Evaluation of Diet Supplementation with Wheat Grass Juice on Growth Performance, Body Composition and Blood Biochemical Profile of Carp (Cyprinus carpio L.)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish Trial
2.2. Wheat Grass Juice Extraction
2.3. Chemical Analysis of Wheat Grass Juice
2.4. Fish Growth Indices
- IBW—initial body weight (g);
- FBW—final body weight (g);
- WG—weight gain (g) = FBW − IBW;
- FCR—feed conversion ratio (g/g) = Feed intake (g)/WG;
- RGR—relative growth rate (g/g day−1) = WG/days of experiment/IBW;
- SGR—specific growth rate (% day−1) = (ln FBW − ln IBW)/days of experiment × 100;
- PER—protein efficiency ratio = WG/total protein;
- CF—condition factor = FBW/body length3 × 100.
2.5. Body Composition
2.6. Blood Parameters
2.7. Statistical Analysis
3. Results
3.1. Yield and Biochemical Composition of Wheat Grass Juice
3.2. Fish Growth Performance
3.3. Body Composition
3.4. Blood Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture (SOFIA); FAO: Rome, Italy, 2020; ISBN 978-92-5-132692-3. [Google Scholar]
- Eurostat. Available online: https://ec.europa.eu/eurostat/web/fisheries/data/database (accessed on 1 July 2021).
- Hoseinifar, S.H.; Rashidian, G.; Ghafarifarsani, H.; Jahazi, M.A.; Soltani, M.; Doan, H.V.; El-Haroun, E.; Paolucci, M. Effects of apple (Malus pomila) pomace-derived pectin on the innate immune responses, expressions of key immune-related genes, growth performance, and digestive enzyme activity of rainbow trout (Oncorhynchus mykiss). Animals 2021, 11, 2117. [Google Scholar] [CrossRef]
- Reverter, M.; Bontemps, N.; Lecchini, D.; Banaigs, B.; Sasal, P. Use of plant extracts in fish aquaculture as an alternative to chemotherapy: Current status and future perspectives. Aquaculture 2014, 433, 50–61. [Google Scholar] [CrossRef]
- Van Hai, N. The use of medicinal plants as immunostimulants in aquaculture: A review. Aquaculture 2015, 446, 88–96. [Google Scholar] [CrossRef]
- Reverter, M.; Tapissier-Bontemps, N.; Sarter, S.; Sasal, P.; Caruso, D. Moving towards more sustainable aquaculture practices: A meta-analysis on the potential of plant-enriched diets to improve fish growth, immunity and disease resistance. Rev. Aquacult. 2021, 13, 537–555. [Google Scholar] [CrossRef]
