eu/eli/dir/2010/63/2019-06-26 (accessed on 7 September 2021).
- Russell, W.M.S.; Burch, R.L. The Principles of Humane Experimental Technique; Methuen: London, UK, 1959. [Google Scholar]
- National Research Council (US). Committee for the Update of the Guide for the Care and Use of Laboratory Animals. In Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Canadian Council on Animal Care. CCAC Guidelines: Rats. 2020. Available online: http://ccac.ca/Documents/Standards/Guidelines/CCAC_Guidelines_Rats.pdf (accessed on 7 September 2021).
- Guillén, J.; Steckler, T. Good Research Practice: Lessons from Animal Care and Use. In Handbook of Experimental Pharmacology; Bespalov, A., Michel, M.C., Steckler, T., Eds.; Springer: Berlin, Germany, 2019; pp. 367–382. [Google Scholar]
- El Ayache, N.; Galligan, J.J. The rat in neuroscience research. In American College of Laboratory Animal Medicine, The Laboratory Rat, 3rd ed.; Mark, A., Suckow, F., Hankenson, C., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 1003–1022. [Google Scholar]
- Ferry, B.; Sandner, G.; Di Scala, G. Neuroanatomical and functional specificity of basolateral amygdaloid nucleus in Taste Potentiated Odor Aversion. Neurobiol. Learn. Mem. 1995, 64, 169–180. [Google Scholar] [CrossRef]
- Ferry, B.; Oberling, P.; Jarrard, L.E.; Di Scala, G. Facilitation of conditioned odor aversion learning by entorhinal cortex lesions in the rat. Behav. Neurosci. 1996, 110, 443–450. [Google Scholar] [CrossRef]
- Ferry, B.; McGaugh, J.L. Clenbuterol administration into the basolateral amygdala post-training enhances retention in an inhibitory avoidance task. Neurobiol. Learn. Mem. 1999, 72, 8–12. [Google Scholar] [CrossRef]
- Ferry, B.; Roozendaal, B.; McGaugh, J.L. Involvement of alpha1-adrenoceptors in the basolateral amygdala in modulation of memory storage. Eur. J. Pharmacol. 1999, 372, 9–16. [Google Scholar] [CrossRef]
- Ferry, B.; Roozendaal, B.; McGaugh, J.L. Basolateral amygdala noradrenergic influences on memory are mediated by an interaction between beta- and alpha1-adrenoceptors. J. Neurosci. 1999, 19, 5119–5123. [Google Scholar] [CrossRef] [PubMed]
- Roozendaal, B.; De Quervain, D.; Ferry, B.; Setlow, B.; McGaugh, J. Basolateral amygdala-nucleus accumbens interactions in mediating glucocorticoid enhancement of memory consolidation. J. Neurosci. 2001, 21, 2518–2525. [Google Scholar] [CrossRef]
- Majchrzak, M.; Ferry, B.; Marchand, A.R.; Herbeaux, K.; Seillier, A.; Barbelivien, A. Entorhinal cortex lesions disrupt fear conditioning to context but spare fear conditioning to a tone. Hippocampus 2006, 16, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Ferry, B.; Ferreira, G.; Traissard, N.; Majchrzak, M. Selective involvement of the lateral entorhinal cortex in the control of the olfactory memory trace during conditioned odor aversion in the rat. Behav. Neurosci. 2006, 120, 1180–1186. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.I.; Rodríguez-García, G.; Reyes-Lopez, J.V.; Ferry, B.; Ferreira, G. Blockade of β-adrenergic receptors in basolateral amygdala or insular cortex disrupts appetitive but not aversive taste. Neurobiol. Learn. Mem. 2008, 90, 54–61. [Google Scholar]
- Ferry, B.; McGaugh, J.L. Involvement of the basolateral amygdala α2-adrenoceptors in memory modulation of inhibitory avoidance in the rat. Learn. Mem. 2008, 15, 238–243. [Google Scholar]
- Ferry, B.; Duchamp-Viret, P. The orexin component of fasting triggers memory processes underlying food selection in the rat Orexin. Learn. Mem. 2014, 21, 185–189. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Boisselier, L.; Ferry, B.; Gervais, R. Selective involvement of NMDA transmission in the lateral entorhinal cortex for the formation of cross-modal olfactory-tactile associations in the rat. Hippocampus 2014, 24, 877–891. [Google Scholar] [CrossRef]
