Addition of Bamboo Charcoal to Selenium (Se)-Rich Feed Improves Growth and Antioxidant Capacity of Blunt Snout Bream (Megalobrama amblycephala)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Diet Preparation
2.3. Experimental Procedure
2.4. Sample Collection and Analysis
2.4.1. Sample Collection
2.4.2. Laboratory Analysis
2.4.3. Determination of Hepatic Antioxidant Parameters
2.4.4. Real-Time PCR Analysis
2.5. Statistical Analysis
3. Results
3.1. Effects of Se-Rich Dietary Bamboo Charcoal Inclusion Levels on Juvenile Blunt Snout Bream Growth Parameters
3.2. Effects of Se-Rich Dietary Bamboo Charcoal Inclusion Levels on the Muscle Nutrient Profile of Juvenile Blunt Snout Bream
3.3. Effects of Se-Rich Dietary Bamboo Charcoal Inclusion Levels on Plasma Physiological and Biochemical Parameters
3.4. Effects of Se-Rich Dietary Bamboo Charcoal Inclusion Levels on Liver Antioxidant Abilities
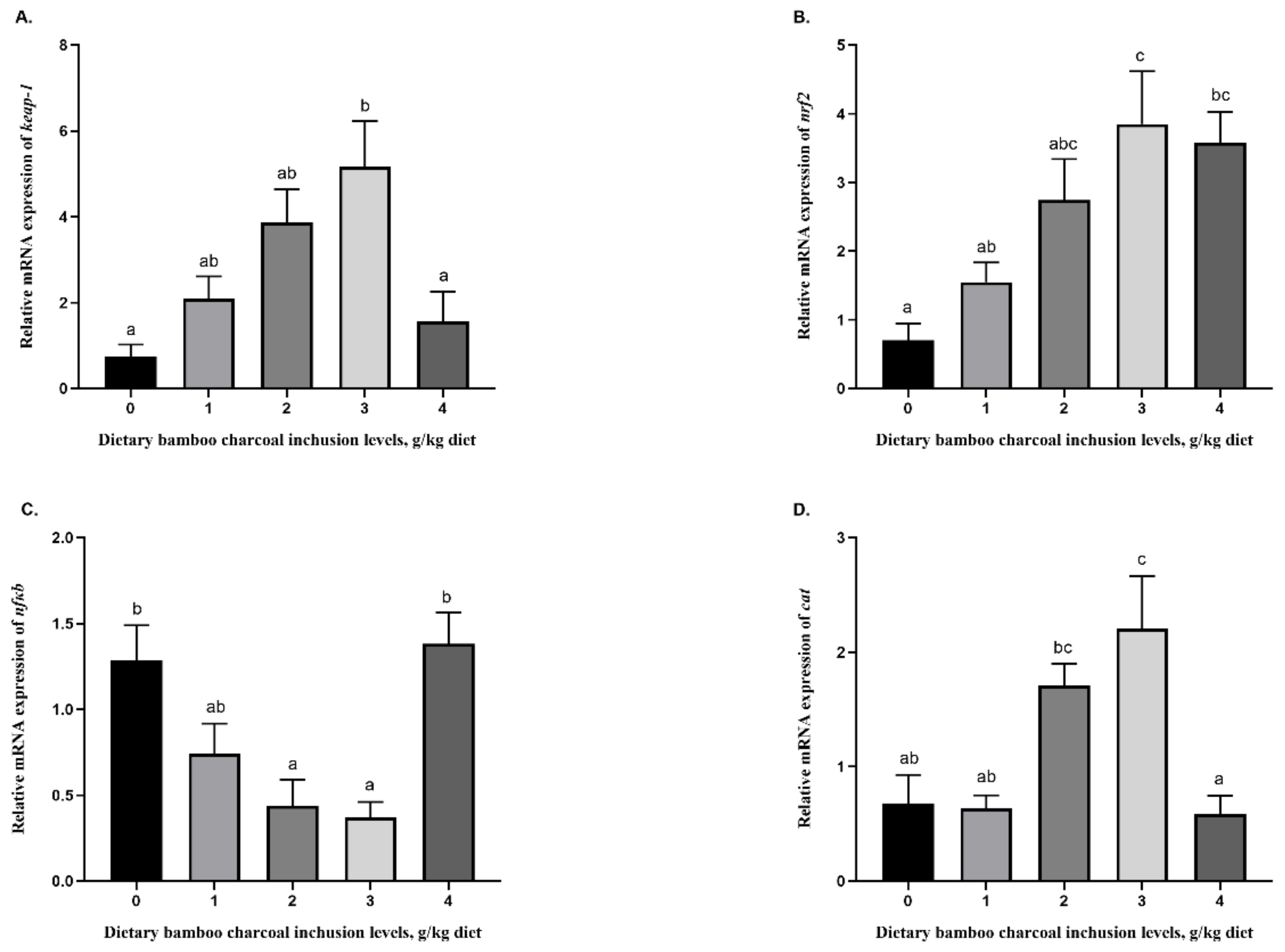
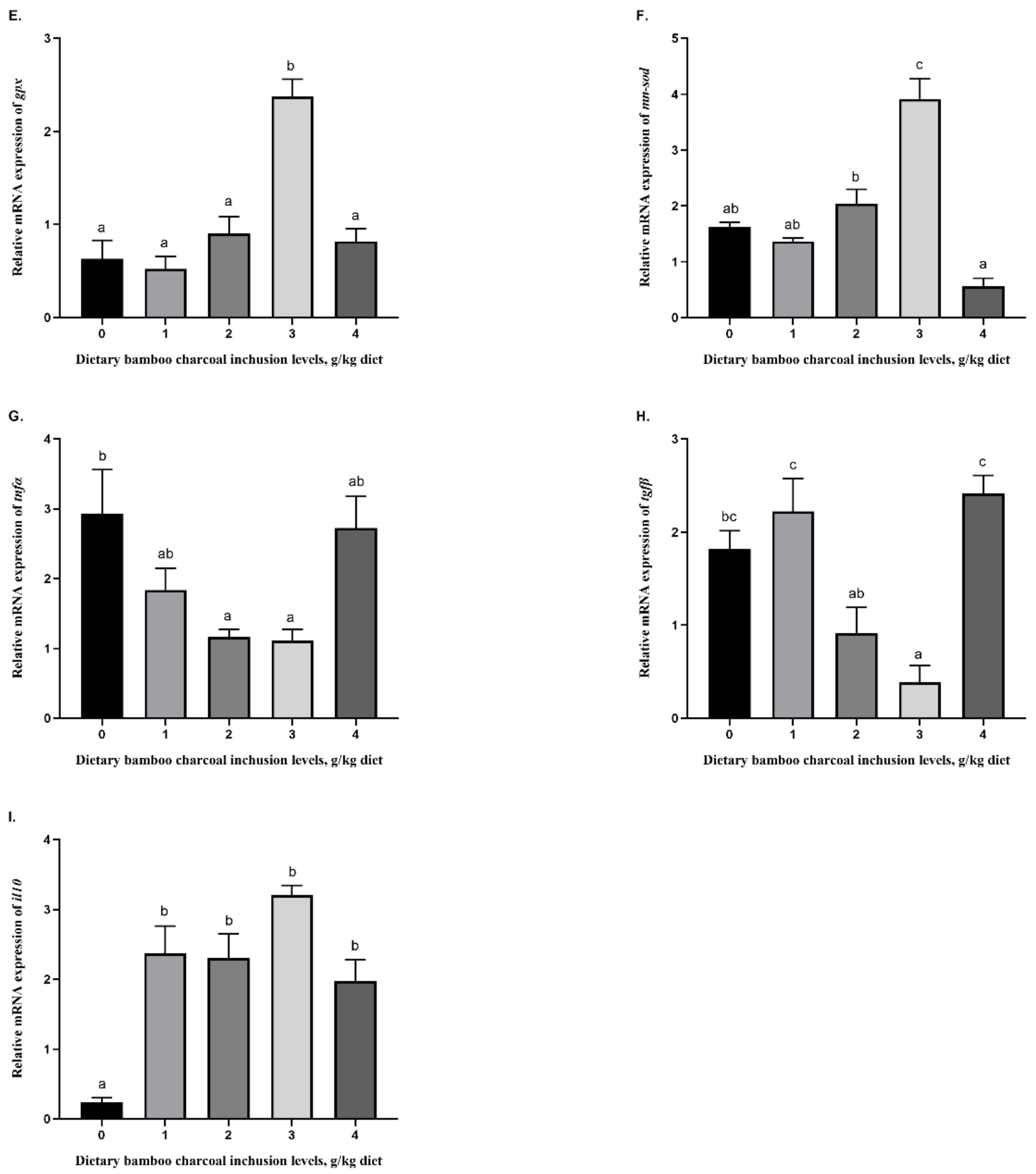
3.5. Effects of Se-Rich Dietary Bamboo Charcoal Inclusion Levels on Anti-Inflammatory Gene Expression in Juvenile Blunt Snout Bream Livers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maier, K.J.; Knight, A.W. Ecotoxicology of selenium in freshwater systems. In Reviews of Environmental Contamination and Toxicology; Ware, G.W., Ed.; Springer: New York, NY, USA, 1994; p. 134. [Google Scholar] [CrossRef]
- Mateo, R.D.; Spallholz, J.E.; Elder, R.; Yoon, I.; Kim, S.W. Efficacy of dietary selenium sources on growth and carcass characteristics of growing-finishing pigs fed diets containing high endogenous selenium. J. Anim. Sci. 2007, 85, 1177–1183. [Google Scholar] [CrossRef]
- Juniper, D.T.; Phipps, R.H.; Ramos-Morales, E.; Bertin, G. Effect of dietary supplementation with selenium-enriched yeast or sodium selenite on selenium tissue distribution and meat quality in beef cattle. J. Anim. Sci. 2008, 86, 3100–3109. [Google Scholar] [CrossRef]
- Neamat-Allah, A.N.; Mahmoud, E.A.; Abd El Hakim, Y. Efficacy of dietary Nano-selenium on growth, immune response, antioxidant, transcriptomic profile and resistance of Nile tilapia, Oreochromis niloticus against Streptococcus iniae infection. Fish Shellfish. Immunol. 2019, 94, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Kiron, V.; Satoh, S. Trace minerals in fish nutrition. Aquaculture 1997, 151, 185–207. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, Y.; Liu, Y.; Yang, H.; Liang, G.; Tian, L. Effect of dietary selenium level on growth performance, body composition and hepatic glutathione peroxidase activities of largemouth bass Micropterus salmoide. Aquac. Res. 2012, 43, 1660–1668. [Google Scholar] [CrossRef]