- Mohammadi Gheisar, M.; Zhao, P.; Kim, I.H. Addition of phytogenic blend in different nutrient density diets of meat-type ducks. J. Appl. Anim. Res. 2018, 46, 854–859. [Google Scholar] [CrossRef] [Green Version]
- Abudabos, A.M.; Alyemni, A.H.; Dafalla, Y.M.; Khan, R.U. The effect of phytogenics on growth traits, blood biochemical and intestinal histology in broiler chickens exposed to Clostridium perfringens challenge. J. Appl. Anim. Res. 2018, 46, 691–695. [Google Scholar] [CrossRef] [Green Version]
- Soltani, M.; Sheikhzadeh, N.; Ebrahimzadeh-Mousavi, H.A.; Zargar, A. Effects of Zataria multiflora essential oil on innate immune responses of common carp (Cyprinus carpio). J. Fish. Aquat. Sci. 2010, 5, 191–199. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Latif, H.M.R.; Abdel-Tawwab, M.; Khafaga, A.; Dawood, M.A.O. Dietary oregano essential oil improved antioxidative status, immune-related genes, and resistance of common carp (Cyprinus carpio L.) to Aeromonas hydrophila infection. Fish Shellfish. Immunol. 2020, 104, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Brum, A.; Pereira, S.A.; Owatari, M.S.; Chagas, E.C.; Chaves, F.C.M.; Mourino, J.L.P. Effect of dietary essential oils of clove basil and ginger on Nile tilapia (Oreochromis niloticus) following challenge with Streptococcus agalactiae. Aquaculture 2017, 468, 235–243. [Google Scholar] [CrossRef]
- Talpur, A.D. Mentha piperita (pepermint) As feed additive enhanced growth performance survival, immune system, and disease resistance of Asian seabass, Lates calcarifer (Bloch) against Vibrio harvey infection. Aquaculture 2014, 420, 71–78. [Google Scholar] [CrossRef]
- Zakia, M.A.; Labib, E.M.; Noura, A.M.; Tonsy, H.D.; Mahmoud, S.H. Effect some medicinal plants diets on mono sex nile tilapia (Oreochromis niloticus), growth performance, feed utilization and physiological parameters. APCBEE Procedia 2012, 4, 220–227. [Google Scholar] [CrossRef] [Green Version]
- Dawood, M.A.O.; Abdel-Tawwab, M.; Abdel-Latif, H.M.R. Lycopene reduces the impacts of aquatic environmental pollutants and physical stressors in fish. Rev. Aquac. 2020, 12, 2511–2526. [Google Scholar] [CrossRef]
- Meyerowitz, S. Wheatgrass Nature’s Finest Medicine: The Complete Guide to Using Grasses to Revitalize Your Health; Sproutman Publications: Great Barrington, MA, USA, 2006; 242p. [Google Scholar]
