Effect of Pre-Milking Teat Foam Disinfection on the Prevention of New Mastitis Rates in Early Lactation
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Quarter Milk Sampling Procedure
2.2. Teat Swabbing Procedure
2.3. Teat Skin Condition Scoring
2.4. Statistical Analysis
3. Results
3.1. Quarter Foremilk Sample Results
3.2. Teat Skin Swab Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goodwin, P.J.; Kenny, G.R.; Josey, M.J.; Imbeah, M. Effectiveness of post-milking teat antisepsis with iodophor, chlorhexidine or dodecyl benzene sulphonic acid. Proc. Aust. Soc. Anim. Prod. 1996, 21, 266–269. [Google Scholar]
- Godden, S.M.; Royster, E.; Knauer, W.; Sorg, J.; Lopez-Benavides, M.; Schukken, Y.; French, E.A. Randomized non-inferiority study evaluating the efficacy of a post-milking teat disinfectant for the prevention of naturally occurring intramammary infections. J. Dairy Sci. 2016, 99, 3675–3687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lago, A.; Bruno, D.R.; Lopez-Benavides, M.; Leibowitz, S. Short communication: Efficacy of glycolic acid-based and iodine-based post-milking barrier teat disinfectants for prevention of new intramammary infections in dairy cattle. J. Dairy Sci. 2016, 99, 7467–7472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pankey, J.W. Pre-milking udder hygiene. J. Dairy Sci. 1989, 72, 1308–1312. [Google Scholar] [CrossRef]
- Galton, D.M.; Petersson, L.G.; Merrill, W.G.; Bandler, D.K.; Shuster, D.E. Effects of pre-milking udder preparation on bacterial population, sediment, and iodine residue in milk. J. Dairy Sci. 1984, 67, 2580–2589. [Google Scholar] [CrossRef]
- Baumberger, C.; Guarin, J.F.; Ruegg, P.L. Effect of 2 different pre-milking teat sanitation routines on reduction of bacterial counts on teat skin of cows on commercial dairy farms. J. Dairy Sci. 2016, 99, 2915–2929. [Google Scholar] [CrossRef] [Green Version]