- Jingyuan, H.; Yan, L.; Wenjing, P.; Wenqiang, J.; Bo, L.; Linghong, M.; Qunlang, Z.; Hualiang, L.; Xianping, G. Dietary selenium enhances the growth and anti-oxidant capacity of juvenile blunt snout bream (Megalobrama amblycephala). Fish Shellfish Immunol. 2020, 101, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.H.; Shiau, S.Y. Dietary selenium requirements of juvenile grouper, Epinephelus malabaricus. Aquaculture 2005, 250, 356–363. [Google Scholar] [CrossRef]
- Lemly, A.D. Symptoms and implications of selenium toxicity in fish: The Belews Lake case example. Aquat. Toxicol. 2002, 57, 39–49. [Google Scholar] [CrossRef]
- Bell, J.G.; Cowey, C.B.; Adron, J.W.; Pirie, B.J.S. Some effects of selenium deficiency on enzyme activities and indices of tissue peroxidation in Atlantic salmon parr (Salmo salar). Aquaculture 1987, 65, 43–54. [Google Scholar] [CrossRef]
- Poston, H.A.; Combs, G.F.; Leibovitz, L. Vitamin E and selenium interrelations in the diet of atlantic salmon (Salmo salar): Gross, histological and biochemical deficiency signs. J. Nutr. 1976, 106, 892–904. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Nambi, R.W.; Won, S.; Katya, K.; Bai, S.C. Dietary selenium requirement and toxicity levels in juvenile Nile tilapia, Oreochromis niloticus. Aquaculture 2016, 464, 153–158. [Google Scholar] [CrossRef]
- Zhao, R.S.; Yuan, J.P.; Jiang, T.; Shi, J.B.; Cheng, C.G. Application of bamboo charcoal as solid-phase extraction adsorbent for the determination of atrazine and simazine in environmental water samples by high-performance liquid chromatography-ultraviolet detector. Talanta 2008, 76, 956–959. [Google Scholar] [CrossRef] [PubMed]
- Hwang, G.S. Effects of carbonization temperatures in an Earthen Kiln on the properties of bamboo charcoal. Taiwan J. For. Sci. 2016, 31, 285–291. [Google Scholar]
- Van, D.T.T.; Mui, N.T.; Ledin, I. Effect of method of processing foliage of Acacia mangium and inclusion of bamboo charcoal in the diet on performance of growing goats. Anim. Feed Sci. Technol. 2006, 130, 242–256. [Google Scholar] [CrossRef]
- Hsieh, M.F.; Wen, H.W.; Shyu, C.L.; Chen, S.H.; Li, W.T.; Wang, W.C.; Chen, W.C. Synthesis, in vitro macrophage response and detoxification of bamboo charcoal beads for purifying blood. J. Biomed. Mater. Res. Part A 2010, 94, 1133–1140. [Google Scholar] [CrossRef]
- Qiu, J.; Fan, H.; Liu, T.; Liang, X.; Meng, F.; Quilliam, M.A.; Li, A. Application of activated carbon to accelerate detoxification of paralytic shellfish toxins from mussels Mytilus galloprovincialis and scallops Chlamys farreri. Ecotoxicol. Environ. Saf. 2018, 148, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Banner, R.E.; Rogosic, J.; Burritt, E.A.; Provenza, F.D. Supplemental barley and charcoal increase intake of sagebrush by lambs. J. Range Manag. 2000, 53, 415–420. [Google Scholar] [CrossRef]