- Padalia, S.; Drabu, S.; Raheja, I.; Gupta, A.; Dhamija, M. Multitude potential of wheatgrass juice (Green Blood): An overview. Indian J. Pharmacol. 2010, 1, 23–28. [Google Scholar]
- Tsai, C.C.; Lin, C.R.; Tsai, H.Y.; Chen, C.J.; Li, W.T.; Yu, H.M.; Ke, Y.Y.; Hsieh, W.Y.; Chang, C.Y.; Wu, C.Y.; et al. The immunologically active oligosaccharides isolated from wheatgrass modulate monocytes via toll-like receptor-2 signaling. J. Biol. Chem. 2013, 288, 1768917697. [Google Scholar] [CrossRef] [Green Version]
- Afroz, R.D.; Nurunnabi, A.S.M.; Khan, M.I.; Jahan, T. Effect of wheatgrass (Triticum aestivum) juice on High Density Lipoprotein (HDL) level in experimentally induced dyslipidaemic male long evans rat. Delta Med. Col. J. 2015, 3, 18–24. [Google Scholar] [CrossRef] [Green Version]
- Fortună, M.E.; Vasilache, V.; Ignat, M.; Silion, M.; Vicol, T.; Patraș, X.; Miron, I.; Lobiuc, A. Elemental and macromolecular modifications in Triticum aestivum L. plantlets under different cultivation conditions. PLoS ONE 2018, 13, e0202441. [Google Scholar] [CrossRef]
- Dumitru, G.; Dirvariu, L.; Barbacariu, C.A.; Miron, I.; Sandu, I.; Todirascu Ciornea, E. The effect of wheatgrass juice administration on physiological state and oxidative stress in carp. Rev. Chim. 2018, 69, 4046–4051. [Google Scholar] [CrossRef]
- Wellburn, A.R. The spectral determination of chlorophyll a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Lobiuc, A.; Vasilache, V.; Oroian, M.; Stoleru, T.; Burducea, M.; Pintilie, O.; Zamfirache, M.M. Blue and red LED illumination improves growth and bioactive compounds contents in acyanic and cyanic Ocimum basilicum L. Microgreens. Molecules 2017, 22, 2111. [Google Scholar] [CrossRef] [Green Version]
- Hoseinifar, S.H.; Sohrabi, A.; Paknejad, H.; Jafari, V.; Paolucci, M.; Van Doan, H. Enrichment of common carp (Cyprinus carpio) fingerlings diet with Psidium guajava: The effects on cutaneous mucosal and serum immune parameters and immune related genes expression. Fish Shellfish. Immunol. 2019, 86, 688–694. [Google Scholar] [CrossRef]
- Sheikhzadeh, N.; Soltani, M.; Ebrahimzadeh-Mousavi, H.A.; Shahbazian, N.; Norouzi, M. Effects of Zataria multiflora and Eucalyptus globolus essential oils on haematological parameters and respiratory burst activity in Cyprinus carpio. Iran. J. Fish. Sci. 2011, 10, 316–323. [Google Scholar]
