Epidemiology and Cost of Peste des Petits Ruminants (PPR) Eradication in Small Ruminants in the United Arab Emirates—Disease Spread and Control Strategies Simulations
Abstract
Simple Summary
Abstract
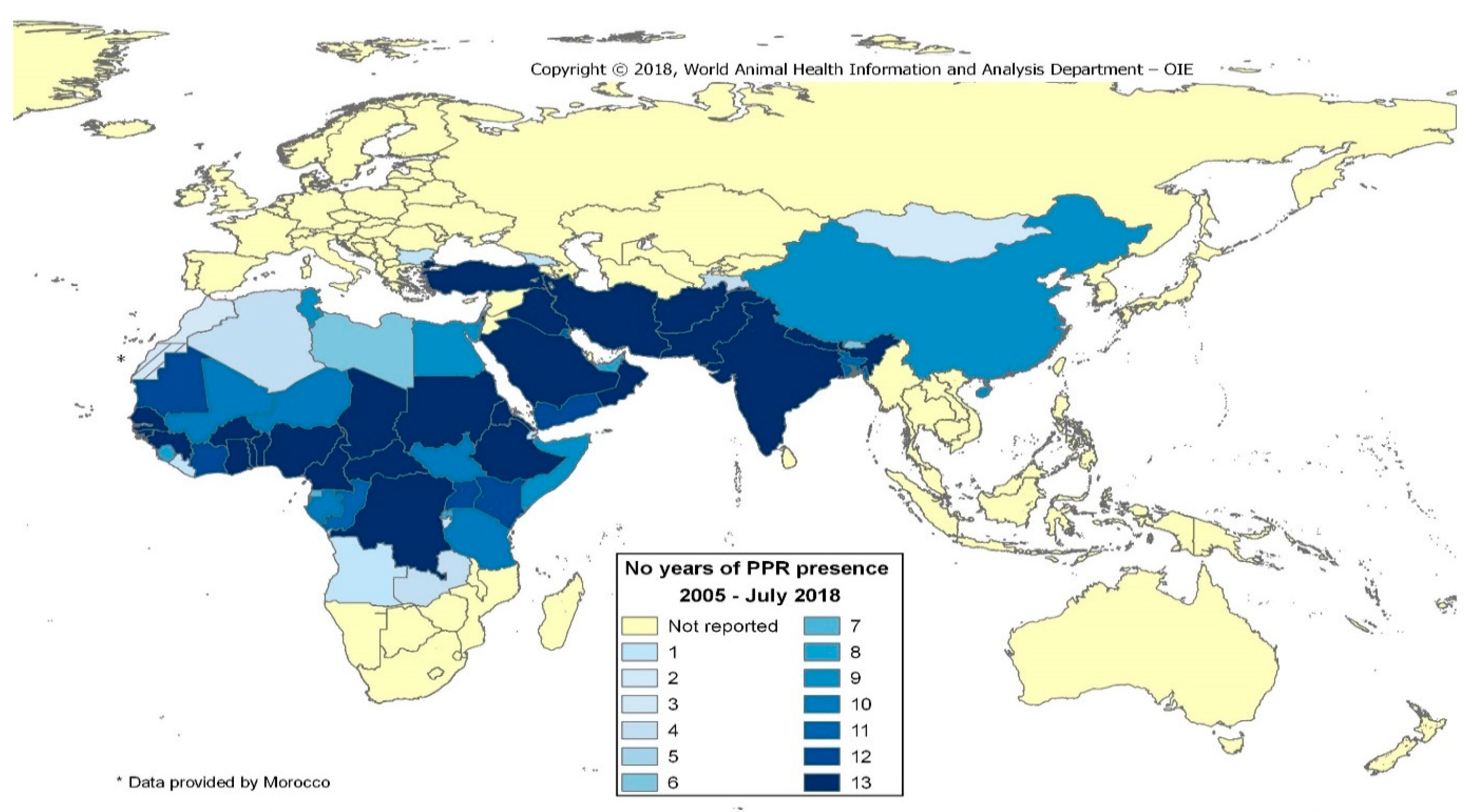
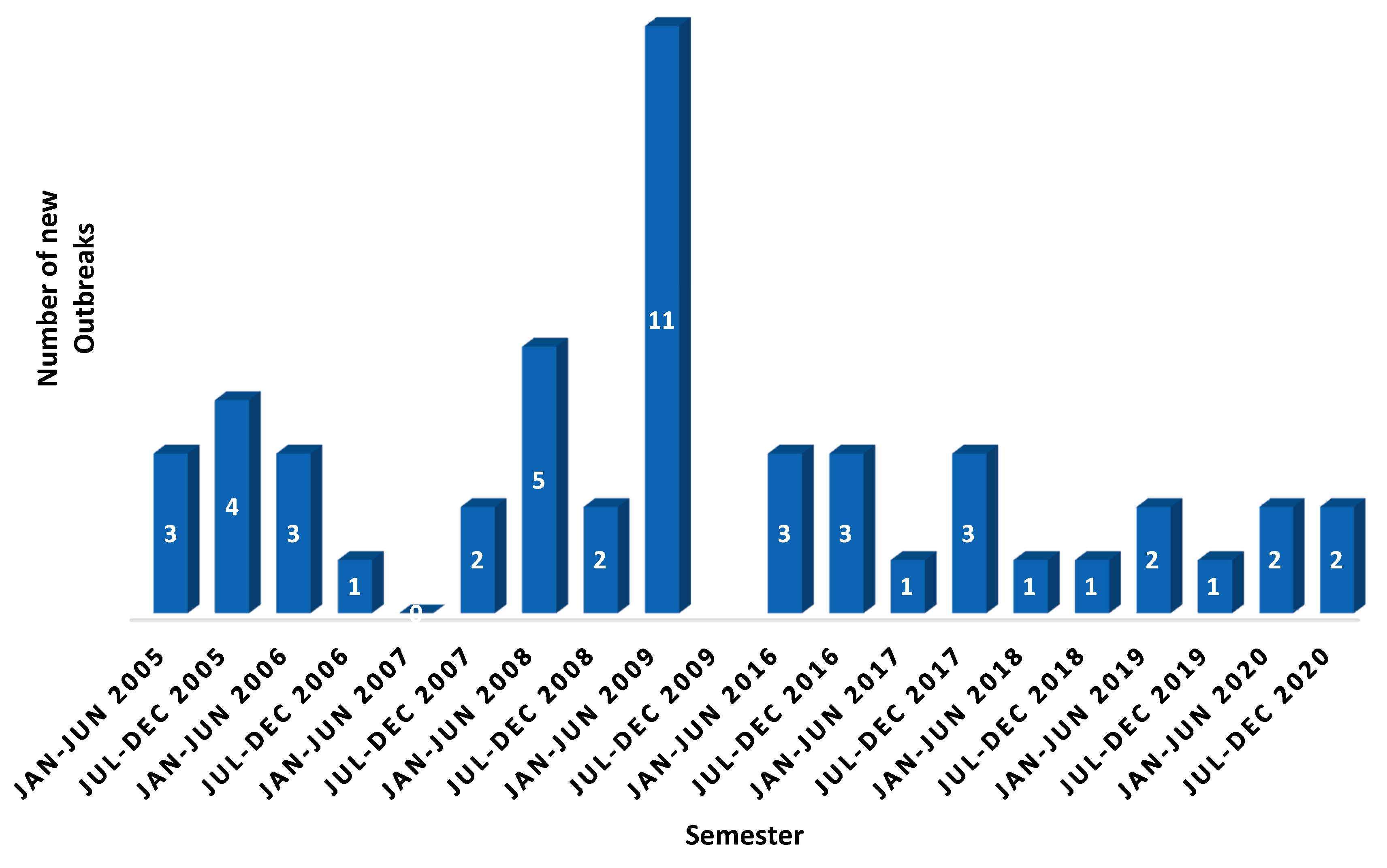
1. Background
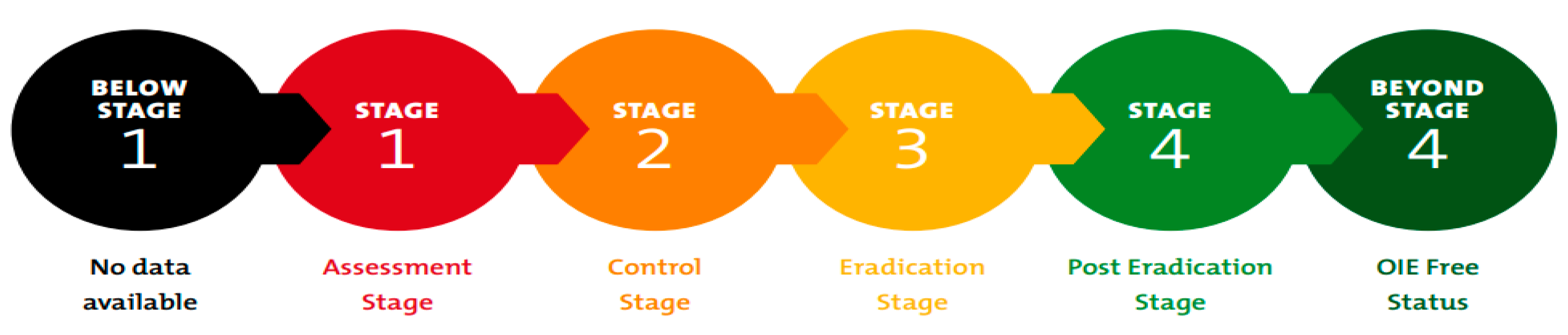
2. Global Strategy to Eradicate Peste des Petits Ruminants (PPR)
3. Objective and Simulation Scenarios
4. Data and Method
4.1. Data
4.2. Methodology and Simulation Scenarios
- This scenario illustrates the case where there is no virus circulation either at the zonal or national level and the country is ready to apply for official OIE recognition of PPR freedom. This scenario represents a strategy that recognizes the initial situations when the country, here the UAE, has already controlled the disease in areas where it is highly endemic, mass vaccination is ceased (no vaccination), and moderate movement restrictions are applied.