- Kutlu, H.R.; Ünsal, I.; Görgülü, M. Effects of providing dietary wood (oak) charcoal to broiler chicks and laying hens. Anim. Feed Sci. Technol. 2001, 90, 213–226. [Google Scholar] [CrossRef]
- Samanya, M.; Yamauchi, K. Morphological changes of the intestinal villi in chickens fed the dietary charcoal powder including wood vinegar compounds. J. Poult. Sci. 2001, 38, 289–301. [Google Scholar] [CrossRef]
- Mekbungwan, A.; Yamauchi, K.; Sakaida, T. Intestinal villus histological alterations in piglets fed dietary charcoal powder including wood vinegar compound liquid. J. Vet. Med. Ser. C Anat. Histol. Embryol. 2004, 33, 11–16. [Google Scholar] [CrossRef]
- Michael, F.R.; Saleh, N.E.; Shalaby, S.M.; Sakr, E.M.; Abd-El-Khalek, D.E.; Abd Elmonem, A.I. Effect of different dietary levels of commercial wood charcoal on growth, body composition and environmental loading of red tilapia hybrid. Aquac. Nutr. 2017, 23, 210–216. [Google Scholar] [CrossRef]
- Thu, M.; Koshio, S.; Ishikawa, M.; Yokoyama, S. Effects of supplementation of dietary bamboo charcoal on growth performance and body composition of juvenile Japanese flounder, Paralichthys olivaceus. J. World Aquac. Soc. 2010, 41, 255–262. [Google Scholar] [CrossRef]
- Quaiyum, M.A.; Jahan, R.; Jahan, N.; Akhter, T.; Sadiqul, I.M. Effects of bamboo charcoal added feed on reduction of ammonia and growth of Pangasius hypophthalmus. J. Aquac. Res. Dev. 2014, 5, 269. [Google Scholar] [CrossRef]
- Mabe, L.T.; Su, S.; Tang, D.; Zhu, W.; Wang, S.; Dong, Z. The effect of dietary bamboo charcoal supplementation on growth and serum biochemical parameters of juvenile common carp (Cyprinus carpio L.). Aquac. Res. 2018, 49, 1142–1152. [Google Scholar] [CrossRef]
- AOAC. International Official Methods of Analysis of AOAC International—20th Edition, 2016; AOAC: Gaithersburg, MD, USA, 2016. [Google Scholar]
- Hilton, J.W.; Hodson, P.V.; Slinger, S.J. The requirement and toxicity of selenium in rainbow trout (Salmo gairdneri). J. Nutr. 1980, 110, 2527–2535. [Google Scholar] [CrossRef]
- Rattanawut, J.; Todsadee, A.; Yamauchi, K.E. Effects of bamboo charcoal powder including vinegar supplementation on performance, eggshell quality, alterations of intestinal villi and intestinal pathogenic bacteria populations of aged laying hens. Ital. J. Anim. Sci. 2017, 16, 259–265. [Google Scholar] [CrossRef]