- Liu, H.W.; Tong, J.M.; Zhou, D.W. Utilization of Chinese herbal feed additives in animal production. Agric. Sci. China 2011, 10, 1262–1272. [Google Scholar] [CrossRef]
- Aas, T.S.; Ytrestøyl, T.; Åsgård, T. Utilization of feed resources in the production of Atlantic salmon (Salmo salar) in Norway: An update for 2016. Aquac. Rep. 2019, 15, 100216. [Google Scholar] [CrossRef]
- Kok, B.; Malcorps, W.; Tlusty, M.F.; Eltholth, M.M.; Auchterlonie, N.A.; Little, D.C.; Harmsen, R.; Newton, R.W.; Davies, S.J. Fish as feed: Using economic allocation to quantify the fish in–fish-out ratio of major fed aquaculture species. Aquaculture 2020, 528, 735474. [Google Scholar] [CrossRef]
- Engle, C.R.; Kumar, G.; Senten, J. Cost drivers and profitability of U.S. pond, raceway, and RAS aquaculture. J. World Aquac. Soc. 2020, 51, 847–873. [Google Scholar] [CrossRef]
- Özköse, A.; Arslan, D.; Acar, A. The comparison of the chemical composition, sensory, phenolic and antioxidant properties of juices from different wheatgrass and turfgrass species. Not. Bot. Horti Agrobot. 2016, 44, 499–507. [Google Scholar] [CrossRef] [Green Version]
- Blicharz-Kania, A.; Andrejko, D.; Kluza, F.; Rydzak, L.; Kobus, Z. Assessment of the potential use of young barley shoots and leaves for the production of green juices. Sustainability 2019, 11, 3960. [Google Scholar] [CrossRef] [Green Version]
- Mishra, V.K.; Bacheti, R.K.; Husen, A. Medicinal uses of chlorophyll: A critical overview. In Chlorophyll: Structure, Function and Medicinal Uses Hauppauge; Le, H., Salcedo, E., Eds.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2011; pp. 1–22. [Google Scholar]
- Ogutu, F.O.; Makori, S.I.; Maringa, C.W.; Lemtukei, D.; Okiko, G.; Luvita, S. Wheat grass: A functional food. Food Sci. Qual. Manag. 2017, 65, 33–38. [Google Scholar]
- Hayes, M.; Ferruzzi, M.G. Update on the bioavailability and chemopreventative mechanisms of dietary chlorophyll derivatives. Nutr. Res. 2020, 81, 19–37. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, G.; Wang, J.; Ba, Q.; Che, H.; Song, Y.; Zhang, P.; Niu, N.; Wang, J.; Ma, S.; et al. The relationship between male sterility and membrane lipid peroxidation and antioxidant enzymes in wheat (Triticum aestivum L.). Turk. J. Field Crop. 2015, 20, 179–187. [Google Scholar] [CrossRef] [Green Version]