- This scenario represents the case where efforts and the control strategy are based on mass vaccination and stamping out the disease to reduce the impact of PPR at the national level to the minimum level possible.
- The full eradication scenario represents a strategy based on mass and ring vaccination when needed (vaccinating detected herds when they occur in the vaccination ring), very strict movement controls, and stamping out the disease.
5. PPR Epidemiology and Cost
- The morbidity rate in susceptible populations can reach 90–100%;
- Mortality rates vary among susceptible animals, but can reach 50–100% in more severe instances;
- Both morbidity and mortality rates are lower in endemic areas and in adult animals when compared to the young;
- The latent period is considered 3 days minimum, 7 days mode, and 10 days maximum using the Beta-PERT probability function;
- The period required for herd-level natural immunity to be achieved, either through infection and recovery or vaccination, is considered to be 1460 days (4 years). Natural immunity was modeled as having a normal distribution;
5.1. PPR Detection and Clinical Diagnosis
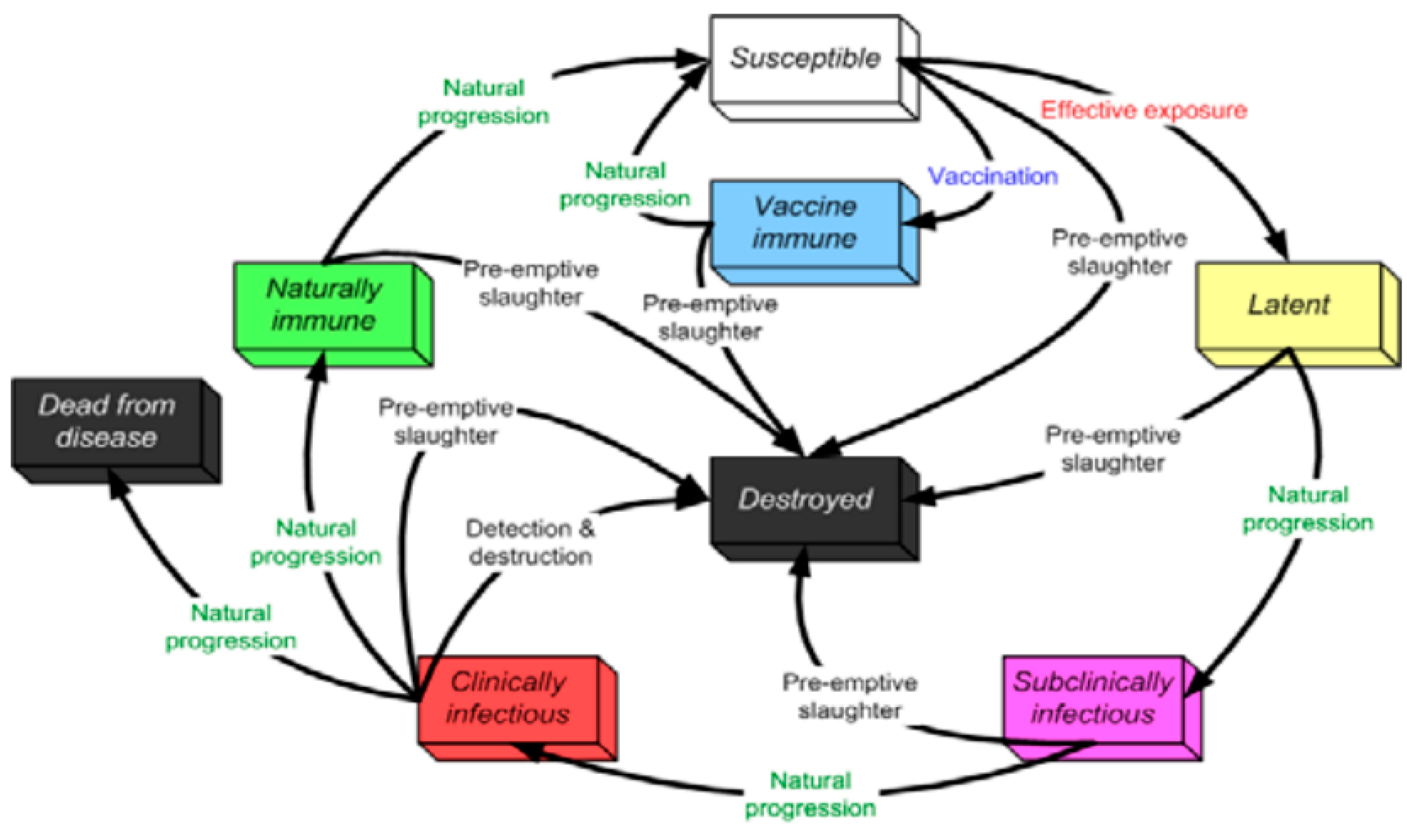
5.2. PPR Disease States
5.3. Animals Movements and Indirect Contacts
5.3.1. Direct Contact Spread Parameters
- Mean rate of animal shipments (number of recipient units per source unit per day);
- Movement distance (probability density function per km);
- Shipping delay (probability density function per days);
- The probability of infection of the recipient unit, given exposure to an infected unit;
- Movement rate multiplier (scalar value as a function of the number of days since the first detection of the outbreak).