- Neave, F.K.; Dodd, F.H.; Kingwill, R.G.; Westgarth, D.R. Control of mastitis in the dairy herd by hygiene and management. J. Dairy Sci. 1969, 52, 696–707. [Google Scholar] [CrossRef]
- Galton, D.M.; Petersson, L.G.; Merrill, W.G. Effects of pre-milking udder preparation practices on bacterial counts in milk and teats. J. Dairy Sci. 1986, 69, 260–266. [Google Scholar] [CrossRef]
- Gleeson, D.; O’Brien, B.; Flynn, J.; O’Callaghan, E.; Galli, F. Effect of pre-milking teat preparation procedures on the microbial count on teats prior to cluster application. Ir. Vet. J. 2009, 62, 461–467. [Google Scholar] [CrossRef] [Green Version]
- Mišeikienė, R.; Rudejevienė, J.; Gerulis, G. Effect of pre-milking antiseptic treatment on the bacterial contamination of cow teats’ skin. Bulg. J. Vet. Med. 2015, 18, 159–166. [Google Scholar] [CrossRef]
- Rowe, S.; Tranter, W.; Laven, R. Effect of pre-milking teat disinfection on clinical mastitis incidence in a dairy herd in Northern Queensland, Australia. Aust. Vet. J. 2018, 96, 69–75. [Google Scholar] [CrossRef]
- O’Connell, A.; Ruegg, P.L.; Gleeson, D.E. Farm management factors associated with the Bacillus cereus count in bulk tank milk. Ir. J. Agric. Food Res. 2013, 52, 229–241. [Google Scholar]
- Schukken, Y.H.; Rauch, B.J.; Morelli, J. Defining standardized protocols for determining the efficacy of a post-milking teat disinfectant following experimental exposure of teats to mastitis pathogens. J. Dairy Sci. 2013, 96, 2694–2704. [Google Scholar] [CrossRef] [Green Version]
- Gleeson, D.; Flynn, J.; O’ Brien, B. Effect of pre-milking teat disinfection on new mastitis infection rates of dairy cows. Ir. Vet. J. 2018, 71, 1–8. [Google Scholar] [CrossRef]