- Burducea, M.; Zheljazkov, V.D.; Dincheva, I.; Lobiuc, A.; Teliban, G.C.; Stoleru, V.; Zamfirache, M.M. Fertilization modifies the essential oil and physiology of basil varieties. Ind. Crop. Prod. 2018, 121, 282–293. [Google Scholar] [CrossRef]
- Burducea, M.; Zheljazkov, V.D.; Lobiuc, A.; Pintilie, C.A.; Virgolici, M.; Silion, M.; Asandulesa, M.; Burducea, I.; Zamfirache, M.M. Biosolids application improves mineral composition and phenolic profile of basil cultivated on eroded soil. Sci. Hortic. 2019, 249, 407–418. [Google Scholar] [CrossRef]
- Baba, E.; Ontas, C.; Kesbic, O.S.; Yılmaz, S. Evaluation of Citrus limon peels essential oil on growth performance, immune response of Mozambique tilapia Oreochromis mossambicus challenged with Edwardsiella tarda. Aquaculture 2016, 465, 13–18. [Google Scholar] [CrossRef]
- Mocanu, E.; Athanasopoulo, L.; Patriche, N.; Tenciu, M.; Jecu, E. Effect of phyto-additives diets on growth parameters and biochemical composition of carp species (Cyprinus carpio) in recirculating system. Sci. Pap. Anim. Sci. Ser. 2018, 71, 139–145. [Google Scholar]
- Nasir, N.A.; Hamed, Q. Growth development of young common carp Cyprinus carpio through dietary sodium chloride supplementation. Mesop. Environ. J. 2016, 2, 12–18. [Google Scholar]
- Gheorghe, C.E.; Radu, D.; Costache, M.; Bucur, C. Testing some protein feed used for the intensive carp raising (Cyprinus carpio L., 1758), during summer II. Analele IBNA 2008, 24, 16–24. [Google Scholar]
- Maas, P.; Grzegrzółka, B.; Kreß, P.; Oberle, M.; Judas, M.; Kremer-Rücker, P.V. Prediction of body composition in mirror carp (Cyprinus carpio) by using linear measurements in vivo and computed tomography post-mortem. Arch. Anim. Breed. 2020, 63, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Bauer, C.; Schlott, G. Fillet yield and fat content in common carp (Cyprinus carpio) produced in three Austrian carp farms with different culture methodologies. J Appl. Ichthyol. 2009, 25, 591–594. [Google Scholar] [CrossRef]
- Ljubojević, D.; Ćirković, M.; Đorđević, V.; Puvača, N.; Trbović, D.; Vukadinov, J.; Plavša, N. Fat quality of marketable fresh water fish species in the Republic of Serbia. Czech. J. Food Sci. 2013, 31, 445–450. [Google Scholar] [CrossRef] [Green Version]