- Pirarat, N.; Boonananthanasarn, S.; Krongpong, L.; Katagiri, T.; Maita, M. Effect of activated charcoal-supplemented diet on growth performance and intestinal morphology of Nile Tilapia (Oreochromis niloticus). Thai J. Vet. Med. 2015, 45, 113–119. [Google Scholar]
- Abdel-Tawwab, M.; El-Sayed, G.O.; Shady, S.H. Effect of dietary active charcoal supplementation on growth performance, biochemical and antioxidant responses, and resistance of Nile tilapia, Oreochromis niloticus (L.) to environmental heavy metals exposure. Aquaculture 2017, 479, 17–24. [Google Scholar] [CrossRef]
- Samadaii, S.; Bahrekazemi, M. The effect of diets containing different levels of active charcoal on growth performance, body composition, haematological parameters and possibility of heavy metals detoxification in big sturgeon (Huso huso). Aquac. Res. 2020, 51, 91–101. [Google Scholar] [CrossRef]
- Akrami, R.; Gharaei, A.; Mansour, M.R.; Galeshi, A. Effects of dietary onion (Allium cepa) powder on growth, innate immune response and hemato-biochemical parameters of beluga (Huso huso Linnaeus, 1754) juvenile. Fish Shellfish Immunol. 2015, 45, 828–834. [Google Scholar] [CrossRef]
- Wang, X.F.; Li, X.Q.; Leng, X.J.; Shan, L.L.; Zhao, J.X.; Wang, Y.T. Effects of dietary cottonseed meal level on the growth, hematological indices, liver and gonad histology of juvenile common carp (Cyprinus carpio). Aquaculture 2014, 428, 79–87. [Google Scholar] [CrossRef]
- Tang, D.; Su, S.; Zhu, W.; Wang, L.; Song, F.; Dong, J.; Zhang, D.; Dong, Z. Effects of dietary addition of bamboo charcoal on muscle fatty acid composition and lipid metabolism related genes of juvenile red tilapia (Oreochromis mossambicus × O. Niloticus). J. Fish. China 2019, 43, 1092–1103. [Google Scholar] [CrossRef]
- Jiang, Z.Y.; Li, L.L.; Tang, L.H.; Fan, J.X.; Wang, S.P.; Yin, Y.L.; Li, T.J. Effects of bamboo charcoal and bamboo vinegar on antioxidant performance and intestinal mucosal morphology of weaned piglets contaminated with Don. J. Northwest A F Univ. 2013, 41, 37–42. [Google Scholar]
- Wang, S.F.; Gao, X.P.; Liu, H.X. Effects of supplementation of active charcoal on growth performance, antioxidation and resistance to environmental heavy metals of tilapia. China Feed 2019, 12, 44–48. [Google Scholar]
- Feng, Y.; Qin, Z.; Dai, Y.; Zhang, Y.; Liu, X.; Zhou, Y.; Lan, J.; Zhao, L.; Lin, L. Cloning and analysis of nuclear factor E2-related factor 2 (Nrf2) and its function in the regulation of respiratory burst in grass carp (Ctenopharyngodon idella). J. Fish. China 2018, 42, 161–177. [Google Scholar] [CrossRef]
- Timme-Laragy, A.R.; Karchner, S.I.; Franks, D.G.; Jenny, M.J.; Harbeitner, R.C.; Goldstone, J.V.; McArthur, A.G.; Hahn, M.E. Nrf2b, novel zebrafish paralog of oxidant-responsive transcription factor NF-E2-related factor 2 (NRF2). J. Biol. Chem. 2012, 287, 4609–4627. [Google Scholar] [CrossRef]