5.3.2. Indirect Contact Spread Parameters
6. Peste des Petits Ruminants (PPR) Control Strategies
6.1. Zoning and Tracing
6.2. Isolation and Quarantine Parameters
6.3. Destruction (Depopulation) Parameters
- Delay to beginning a destruction program (fixed integer value per days)
- Destruction capacity (relational function: number of units that can be destroyed as a function of the number of days since the first detection of an outbreak);
- Destruction priorities (rank order of reasons for unit destruction, as described in the text);
- The radius of the destruction ring, if a ring is triggered;
- Destruction capacity is assumed to be 100 units/herd per week
6.4. Vaccination and Immunity Parameters
- Number of units that must be detected before vaccination begins (number of detected units), which is assumed to be one unit/herd detected;
- Vaccination capacity (relational function: number of units that can be vaccinated as a function of the number of days since the first detection of an outbreak). The vaccination capacity in the model is assumed to 100 units/herd per week;
- Vaccination priorities (Rank order of reasons for unit vaccination)—vaccinate detected unit when they occur in the vaccination rings;
- The radius of the vaccination ring, if a ring is triggered and other parameters, which is assumed to be 3 km.
7. Costs Estimation Parameters
- Appraisal;
- Cleaning and disinfection;
- Euthanasia;
- Indemnification;
- Carcass disposal;
- Parameters associated with cost of vaccination;
- Number of animals that can be vaccinated at the baseline cost;
- The baseline cost of vaccination;
- Additional cost incurred when the number of animals vaccinated exceeds the threshold set;
- Cost of vaccination site set-up.
8. Peste des Petits Ruminants (PPR) Simulation Results and Discussion
9. Direct Government Costs Comparison of PPR Eradication Scenarios Results
10. Conclusions
11. Limitations and Future Research
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| A- Destruction and Cleaning and Disinfection (C&D): | Unit/Animal | Camels | Sheep and Goats | Small Ruminants Farms | |
|---|---|---|---|---|---|
| Appraisal | Unit | 1183 | 29.7 | 19.71 | |
| Cleaning and Disinfection | Unit | 217,983 | 779.49 | 591.3 | |
| Indemnification | Animal | 1232 | 168.48 | 98.55 | |
| Euthanasia | Animal | 72 | 71.82 | 71.82 | |
| Caracas Disposal | Animal | 30 | 9.99 | 9.99 | |
| B- Vaccination: | |||||
| Site Set-up | Unit | 11,517 | 533.25 | 492.75 | |
| Baseline Vaccination | Animal | 3 | 1.89 | 1.08 | |
| Number of Animals Before Vac. Cost Increases | Number of Heads | 2700 | 135 | 135 | |
| Additional Cost beyond Threshold | Animal | 1 | 1.08 | 1.08 | |
| C- Zoning: | |||||
| High Risk Zone | Animal | 79 | 23.76 | 19.71 | |
| Low Risk Zone | Animal | 59 | 9.99 | 9.99 |
References
- Kinne, J.; Kreutzer, R.; Kreutzer, M.; Wernery, U.; Wohlsein, P. Peste des petits ruminants in Arabian wildlife. Epidemiol. Infect. 2010, 138, 1211–1214. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Maherchandani, S.; Kashyap, S.K.; Singh, S.V.; Sharma, S.; Chaubey, K.K.; Ly, H. Peste des petits ruminants virus infection of small ruminants: A comprehensive review. Viruses 2014, 6, 2287–2327. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization (FAO); World Organization for Animal Health (OIE). Global Strategy for the Control and Eradication of PPR; FAO: Rome, Italy; OIE: Paris, France, 2015. [Google Scholar]
- Furley, C.W.; Taylor, W.P.; Obi, T.U. An outbreak of peste des petits ruminants in a zoological collection. Vet. Rec. 1987, 121, 443–447. [Google Scholar] [CrossRef] [PubMed]
- World Organization for Animal Health. World Animal Health Information System OIE-WAHIS. Quantitative Data. Available online: https://wahis.oie.int/#/dashboards/qd-dashboard (accessed on 10 June 2021).