- Zeitler, M.H.; Kirchgessner, M.; Schwarz, F.J. Effects of different protein and energy supplies on carcass composition of carp (Cyprinus carpio L.). Aquaculture 1984, 36, 37–48. [Google Scholar] [CrossRef]
- Seibel, H.; Baßmann, B.; Rebl, A. Blood will tell: What hematological analyses can reveal about fish welfare. Front. Vet. Sci. 2021, 8, 194. [Google Scholar] [CrossRef] [PubMed]
- Latif, M.; Faheem, M.; Asmatullah; Hoseinifar, S.H.; Van Doan, H. Dietary Black Seed Effects on Growth Performance, Proximate Composition, Antioxidant and Histo-Biochemical Parameters of a Culturable Fish, Rohu (Labeo rohita). Animals 2021, 11, 48. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Chen, Y.; Li, H.; Zhang, J.; Wu, P.; Ye, K.; Ai, H.; Chu, W. Dietary Ginkgo biloba leaf extract alters immune-related gene expression and disease resistance to Aeromonas hydrophila in common carp Cyprinus carpio. Fish Shellfish. Immunol. 2019, 49, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.L.; Chen, H.C. Effects of gallium on common carp (Cyprinus carpio): Acute test, serum biochemistry, and erythrocyte morphology. Chemosphere 2003, 53, 877–882. [Google Scholar] [CrossRef]
- Kesbic, O.S. Effects of juniper berry oil on growth performance and blood parameters in common carp (Cyprinus carpio). Aquac. Res. 2018, 50, 342–349. [Google Scholar] [CrossRef] [Green Version]
| Parameters | Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | Week 6 | Week 7 |
|---|---|---|---|---|---|---|---|
| Temperature (°C) | 22 | 22 | 22 | 22 | 22 | 22 | 22 |
| pH (pH units) | 7.9 | 7.8 | 7.8 | 7.9 | 8 | 7.9 | 7.9 |
| Dissolved oxygen (mg/L) | 9.79 | 9.01 | 8.78 | 8.58 | 8.90 | 9.12 | 9.40 |
| Conductivity (µS/cm3) | 1271 | 1240 | 1222 | 1305 | 1277 | 1295 | 1283 |
| Nitrates (NO3−)(mg/L) | 39.6 | 37.1 | 44 | 35.2 | 26.4 | 30.8 | 35.2 |
| Nitrites (NO−) (mg/L) | 0.10 | 0.11 | 0.13 | BD | BD | 0.06 | BD |
| Ammonia (NH3+) (mg/L) | BD | BD | BD | BD | BD | BD | BD |
| Phosphates (mg/L) | 0.01 | 0.01 | 0.006 | 0.006 | 0.01 | 0.002 | 0.006 |
| Parameters | Feed + H2O (Control) | Feed + WGJ 1% (V1) | Feed + WGJ 2% (V2) | Feed + WGJ 4% (V3) | p-Value |
|---|---|---|---|---|---|
| Moisture | 9.36 ± 0.01 | 8.95 ± 0.03 | 9.11 ± 0.03 | 9.96 ± 0.03 | 0.00 |