- Ferry, B.; Parrot, S.; Marien, M.; Lazarus, C.; Cassel, J.-C.; McGaugh, J.L. Noradrenergic influences in the basolateral amygdala on inhibitory avoidance memory are mediated by an action on α2-adrenoceptors. Psychoneuroendocrinology 2015, 51, 68–79. [Google Scholar] [CrossRef]
- Ferry, B.; Herbeaux, K.; Javelot, H.; Majchrzak, M. The entorhinal cortex is involved in conditioned odor and context aversions. Front. Neurosci. Res. 2015, 9, 342. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Boisselier, L.; Ferry, B.; Gervais, R. Respective role of the dorsal hippocampus and the entorhinal cortex during the recombination of previously learned olfactory-tactile associations in the rat. Learn. Mem. 2017, 24, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Estrade, L.; Cassel, J.C.; Parrot, S.; Duchamp-Viret, P.; Ferry, B. Microdialysis unveils the role of the alpha2-adrenergic system in the basolateral amygdala during acquisition of conditioned odor aversion in the rat. ACS Chem. Neurosci. 2019, 10, 1929–1934. [Google Scholar] [CrossRef] [PubMed]
- Harrison, N.L. Knockin’ on the door of general anesthetic mechanisms: But will US researchers be shut out? Anesth. Analg. 2003, 97, 616–618. [Google Scholar] [CrossRef]
- Liles, J.H.; Flecknell, P.A. The effects of buprenorphine, nalbuphine and butorphanol alone or following halothane anaesthesia on food and water consumption and locomotor movement in rats. Lab Anim. 1992, 2, 180–189. [Google Scholar] [CrossRef]
- Roughan, J.V.; Flecknell, P.A. Buprenorphine: A reappraisal of its antinociceptive effects and therapeutic use in alleviating post-operative pain in animals. Lab Anim. 2002, 36, 322–343. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 2nd ed.; Academic Press: Sydney, Australia, 1982. [Google Scholar]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 3rd ed.; Academic Press: San Diego, CA, USA, 1996. [Google Scholar]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 5th ed.; Academic Press: San Diego, CA, USA, 2004. [Google Scholar]
- Hekmatpanah, J. Cerebral microvessel perfusion and pathologic alteration of the brain during drowsiness and coma caused by brain tumor: A laboratory study on rats. Surg. Neurol. 2007, 67, 564–571. [Google Scholar] [CrossRef]
- Ferry, B.; Gervasoni, D.; Vogt, C. Stereotaxic Neurosurgery in Laboratory Rodent-Handbook on Best Practices; Springer: New York, NY, USA, 2014. [Google Scholar]
- Gervasoni, D.; Darracq, L.; Fort, P.; Soulière, F.; Chouvet, G.; Luppi, P.-H. Electrophysiological evidence that noradrenergic neurons of the rat locus coeruleus are tonically inhibited by GABA during sleep. Eur. J. Neurosci. 1998, 10, 964–970. [Google Scholar] [CrossRef] [PubMed]
- Azimi, N.; Yadollahikhales, G.; Argenti, J.P.; Cunningham, M.G. Discrepancies in stereotaxic coordinate publications and improving precision using an animal-specific atlas. J. Neurosci. Methods 2017, 284, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Wirth, S.; Ferry, B.; Di Scala, G. Facilitation of olfactory recognition by entorhinal cortex lesions in the rat. Behav. Brain Res. 1998, 91, 49–59. [Google Scholar] [CrossRef]
- Stokes, E.L.; Flecknell, P.A.; Richardson, C.A. Reported analgesic and anaesthetic administration to rodents undergoing experimental surgical procedures. Lab. Anim. 2009, 43, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Rehbinder, C.; Baneux, P.; Forbes, D. FELASA recommendations for the health monitoring of mouse, rat, hamster, gerbil, guinea pig and rabbit experimental units. Lab. Anim. 1995, 30, 193–208. [Google Scholar] [CrossRef]
- Nicklas, W.; Baneux, P.; Boot, R.; Decelle, T.; Deeny, A.A.; Fumanelli, M.; Illgen-Wilcke, B. Recommendations for the health monitoring of rodent and rabbit colonies in breeding and experimental units. Lab. Anim. 2002, 36, 20–42. [Google Scholar] [CrossRef]
- Flecknell, P. Laboratory Animals Anesthesia, 3rd ed.; Academic Press: San Diego, CA, USA, 2009. [Google Scholar]