- Mitchell, M.D.; Beyeler, W.E.; Finley, P.; Finley, M. Modeling Peste des Petits Ruminants (PPR) Disease Propagation and Control Strategies Using Memoryless State Transitions. Appl. Sci. Innov. Res. 2017, 1. [Google Scholar] [CrossRef][Green Version]
- World Organization for Animals Health (OIE). Peste des Petits Ruminants Global Eradication Programme. In Contributing to Food Security, Poverty Alleviation and Resilience; Food and Agriculture Organization of the United Nations: Rome, Italy; World Organisation for Animal Health: Paris, France, 2016. [Google Scholar]
- Lyons, N.A.; Jemberu, W.T.; Chaka, H.; Salt, J.S.; Rushton, J. Field-derived estimates of costs for Peste des Petits Ruminants vaccination in Ethiopia. Prev. Vet. Med. 2019, 163, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Fournié, G.; Waret-Szkuta, A.; Camacho, A.; Yigezu, L.M.; Pfeiffer, D.U.; Roger, F. A dynamic model of transmission and elimination of peste des petits ruminants in Ethiopia. Proc. Natl. Acad. Sci. USA 2018, 115, 8454–8459. [Google Scholar] [CrossRef] [PubMed]
- The North American Animal Disease Spread Model NAADSM. Available online: https://pubmed.ncbi.nlm.nih.gov/17614148/ (accessed on 15 February 2021).
- Zakian, A.; Nouri, M.; Kahroba, H.; Mohammadian, B.; Mokhber-Dezfouli, M.-R. The first report of peste des petits ruminants (PPR) in camels (Camelus dromedarius) in Iran. Trop. Anim. Health Prod. 2016, 48, 1215–1219. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.-U.; Dhama, K.; Ali, Q.; Hussain, I.; Oneeb, M.; Chaudhary, U.; Wensman, J.J.; Shabbir, M.Z. Peste des petits ruminants in large ruminants, camels and unusual hosts. Vet. Q. 2020, 40, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Bates, T.W.; Thurmond, M.C.; Carpenter, T.E. Results of epidemic simulation modeling to evaluate strategies to control an outbreak of foot-and-mouth disease. Am. J. Vet. Res. 2003, 64, 205–210. [Google Scholar] [CrossRef]
- Garner, M.G.; Beckett, S. Modelling the spread of foot--and--mouth disease in Australia. Aust. Vet. J. 2005, 83, 758–766. [Google Scholar] [CrossRef] [PubMed]
- Harvey, N.; Reeves, A.; Schoenbaum, M.A.; Zagmutt-Vergara, F.J.; Dubé, C.; Hill, A.E.; Corso, B.A.; McNab, W.B.; Cartwright, C.I.; Salman, M.D. The North American Animal Disease Spread Model: A simulation model to assist decision making in evaluating animal disease incursions. Prev. Vet. Med. 2007, 82, 176–197. [Google Scholar] [CrossRef] [PubMed]
- The Center for Food Security and Public Health. Fact Sheets on Peste des Petit Ruminants. August 2015. Available online: https://www.cfsph.iastate.edu/Factsheets/pdfs/peste_des_petits_ruminants.pdf (accessed on 12 June 2021).
- Niu, B.; Liang, R.; Zhou, G.; Zhang, Q.; Su, Q.; Qu, X.; Chen, Q. Prediction for global Peste des petits ruminants outbreaks based on a combination of random forest algorithms and meteorological data. Front. Vet. Sci. 2020, 7. [Google Scholar] [CrossRef]
- Niedbalski, W. Eradication of peste des petits ruminants: Application of new research to guide and facilitate the global elimination of the disease. Med. Weter. 2020, 76. [Google Scholar] [CrossRef]
- Cameron, A.R. Strategies for the global eradication of peste des petits ruminants: An argument for the use of guerrilla rather than trench warfare. Front. Vet. Sci. 2019, 6, 331. [Google Scholar] [CrossRef] [PubMed]
- OIE; FAO; GF TADs. The Inter Regional Consultative Meeting on FMD and PPR Situation Progress. 2014. Available online: http://www.fao.org/eufmd/meetings-and-events/detail/ar/c/1177008 (accessed on 23 June 2021).