| Protein | 28.12 ± 0.11 | 27.54 ± 0.17 | 27.6 ± 0.16 | 26.97 ± 0.18 | 0.00 |
| Fat | 4 ± 0 | 4.17 ± 0.03 | 4.02 ± 0 | 3.93 ± 0.03 | 0.00 |
| Ash | 9.02 ± 0.03 | 9.46 ± 0.22 | 9.63 ± 0.24 | 9.39 ± 0.14 | 0.18 |
| Phosphorus | 1.06 ± 0.02 | 1.09 ± 0.01 | 1.07 ± 0.02 | 1.02 ± 0.01 | 0.04 |
| Fiber | 2.47 ± 0.04 | 2.45 ± 0.01 | 2.47 ± 0.04 | 2.72 ± 0.04 | 0.00 |
| Parameters | Values |
|---|---|
| Fresh yield (%) | 78.5 ± 2.01 |
| Chlorophyll a (mg mL−1) | 2.34 ± 0.03 |
| Chlorophyll b (mg mL−1) | 2.01 ± 0.03 |
| Carotenoids (mg mL−1) | 0.35 ± 0.01 |
| Total chlorophyll (mg mL−1) | 4.71 ± 0.06 |
| Total flavonoids (QE µg mL−1) | 289.24 ± 4.72 |
| Total phenols (GAE µg mL−1) | 164.81 ± 2.99 |
| Antioxidant activity (DPPH% inhibition) | 67.78 ± 4.08 |
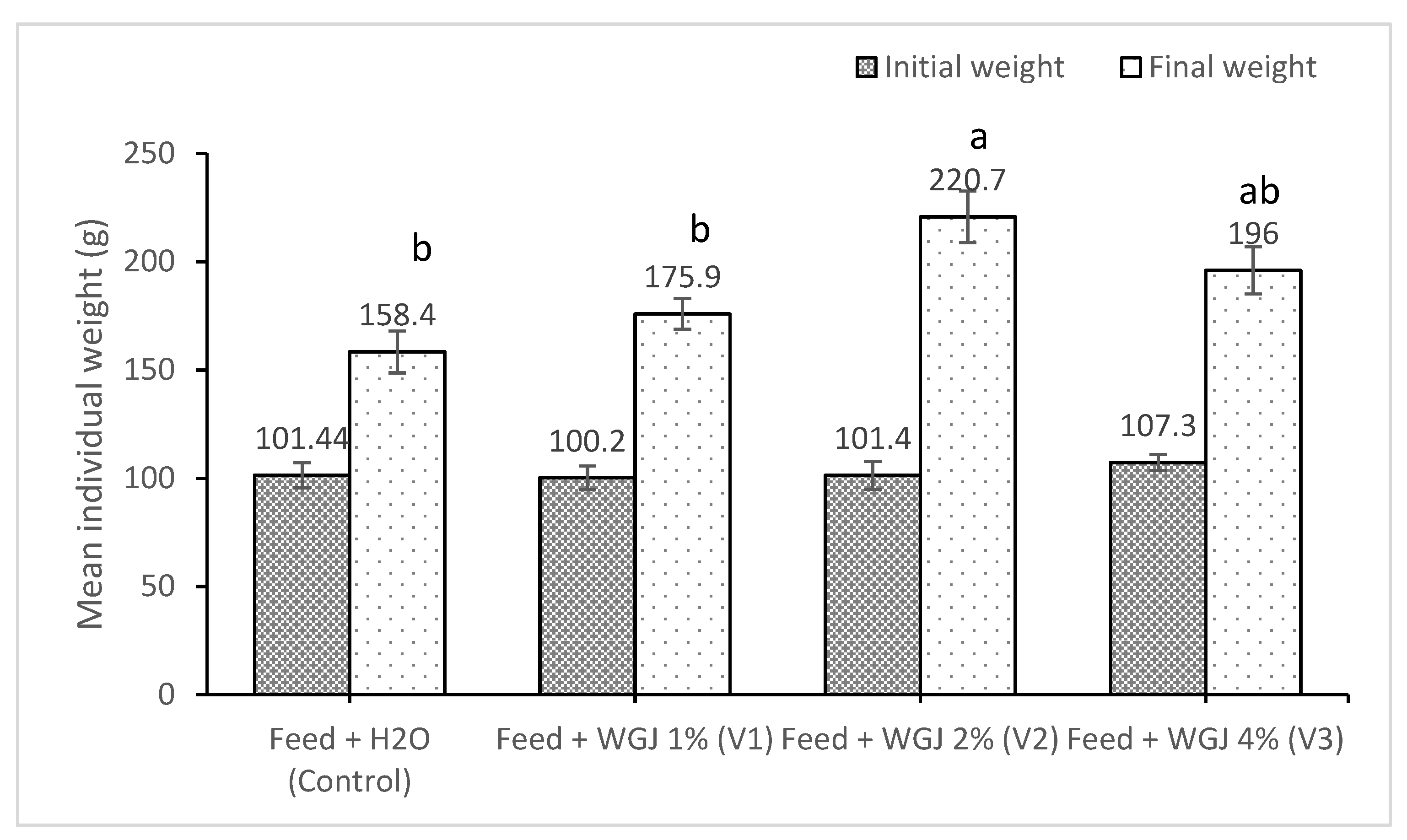
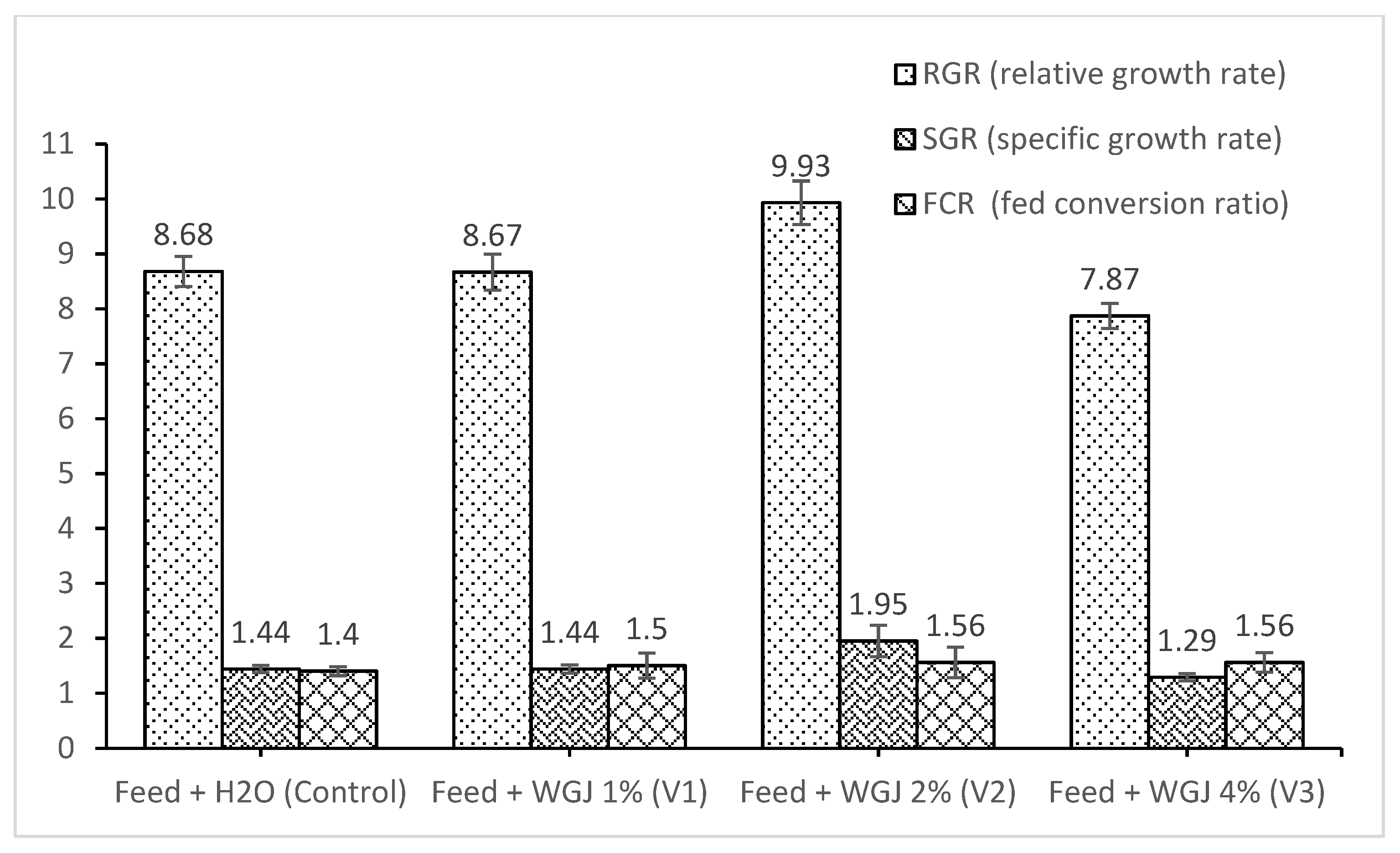
| Indices | Feed + H2O (Control) | Feed + WGJ 1% (V1) | Feed + WGJ 2% (V2) | Feed + WGJ 4% (V3) | p-Value |
|---|---|---|---|---|---|
| IBL (cm) | 17.63 ± 0.28 | 17.52 ± 0.33 | 17.45 ± 0.35 | 17.35 ± 0.37 | 0.22 |
| FBL (cm) | 19.98 ± 0.3 b | 20.26 ± 0.24 b | 21.34 ± 0.27 a | 20.96 ± 0.27 ab | 0.00 |
| WG (g) | 56.96 ± 3.88 b | 75.7 ± 1.64 b | 119.3 ± 5.45 a | 88.7 ± 7.14 ab | 0.00 |
| PER | 0.54 ± 0.02 | 0.57 ± 0.07 | 0.6 ± 0.15 | 0.46 ± 0.06 | 0.77 |
| CF | 1.99 ± 0.05 | 2 ± 0.03 | 2.12 ± 0.04 | 2.05 ± 0.03 | 0.14 |
| Indices | Feed + H2O (Control) | Feed + WGJ 1% (V1) | Feed + WGJ 2% (V2) | Feed + WGJ 4% (V3) | p-Value |
|---|---|---|---|---|---|
| Fat (%) | 2.53 ± 0.03 b | 2.97 ± 0.03 a | 0.9 ± 0.06 d | 1.27 ± 0.03 c | 0.00 |
| Protein (%) | 15.3 ± 0.26 | 15.17 ± 0.03 | 15.03 ± 0.24 | 15.4 ± 0.29 | 0.70 |
| Collagen (%) | 1.3 ± 0.15 ab | 1.13 ± 0.03 b | 1.7 ± 0.1 a | 1.27 ± 0.09 ab | 0.02 |
| Ash (%) | 1.9 ± 0.12 ab | 1.07 ± 0.03 b | 2.2 ± 0.15 a | 1.77 ± 0.37 ab | 0.02 |
| Parameter | Feed + H2O (Control) | Feed + WGJ 1% (V1) | Feed + WGJ 2% (V2) | Feed + WGJ 4% (V3) | p-Value |
|---|---|---|---|---|---|
| ALB (g/dL) | 1.5 ± 0.06 b | 1.73 ± 0.03 a | 1.57 ± 0.06 ab | 1.7 ± 0.07 ab | 0.02 |
| TP (g/dL) | 3.4 ± 0.06 b | 3.53 ± 0.03a b | 3.57 ± 0.03 ab | 3.63 ± 0.06 a | 0.04 |
| GLO (g/dL) | 1.67 ± 0.09 b | 1.87 ± 0.09 ab | 1.93 ± 0.12 ab | 2.1 ± 0.03 a | 0.02 |
| A/G | 0.87 ± 0.03 | 0.93 ± 0.03 | 0.83 ± 0.03 | 0.83 ± 0.03 | 0.19 |