- Albrecht, M.; Henke, J.; Tacke, S.; Markert, M.; Guth, B. Influence of repeated anaesthesia on physiological parameters in male Wistar rats: A telemetric study about isoflurane, ketamine-xylazine and a combination of medetomidine, midazolam and fentanyl. BMC Vet. Res. 2014, 10, 310. [Google Scholar] [CrossRef]
- Bär, T.; Scheck, T. The vascular system of the rat cerebral cortex-basic pattern of leptomeningeal vessels and numerical densities of neocortical arteries and veins. In The Cerebral Veins—An Experimental and Clinical Update; Auer, L.M., Loew, E.E., Eds.; Springer: Wien, Austria, 1983; pp. 65–72. [Google Scholar]
- Scremin, O.U. Cerebral Vascular System. In The Rat Nervous System; Paxinos, G., Ed.; Academic Press: New York, NY, USA, 2004; pp. 1167–1202. [Google Scholar]
- Dashwood, M.R.; Loesch, A. Endothelin-1 as a neuropeptide: Neurotransmitter or vascular effects? J. Cell Commun. Signal 2010, 4, 51–62. [Google Scholar] [CrossRef]
- Sarabia-Estrada, R.; Cowan, A.; Tyler, B.; Guarnieri, M. Association of nausea with buprenorphine analgesia for rats. Lab. Anim. 2017, 46, 242–244. [Google Scholar] [CrossRef]
- Cowan, A.; Doxey, J.C.; Harry, E.J. The animal pharmacology of buprenorphine, an oripavine analgesic agent. Br. J. Pharmacol. 1977, 60, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Kuo, A.; Smith, M.T. In vivo profiling of four centrally administered opioids for antinociception, constipation and respiratory depression: Between-colony differences in Sprague Dawley rats. Clin. Exp. Pharmacol. Physiol. 2018, 45, 1056–1066. [Google Scholar] [CrossRef]
- Sonner, J.M.; Antognini, J.F.; Dutton, R.C.; Flood, P.; Andrew, T.; Harris, A.R.; Homanics, G.E.; Kendig, J.; Orser, B.; Raines, D.; et al. Inhaled anesthetics and immobility: Mechanisms, mysteries and MAC. Anesth. Analg. 2003, 97, 718–740. [Google Scholar] [CrossRef]
- Jensen, E.W.; Litvan, H.; Struys, M.; Martinez Vazquez, P. Pitfalls and challenges when assessing the depth of hypnosis during general anesthesia by clinical signs and electronic indices. Acta Anaesthesiol. Scand. 2004, 48, 1267–1269. [Google Scholar] [CrossRef] [PubMed]
- Messier, C.; Emond, S.; Ethier, K. New techniques in stereotaxic surgery and anesthesia in the mouse. Pharmacol. Biochem. Behav. 1999, 63, 313–318. [Google Scholar] [CrossRef]
- Jurd, R.; Arras, M.; Lambert, S.; Drexler, B.; Siegwart, R.; Crestani, F.; Zaugg, M.; Vogt, K.E.; Ledermann, B.; Antkowiak, B.; et al. General anesthetic actions in vivo strongly attenuated by a point mutation in the GABA(A) receptor beta3 subunit. Fed. Am. Soc. Exp. Biol. J. 2003, 17, 250–252. [Google Scholar]
- Fornari, R.V.; Wichmann, R.; Atsak, P.; Atucha, E.; Barsegyan, A.; Beldjoud, H.; Messanvi, F.; Thuring, C.M.A.; Roozendaal, B. Rodent stereotaxic surgery and animal welfare outcome improvements for behavioral neuroscience. J. Vis. Exp. 2012, 59, e3528. [Google Scholar] [CrossRef] [PubMed]
- De Vloo, P.; Nuttin, B. Stereotaxy in rat models: Current state of the art, proposals to improve targeting accuracy and reporting guideline. Behav. Brain Res. 2019, 364, 457–463. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferry, B.; Gervasoni, D. Improving Stereotaxic Neurosurgery Techniques and Procedures Greatly Reduces the Number of Rats Used per Experimental Group—A Practice Report. Animals 2021, 11, 2662. https://doi.org/10.3390/ani11092662
Ferry B, Gervasoni D. Improving Stereotaxic Neurosurgery Techniques and Procedures Greatly Reduces the Number of Rats Used per Experimental Group—A Practice Report. Animals. 2021; 11(9):2662. https://doi.org/10.3390/ani11092662
Chicago/Turabian StyleFerry, Barbara, and Damien Gervasoni. 2021. "Improving Stereotaxic Neurosurgery Techniques and Procedures Greatly Reduces the Number of Rats Used per Experimental Group—A Practice Report" Animals 11, no. 9: 2662. https://doi.org/10.3390/ani11092662
APA StyleFerry, B., & Gervasoni, D. (2021). Improving Stereotaxic Neurosurgery Techniques and Procedures Greatly Reduces the Number of Rats Used per Experimental Group—A Practice Report. Animals, 11(9), 2662. https://doi.org/10.3390/ani11092662