- Oliver, S.P.; Lewis, M.J.; Ingle, T.L.; Gillespie, B.E.; Matthews, K.R. Prevention of bovine mastitis by a pre-milking teat disinfectant containing chlorous acid and chlorine dioxide. J. Dairy Sci. 1993, 76, 287–292. [Google Scholar] [CrossRef]
- Oliver, S.P.; Lewis, M.J.; Ingle, T.L.; Gillespie, B.E.; Matthews, K.R.; Dowlen, H.H. Pre-milking teat disinfection for the prevention of environmental pathogen intramammary infections. J. Food Prot. 1993, 56, 852–855. [Google Scholar] [CrossRef]
- Oliver, S.P.; Gillespie, B.E.; Lewis, M.J.; Ivey, S.J.; Almeida, R.A.; Luther, D.A.; Dowlen, H.H. Efficacy of a new pre-milking teat disinfectant containing a phenolic combination for the prevention of mastitis. J. Dairy Sci. 2001, 84, 1545–1549. [Google Scholar] [CrossRef]
- Williamson, J.H.; Lacy-Hulbert, S.J. Effect of disinfecting teats post-milking or pre- and post-milking on intramammary infection and somatic cell count. N. Z. Vet. J. 2013, 61, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Morton, J.M.; Penry, J.F.; Malmo, J.; Mein, G.A. Pre-milking teat disinfection: Is it worthwhile in pasture-grazed dairy herds? J. Dairy Sci. 2014, 97, 7525–7537. [Google Scholar] [CrossRef] [Green Version]
- Gibson, H.; Sinclair, L.A.; Brizuela, C.M.; Worton, H.L.; Protheroe, R.G. Effectiveness of selected pre-milking teat-cleaning regimes in reducing teat microbial load on commercial dairy farms. Lett. Appl. Microbiol. 2008, 46, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, S.R.; Garvey, M.; Flynn, J.; Jordan, K.; Gleeson, D. Are some teat disinfectant formulations more effective against specific bacteria isolated on teat skin than others? Acta Vet. Scand. 