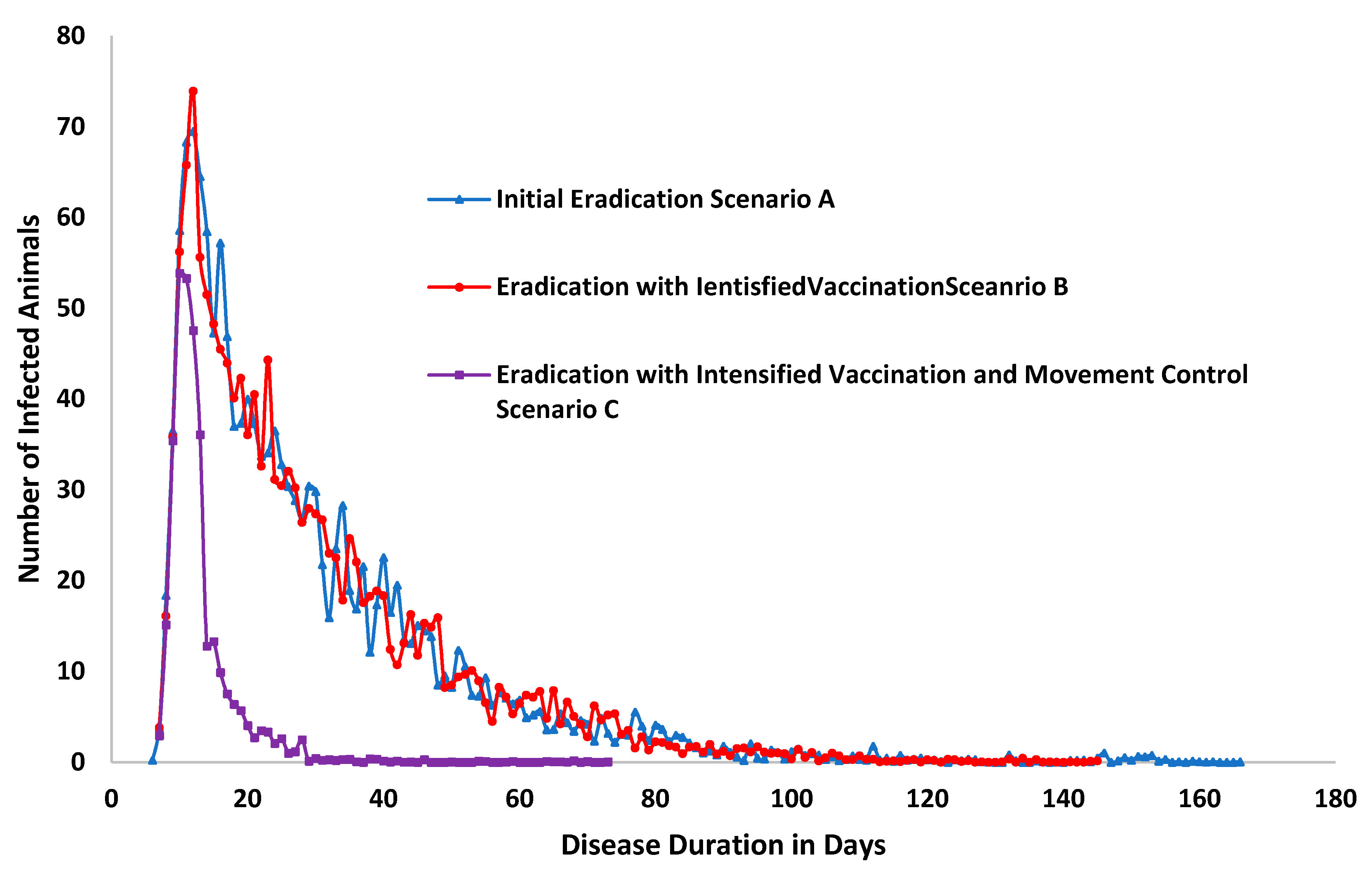
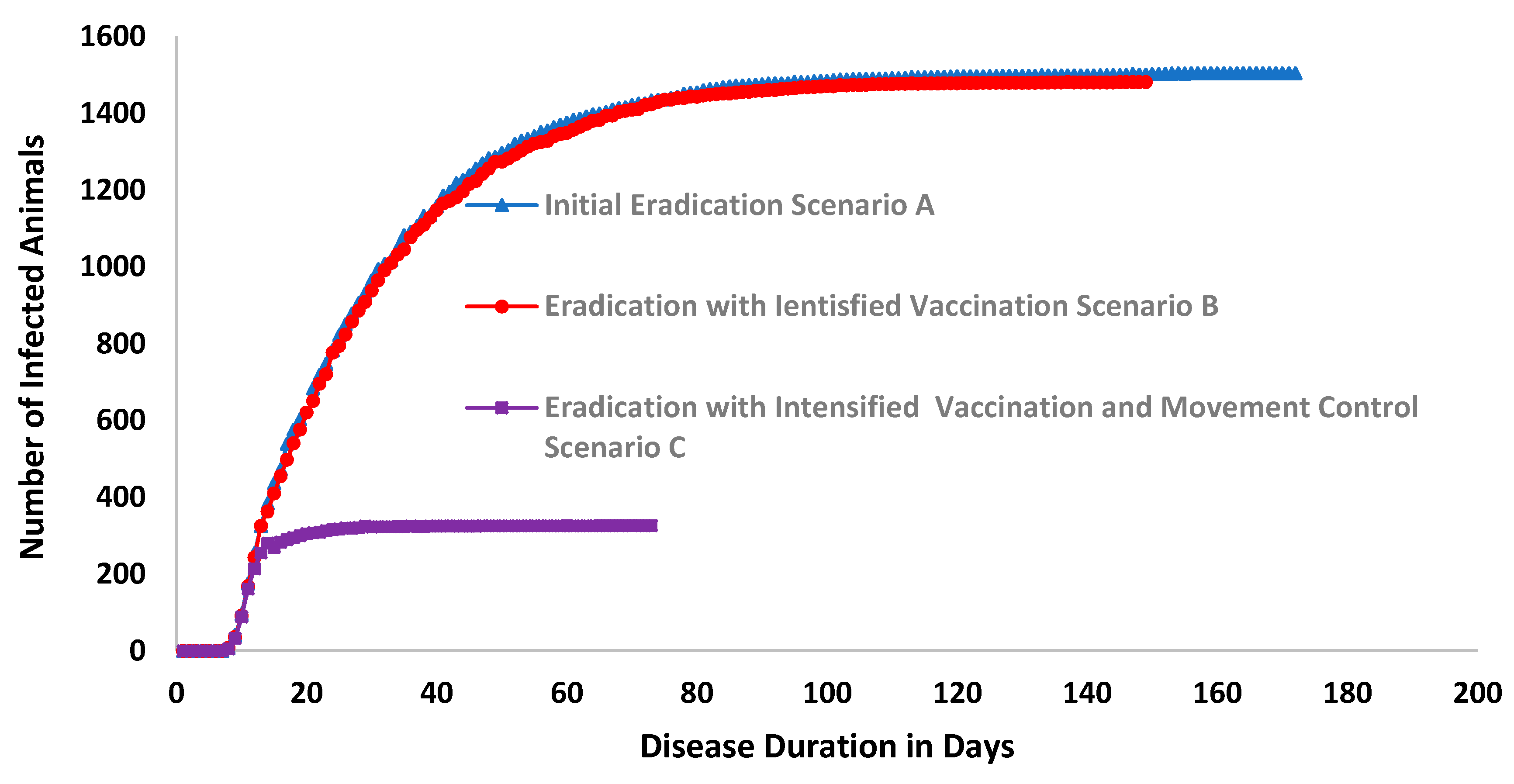
| Unit | Scenario A | Scenario B | Scenario C | |
|---|---|---|---|---|
| Direct Contact: Main Base-line Contact Rate: | Shipments of Units/Unit/Day | |||
| Sheep and Goat Commercial Farms | 0.1 | 0.1 | 0.015 | |
| Small and Goat Small Farms | 0.1 | 0.1 | 0.015 | |
| Camels Farms | 0.5 | 0.5 | 0.015 | |
| Indirect Contact: Probability of Infection Transfer: | 0–1 probability | |||
| Sheep and Goat Commercial Farms | 0.5 | 0.5 | 0.25 | |
| Small and Goat Small Farms | 0.5 | 0.5 | 0.25 | |
| Camel Farms | 0.25 | 0.25 | 0.25 | |
| Distance Distribution of Recipients Units: | Beta-PERT Distribution (Minimum. Mode, and Maximum) in Kilometers | |||
| Sheep and Goat Commercial Farms | 0, 20, 400 | 0, 20, 400 | 0, 20, 400 | |
| Small and Goat Small Farms | 0, 20, 400 | 0, 20, 400 | 0, 20, 400 | |
| Camel Farms | 0, 20, 400 | 0, 20, 400 | 0, 20, 400 |
| Outcome over the Iterations (Number of Animals in all Units or Duration in Days) | Initial Eradication Scenario (Scenario A) | Eradication with Intensified Vaccination Scenario (Scenarios B) | Percentage Change in Scenario B from Initial Scenario A (Scenario B—Scenario A)/Scenario A | Complete Eradication (Intensified Vaccination and Movement Control (Scenario C) | Percentage Change in Scenario C from Initial Scenario A (Scenario C—Scenario A)/Scenario A |
|---|---|---|---|---|---|
| Number of susceptible animals | 2,954,213 | 2,954,213 | - | 2,954,213 | - |
| Number of latent animals | 1327 | 1316 | −1% | 315 | −76% |
| Number of animals showing subclinical signs | 887 | 889 | 0% | 271 | −69% |
| Number of animals showing clinical signs | 690 | 697 | 1% | 255 | −63% |
| Number of animals that are destroyed | 1384 | 1370 | −1% | 321 | −77% |
| Number of animals becoming infected (not including initially infected units) | 1103 | 1092 | −1% | 170 | −85% |