- Zeng, L.; Ai, C.X.; Wang, Y.H.; Zhang, J.S.; Wu, C.W. Abrupt salinity stress induces oxidative stress via the Nrf2-Keap1 signaling pathway in large yellow croaker Pseudosciaena crocea. Fish Physiol. Biochem. 2017, 43, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Feng, L.; Liu, Y.; Jiang, W.; Wu, P.; Jiang, J.; Zhang, Y.; Zhou, X. Effect of dietary isoleucine on the immunity, antioxidant status, tight junctions and microflora in the intestine of juvenile Jian carp (Cyprinus carpio var. Jian). Fish Shellfish Immunol. 2014, 41, 663–673. [Google Scholar] [CrossRef]
- Wang, M.; Zhu, Z. Nrf2 is involved in osmoregulation, antioxidation and immunopotentiation in Coilia nasus under salinity stress. Biotechnol. Biotechnol. Equip. 2019, 33, 1453–1463. [Google Scholar] [CrossRef][Green Version]
- Jia, Z.L.; Cen, J.; Wang, J.B.; Zhang, F.; Xia, Q.; Wang, X.; Chen, X.Q.; Wang, R.C.; Hsiao, C.D.; Liu, K.C.; et al. Mechanism of isoniazid-induced hepatotoxicity in zebrafish larvae: Activation of ROS-mediated ERS, apoptosis and the Nrf2 pathway. Chemosphere 2019, 227, 541–550. [Google Scholar] [CrossRef]
- Pan, W.; Miao, L.; Lin, Y.; Huang, X.; Ge, X.; Moosa, S.L.; Liu, B.; Ren, M.; Zhou, Q.; Liang, H.; et al. Regulation mechanism of oxidative stress induced by high glucose through PI3K/Akt/Nrf2 pathway in juvenile blunt snout bream (Megalobrama amblycephala). Fish Shellfish Immunol. 2017, 70, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, S.; Jiang, H.; Liu, B.; Lv, Z.; Guo, C.; Zhang, H. Effects of selenium on apoptosis and abnormal amino acid metabolism induced by excess fatty acid in isolated rat hepatocytes. Mol. Nutr. Food Res. 2017, 61, 1700016. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhang, F.; Lu, D.; Zhang, H. Selenium bioavailability from shrimps (Penaeus vannamei Boone) and its effect on the metabolism of phospholipid and cholesterol ester. J. Funct. Foods 2014, 6, 186–195. [Google Scholar] [CrossRef]
- Stapleton, S.R. Selenium: An insulin-mimetic. Cell. Mol. Life Sci. 2000, 57, 1874–1879. [Google Scholar] [CrossRef]
- Zhang, D.M.; Guo, Z.X.; Zhao, Y.L.; Wang, Q.J.; Gao, Y.S.; Yu, T.; Chen, Y.K.; Chen, X.M.; Wang, G.Q. L-carnitine regulated Nrf2/Keap1 activation in vitro and in vivo and protected oxidized fish oil-induced inflammation response by inhibiting the NF-κB signaling pathway in Rhynchocypris lagowski Dybowski. Fish Shellfish Immunol. 2019, 93, 1100–1110. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Jia, R.; Cao, L.; Du, J.; Gu, Z.; He, Q.; Xu, P.; Yin, G. Effects of chronic glyphosate exposure on antioxdative status, metabolism and immune response in tilapia (GIFT, Oreochromis niloticus). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 239, 108878. [Google Scholar] [CrossRef]