2019, 61, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Keane, O.M.; Budd, K.E.; Flynn, J.; McCoy, F. Pathogen profile of clinical mastitis in Irish milk-recording herds reveals a complex aetiology. Vet. Rec. 2013, 173, 17–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morrill, K.M.; Scillieri Smith, J.C.; Dann, H.M.; Gauthier, H.M.; Ballard, C.S. Evaluation of powdered 0.5% chlorhexidine acetate-based post-milking teat dip compared with a foamed 1% iodine-based post-milking teat dip under cold weather conditions in northern New York. J. Dairy Sci. 2019, 10, 2507–2514. [Google Scholar] [CrossRef] [Green Version]
- Cook, N.B.; Reinemann, D.J. A toolbox for assessing cow, udder and teat hygiene. In Proceedings of the 46th National Mastitis Council, San Antonio, TX, USA, 21–24 January 2007; pp. 31–43. [Google Scholar]
- Haltia, L.; Honkanen-Buzalski, T.; Spiridonova, I.; Olkonen, A.; Myllys, V. A study of bovine mastitis, milking procedures and management practices on 25 Estonian dairy herds. Acta Vet. Scand. 2006, 48, 22. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Salam, Z.; Attala, S.A.; Daoud, E.; Harith, M.A. Monitoring of somatic cells in milk via laser analytical techniques for the early detection of mastitis. Dairy Sci. Technol. 2015, 95, 331–340. [Google Scholar] [CrossRef] [Green Version]
- Goldberg, J.J.; Murdough, P.A.; Howard, A.B.; Drechsler, P.A.; Pankey, J.W.; Ledbetter, G.A.; Day, J.D. Winter evaluation of a post-milking powdered teat dip. J. Dairy Sci. 1994, 77, 748–758. [Google Scholar] [CrossRef]
- Oura, L.Y.; Fox, L.K.; Warf, C.C.; Kemp, G.K. Efficacy of two acidified chlorite postmilking teat disinfectants with sodium dodecylbenzene sulfonic acid on prevention of contagious mastitis using an experimental challenge protocol. J. Dairy Sci. 2002, 85, 252–257. [Google Scholar] [CrossRef]