| Ca (mg/dL) | 7.5 ± 0.2 b | 8.3 ± 0.2 ab | 8.47 ± 0.07 a | 8.1 ± 0.07 ab | 0.04 |
| GLU (mg/dL) | 95 ± 1 | 95.33 ± 2.96 | 92.33 ± 0.88 | 97.33 ± 2.73 | 0.40 |
| BUN (mg/dL) | 2.98 ± 0.09 b | 2.35 ± 0.04 c | 3.78 ± 0.43 a | 3.02 ± 0.31 b | 0.00 |
| AMY (U/L) | 64.33 ± 1.2 a | 71.67 ± 1.2 a | 46.33 ± 6.84 b | 64 ± 7.75 a | 0.00 |
| CHOL (mg/dL) | 214.67 ± 0.33 a | 218.33 ± 2.03 a | 190.33 ± 7.81 b | 213.67 ± 10.07 a | 0.00 |
| ALT (U/L) | 45.67 ± 3.18 | 35.33 ± 2.91 | 38 ± 1.67 | 38.67 ± 1.2 | 0.07 |
| TBIL (mg/dL) | 0.19 ± 0.01 | 0.23 ± 0.01 | 0.23 ± 0.01 | 0.24 ± 0.01 | 0.10 |
| ALP (U/L) | 34 ± 1 | 31.67 ± 1.67 | 32.33 ± 1.67 | 30.67 ± 0.67 | 0.07 |
| CRE (mg/dL) | 0.69 ± 0.04 | 0.68 ± 0.01 | 0.66 ± 0.04 | 0.62 ± 0.06 | 0.63 |
| CK (U/L) | 1745 ± 81.07 | 1962 ± 136.07 | 1834 ± 87.44 | 1976.67 ± 83.67 | 0.28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbacariu, C.-A.; Burducea, M.; Dîrvariu, L.; Oprea, E.; Lupu, A.-C.; Teliban, G.-C.; Agapie, A.L.; Stoleru, V.; Lobiuc, A. Evaluation of Diet Supplementation with Wheat Grass Juice on Growth Performance, Body Composition and Blood Biochemical Profile of Carp (Cyprinus carpio L.). Animals 2021, 11, 2589. https://doi.org/10.3390/ani11092589
Barbacariu C-A, Burducea M, Dîrvariu L, Oprea E, Lupu A-C, Teliban G-C, Agapie AL, Stoleru V, Lobiuc A. Evaluation of Diet Supplementation with Wheat Grass Juice on Growth Performance, Body Composition and Blood Biochemical Profile of Carp (Cyprinus carpio L.). Animals. 2021; 11(9):2589. https://doi.org/10.3390/ani11092589
Chicago/Turabian StyleBarbacariu, Cristian-Alin, Marian Burducea, Lenuta Dîrvariu, Eugen Oprea, Andrei-Cristian Lupu, Gabriel-Ciprian Teliban, Alina Laura Agapie, Vasile Stoleru, and Andrei Lobiuc. 2021. "Evaluation of Diet Supplementation with Wheat Grass Juice on Growth Performance, Body Composition and Blood Biochemical Profile of Carp (Cyprinus carpio L.)" Animals 11, no. 9: 2589. https://doi.org/10.3390/ani11092589
APA StyleBarbacariu, C.-A., Burducea, M., Dîrvariu, L., Oprea, E., Lupu, A.-C., Teliban, G.-C., Agapie, A. L., Stoleru, V., & Lobiuc, A. (2021). Evaluation of Diet Supplementation with Wheat Grass Juice on Growth Performance, Body Composition and Blood Biochemical Profile of Carp (Cyprinus carpio L.). Animals, 11(9), 2589. https://doi.org/10.3390/ani11092589