- Feng, L.; Li, W.; Liu, Y.; Jiang, W.D.; Kuang, S.Y.; Wu, P.; Jiang, J.; Tang, L.; Tang, W.N.; Zhang, Y.A.; et al. Protective role of phenylalanine on the ROS-induced oxidative damage, apoptosis and tight junction damage via Nrf2, TOR and NF-κB signalling molecules in the gill of fish. Fish Shellfish Immunol. 2017, 60, 185–196. [Google Scholar] [CrossRef]
- Jiang, W.D.; Feng, L.; Qu, B.; Wu, P.; Kuang, S.Y.; Jiang, J.; Tang, L.; Tang, W.N.; Zhang, Y.A.; Zhou, X.Q.; et al. Changes in integrity of the gill during histidine deficiency or excess due to depression of cellular anti-oxidative ability, induction of apoptosis, inflammation and impair of cell-cell tight junctions related to Nrf2, TOR and NF-κB signaling in fish. Fish Shellfish Immunol. 2016, 56, 111–122. [Google Scholar] [CrossRef]
- Song, C.; Liu, B.; Xu, P.; Ge, X.; Zhang, H. Emodin ameliorates metabolic and antioxidant capacity inhibited by dietary oxidized fish oil through PPARs and Nrf2-Keap1 signaling in Wuchang bream (Megalobrama amblycephala). Fish Shellfish Immunol. 2019, 94, 842–851. [Google Scholar] [CrossRef]
- Shi, X.; Zhou, B. The role of Nrf2 and MAPK pathways in PFOS-induced oxidative stress in zebrafish embryos. Toxicol. Sci. 2010, 115, 391–400. [Google Scholar] [CrossRef]
| Ingredients (g/kg) | Dietary Bamboo Charcoal Inclusion Levels | ||||
|---|---|---|---|---|---|
| 0 (Control) | 1.0 (BC1) | 2.0 (BC2) | 3.0 (BC3) | 4.0 (BC4) | |
| Bamboo charcoal powder 1 | 0 | 1 | 2 | 3 | 4 |
| Sodium selenite (mg/kg) 2 | 4 | 4 | 4 | 4 | 4 |
| Fish meal 3 | 40 | 40 | 40 | 40 | 40 |
| Soybean meal 3 | 390 | 390 | 390 | 390 | 390 |
| Rapeseed meal 3 | 150 | 150 | 150 | 150 | 150 |
| Cottonseed meal 3 | 100 | 100 | 100 | 100 | 100 |
| Wheat meal 3 | 150 | 150 | 150 | 150 | 150 |
| Soybean oil | 60 | 60 | 60 | 60 | 60 |
| Vitamin premix 4 | 5 | 5 | 5 | 5 | 5 |
| Mineral premix (Se-free) 5 | 5 | 5 | 5 | 5 | 5 |
| Carboxymethyl cellulose | 80 | 79 | 78 | 77 | 76 |
| Monocalcium phosphate | 10 | 10 | 10 | 10 | 10 |
| Vitamin C | 10 | 10 | 10 | 10 | 10 |
| Sum | 1000 | 1000 | 1000 | 1000 | 1000 |
| Nutrition composition, % fresh | |||||
| Crude protein | 36.2 | 36.1 | 36.8 | 36.0 | 36.2 |
| Crude lipid | 8.8 | 8.6 | 8.7 | 8.7 | 8.6 |
| Ash | 7.8 | 7.3 | 7.9 | 7.8 | 7.6 |
| Total Se concentration (mg/kg) | 1.48 | 1.46 | 1.46 | 1.44 | 1.44 |
| Primer | Sequences | |
|---|---|---|
| Forward Primer (5′−3′) | Reverse Primer (5′−3′) | |