| Number of animals exposed to an infected animal | 2454 | 2476 | 1% | 227 | −91% |
| Number of animals directly exposed that could possibly have been traced forward | 1833 | 1837 | 0% | 71 | −96% |
| Total number of animals in units successfully identified by tracing (either forward or back) after direct contact | 1638 | 1644 | 0% | 63 | −96% |
| Number of animals in units successfully identified by tracing (either forward or back) after contact (either direct or indirect) | 2220 | 2248 | 1% | 208 | −91% |
| Number of animals in unique units detected for any reason | 1384 | 1370 | −1% | 321 | −77% |
| Number of animals subjected to diagnostic testing after a trace forward or trace back after direct contact | 1444 | 1461 | 1% | 56 | −96% |
| Number of animals subjected to diagnostic testing after a trace forward or trace back after (either direct or indirect contact) contact | 1962 | 2007 | 2% | 421 | −79% |
| Number of animals in tested units with a true negative diagnostic test result | 1046 | 1111 | 6% | 1 | −100% |
| Total number of animals vaccinated over the iterations. | - | 35,061 | 100% | 2704 | −92% |
| Duration of outbreak in days | 171 | 148 | −13% | 73 | −57% |
| Cost Item | Initial Eradication Scenario | Eradication with Intensified Vaccination Scenario | Percentage Change from Initial Scenario (Scenario B—Scenario A)/Scenario A | Complete Eradication (Intensified Vaccination and Movement Control) Scenario | Percentage Change from Vaccination Triggered Ring Scenario (Scenario C—Scenario B)/Scenario B |
|---|---|---|---|---|---|
| Depopulation Appraisal | 2554 | 2603 | 2% | 399 | −85% |
| Depopulation Cleaning and Disinfection | 434,677 | 442,157 | 2% | 66,218 | −85% |
| Depopulation Euthanasia | 99,414 | 98,411 | −1% | 23,003 | −77% |
| Depopulation Indemnification | 466,200 | 432,833 | −7% | 83,323 | −81% |