| β-actin | TCGTCCACCGCAAATGCTTCTA | CCGTCACCTTCACCGTTCCAGT |
| keap-1 | AATATCCGCCGGCTGTGTAG | TGAGTCCGAGGTGTTTCGTG |
| nrf2 | GGGGAAGTCCTTGAACGGAG | AACCAGCGGGAATATCTCGG |
| nfκb | AGTCCGATCCATCCGCACTA | ACTGGAGCCGGTCATTTCAG |
| cat | CAGTGCTCCTGATACCCAGC | TTCTGACACAGACGCTCTCG |
| gpx | GAACGCCCACCCTCTGTTTG | CGATGTCATTCCGGTTCACG |
| mn-sod | AGCTGCACCACAGCAAGCAC | TCCTCCACCATTCGGTGACA |
| tnfα | TGGAGAGTGAACCAGGACCA | AGAGACCTGGCTGTAGACGA |
| tgfβ | ACTGGACAAACAGAGAGGCG | CAGGGGAGTTGCCGTTAGAG |
| il10 | GTGTTTTCGGGTGCAAGTGG | ATGAACGAGATCCTGCGCTT |
| Dietary Bamboo Charcoal Powder Inclusion Levels, g/kg Diet | p-Value | ||||||
|---|---|---|---|---|---|---|---|
| 0 (Control) | 1.0 (BC1) | 2.0 (BC2) | 3.0 (BC3) | 4.0 (BC4) | Linear | Quadratic | |
| Initial weight (g) | 16.67 ± 0.06 | 16.76 ± 0.02 | 16.76 ± 0.09 | 16.58 ± 0.10 | 16.67 ± 0.05 | 0.439 | 0.596 |
| Final weight (g) | 43.77 ± 0.44 a | 44.24 ± 0.39 ab | 45.93 ± 0.34 b | 45.94 ± 0.65 b | 44.45 ± 0.57 ab | 0.129 | 0.010 |
| WGR (%) | 162.61 ± 2.20 a | 163.93 ± 2.04 ab | 174.15 ± 1.40 bc | 177.13 ± 3.16 c | 166.67 ± 3.16 abc | 0.065 | 0.007 |
| SGR (%/day) | 1.72 ± 0.01 a | 1.73 ± 0.01 ab | 1.80 ± 0.01 bc | 1.82 ± 0.02 c | 1.75 ± 0.02 abc | 0.065 | 0.007 |
| FCR | 1.62 ± 0.01 a | 1.68 ± 0.01 b | 1.73 ± 0.01 bc | 1.74 ± 0.02 c | 1.78 ± 0.01 c | <0.001 | <0.001 |
| PER | 1.69 ± 0.01 c | 1.66 ± 0.01 bc | 1.61 ± 0.01 ab | 1.59 ± 0.02 a | 1.57 ± 0.01 a | <0.001 | <0.001 |
| Dietary Bamboo Charcoal Powder Inclusion Levels, g/kg Diet | p-Value | ||||||
|---|---|---|---|---|---|---|---|
| 0 (Control) | 1.0 (BC1) | 2.0 (BC2) | 3.0 (BC3) | 4.0 (BC4) | Linear | Quadratic | |
| Moisture (g/kg) | 766.84 ± 1.83 | 762.44 ± 1.97 | 763.94 ± 1.64 | 759.37 ± 3.26 | 766.08 ± 2.70 | 0.451 | 0.160 |
| Crude protein (g/kg) | 185.66 ± 1.68 ab | 189.95 ± 1.59 b | 182.91 ± 1.24 a | 187.03 ± 1.26 ab | 188.05 ± 1.93 ab | 0.666 | 0.695 |
| Crude lipid (g/kg) | 31.48 ± 1.17 | 30.55 ± 2.43 | 30.75 ± 1.84 | 29.81 ± 1.94 | 29.85 ± 2.08 | 0.505 | 0.797 |
| Ash (g/kg) | 20.83± 0.79 | 20.59 ± 0.81 | 18.36 ± 0.51 | 19.86± 0.46 | 20.73 ± 0.59 | 0.656 | 0.061 |
| Se (mg/kg) | 0.57± 0.03 c | 0.54 ± 0.03 bc | 0.49 ± 0.01 abc | 0.46 ±0.01 ab | 0.42 ± 0.03 a | <0.001 | <0.001 |
| Dietary Bamboo Charcoal Powder Inclusion Levels, g/kg Diet | p-Value | ||||||
|---|---|---|---|---|---|---|---|
| 0 (Control) | 1.0 (BC1) | 2.0 (BC2) | 3.0 (BC3) | 4.0 (BC4) | Linear | Quadratic | |
| ALB (g/L) | 13.61 ± 0.48 ab | 12.25 ± 0.51 a | 14.18 ± 0.66 ab | 14.85 ± 0.60 b | 13.52 ± 0.48 ab | 0.206 | 0.370 |
| GLU (mmol/L) | 9.62 ± 0.32 ab | 9.89 ± 0.65 ab | 11.62 ± 0.74 b | 11.95 ± 0.65 b | 8.93 ± 0.62 a | 0.665 | 0.006 |
| ALP (U/L) | 37.88 ± 3.05 ab | 49.01 ± 4.53 b | 38.67 ± 4.15 ab | 32.55 ± 2.30 a | 33.89 ± 3.24 a | 0.031 | 0.059 |
| TP (g/L) | 31.99 ± 0.91 | 32.91 ± 1.77 | 32.75 ± 1.19 | 33.15 ± 1.09 | 30.95 ± 1.35 | 0.685 | 0.463 |
| TC (mmol/L) | 6.80 ± 0.14 | 7.66 ± 0.39 | 7.26 ± 0.34 | 6.99 ± 0.30 | 6.95± 0.29 | 0.647 | 0.311 |
| TG (mmol/L) | 2.83 ± 0.06 | 2.80 ± 0.17 | 2.79 ± 0.13 | 3.06 ± 0.12 | 2.65 ± 0.016 | 0.962 | 0.536 |
| ALT (U/L) | 7.54 ± 1.03 | 8.83 ± 0.88 | 6.12 ± 0.50 | 6.33 ± 0.69 | 6.85 ± 0.95 | 0.159 | 0.338 |
| AST (U/L) | 259.58 ± 10.75 | 256.08 ± 24.10 | 275.11 ± 26.86 | 217.9 ± 22.59 | 218.15 ± 21.10 | 0.090 | 0.185 |
| Dietary Bamboo Charcoal Powder Inclusion Levels, g/kg Diet | p-Value | ||||||
|---|---|---|---|---|---|---|---|
| 0 (Control) | 1.0(BC1) | 2.0(BC2) | 3.0(BC3) | 4.0(BC4) | Linear | Quadratic | |
| CAT (U/mgprot) | 11.68 ± 0.94 a | 15.52 ± 1.70 a | 16.07 ± 1.47 a | 22.94 ± 1.51 b | 14.57 ± 1.33 a | 0.009 | 0.001 |
| T-SOD (U/mgprot) | 60.84 ± 3.01 a | 52.87 ± 5.19 a | 57.12 ± 5.02 a | 81.38 ± 5.05 b | 61.95 ± 3.95 a | 0.037 | 0.114 |
| T-AOC (U/mgprot) | 0.14 ± 0.02 a | 0.46 ± 0.09 a | 0.45 ± 0.09 a | 0.98 ± 0.10 b | 0.44 ± 0.09 a | 0.002 | <0.001 |
| GSH-Px (U/mgprot) | 88.76 ± 6.07 ab | 85.50 ± 7.50 a | 129.87 ± 11.55 b | 180.76 ± 10.77 c | 120.79 ± 6.23 ab | 0.002 | 0.002 |
| GSH (mg/gprot) | 4.43 ± 0.64 | 5.21 ± 1.00 | 3.60 ± 0.74 | 3.54 ± 0.47 | 5.19 ± 0.87 | 0.911 | 0.501 |
| MDA (nmol/mgprot) | 7.08 ± 0.47 a | 11.62 ± 0.86 b | 9.18 ± 0.85 ab | 7.08 ± 0.57 a | 7.96 ± 0.61 a | 0.311 | 0.028 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, F.; Lin, Y.; Miao, L.; Hao, J. Addition of Bamboo Charcoal to Selenium (Se)-Rich Feed Improves Growth and Antioxidant Capacity of Blunt Snout Bream (Megalobrama amblycephala). Animals 2021, 11, 2585. https://doi.org/10.3390/ani11092585
Jiang F, Lin Y, Miao L, Hao J. Addition of Bamboo Charcoal to Selenium (Se)-Rich Feed Improves Growth and Antioxidant Capacity of Blunt Snout Bream (Megalobrama amblycephala). Animals. 2021; 11(9):2585. https://doi.org/10.3390/ani11092585
Chicago/Turabian StyleJiang, Fang, Yan Lin, Linghong Miao, and Jingyuan Hao. 2021. "Addition of Bamboo Charcoal to Selenium (Se)-Rich Feed Improves Growth and Antioxidant Capacity of Blunt Snout Bream (Megalobrama amblycephala)" Animals 11, no. 9: 2585. https://doi.org/10.3390/ani11092585
APA StyleJiang, F., Lin, Y., Miao, L., & Hao, J. (2021). Addition of Bamboo Charcoal to Selenium (Se)-Rich Feed Improves Growth and Antioxidant Capacity of Blunt Snout Bream (Megalobrama amblycephala). Animals, 11(9), 2585. https://doi.org/10.3390/ani11092585