| Depopulation Disposal | 18,271 | 17,563 | −4% | 3755 | −79% |
| Depopulation Subtotal | 1,021,116 | 993,568 | −3% | 176,699 | −82% |
| Vaccination Setup | n/a | 424,381 | 100% | 31,505 | −93% |
| Vaccination | n/a | 100,160 | 100% | 7284 | −93% |
| Vaccination Subtotal | n/a | 524,542 | 100% | 38,789 | −93% |
| Total Costs | 1,021,116 | 1,518,110 | 49% | 215,488 | −86% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fathelrahman, E.M.; Reeves, A.; Mohamed, M.S.; Ali, Y.M.E.; El Awad, A.I.; Bensalah, O.-K.; Abdalla, A.A. Epidemiology and Cost of Peste des Petits Ruminants (PPR) Eradication in Small Ruminants in the United Arab Emirates—Disease Spread and Control Strategies Simulations. Animals 2021, 11, 2649. https://doi.org/10.3390/ani11092649
Fathelrahman EM, Reeves A, Mohamed MS, Ali YME, El Awad AI, Bensalah O-K, Abdalla AA. Epidemiology and Cost of Peste des Petits Ruminants (PPR) Eradication in Small Ruminants in the United Arab Emirates—Disease Spread and Control Strategies Simulations. Animals. 2021; 11(9):2649. https://doi.org/10.3390/ani11092649
Chicago/Turabian StyleFathelrahman, Eihab M., Aaron Reeves, Meera S. Mohamed, Yassir M. Eltahir Ali, Adil I. El Awad, Oum-Keltoum Bensalah, and Afra A. Abdalla. 2021. "Epidemiology and Cost of Peste des Petits Ruminants (PPR) Eradication in Small Ruminants in the United Arab Emirates—Disease Spread and Control Strategies Simulations" Animals 11, no. 9: 2649. https://doi.org/10.3390/ani11092649
APA StyleFathelrahman, E. M., Reeves, A., Mohamed, M. S., Ali, Y. M. E., El Awad, A. I., Bensalah, O.-K., & Abdalla, A. A. (2021). Epidemiology and Cost of Peste des Petits Ruminants (PPR) Eradication in Small Ruminants in the United Arab Emirates—Disease Spread and Control Strategies Simulations. Animals, 11(9), 2649. https://doi.org/10.3390/ani11092649