- Merle, R.; Schröder, A.; Hamann, J. Cell function in the bovine mammary gland: A preliminary study on interdependence of healthy and infected udder quarters. J. Dairy Sci. 2007, 74, 174–179. [Google Scholar] [CrossRef]
- IDF. Bulletin of the IDF N° 466/2013: Guidelines for the Use and Interpretation of Bovine Milk Somatic Cell Counts (SCC) in the Dairy Industry; IDF: Brussels, Belgium, 2013. [Google Scholar]
- Schwarz, D.; Diesterbeck, U.S.; Failing, K.; König, S.; Brügemann, K.; Zschöck, M.; Wolter, W.; Czerny, C.P. Somatic cell counts and bacteriological status in quarter foremilk samples of cows in Hesse, Germany—A longitudinal study. J. Dairy Sci. 2010, 93, 5716–5728. [Google Scholar] [CrossRef] [Green Version]
- Souza, F.N.; Cunha, A.F.; Rosa, D.L.; Brito, M.A.V.; Guimarães, A.S.; Mendonça, L.C.; Souza, G.N.; Lage, A.P.; Blagitz, M.G.; Della Libera, A.M.M.P.; et al. Somatic cell count and mastitis pathogen detection in composite and single or duplicate quarter milk samples. Pesqui. Vet Bras. 2016, 36, 811–818. [Google Scholar] [CrossRef]
- McParland, S.; O’Brien, B.; McCarthy, J. The association between herd- and cow-level factors and somatic cell count of Irish dairy cows. Ir. J. Agric. Food Res. 2013, 52, 151–158. [Google Scholar]
- Bruckmaier, R.M.; Blum, J.W. Simultaneous recording of oxytocin release, milk ejection and milk flow during milking of dairy cows with and without pre-stimulation. J. Dairy Res. 2009, 63, 201–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
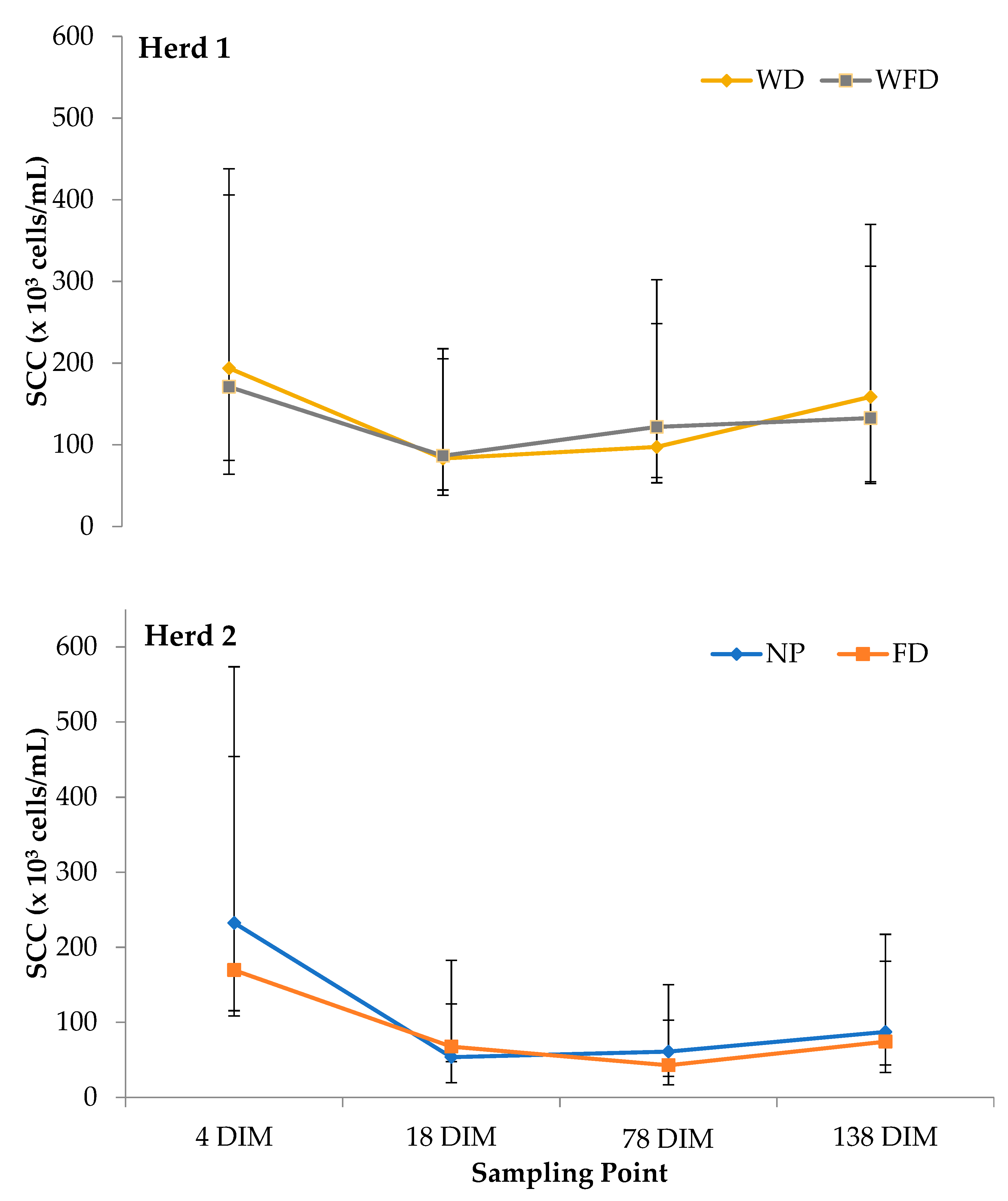
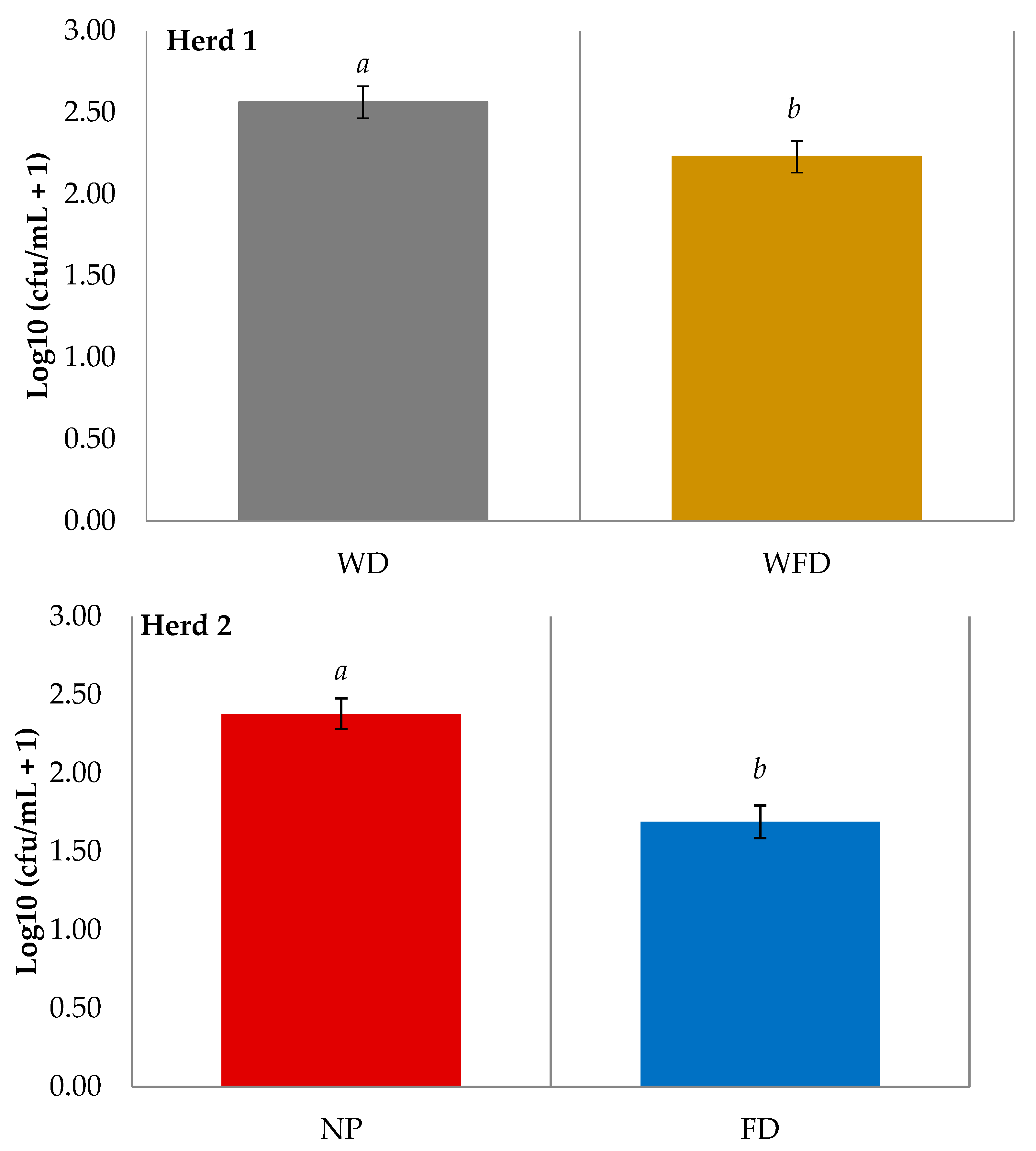
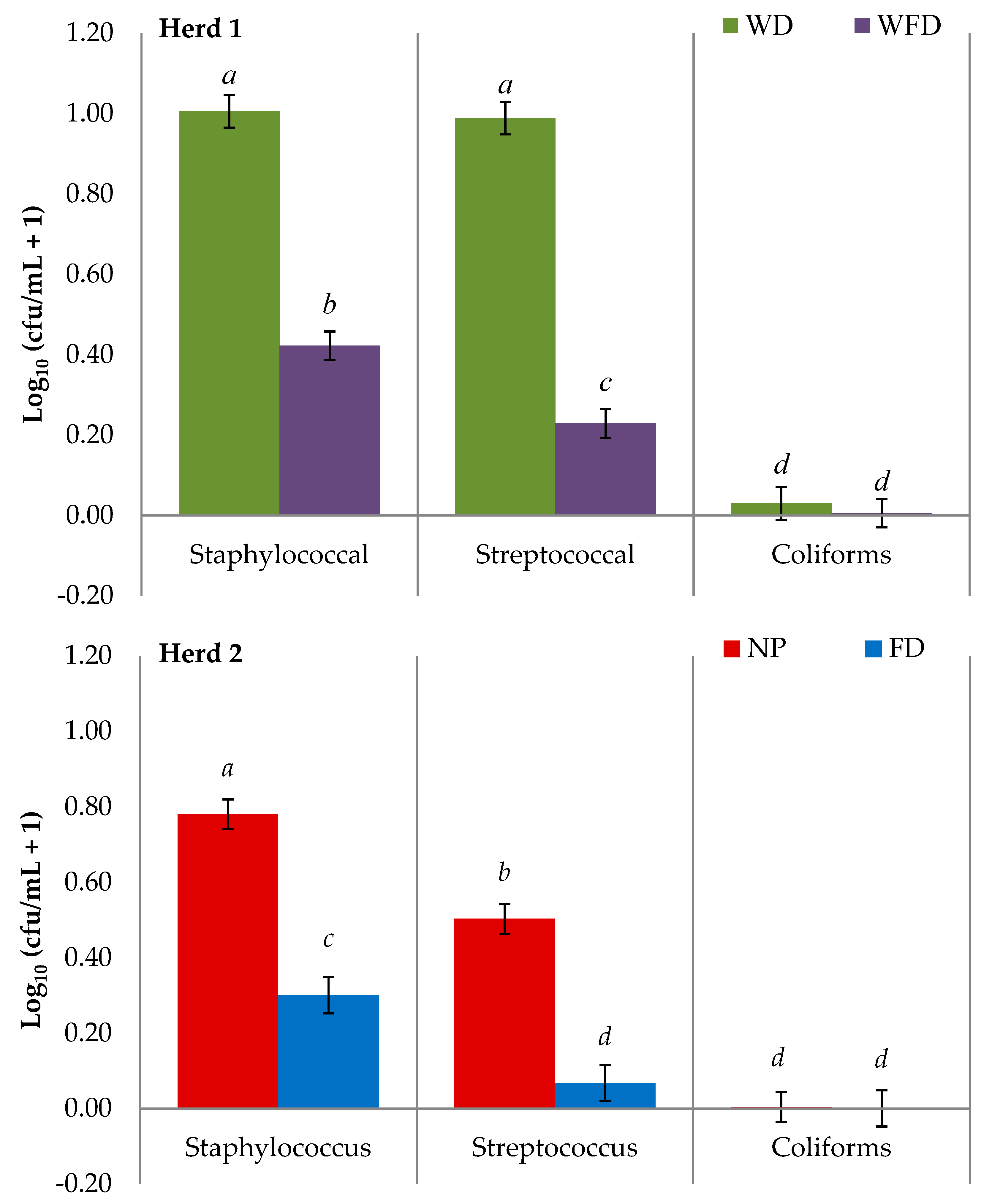
| Herd 1 | WD vs. WFD | p-Value | Herd 2 | NP vs. FD | p-Value |
|---|---|---|---|---|---|
| 4 DIM | 1.20 (0.99–1.45) | 0.06 | 4 DIM | 0.75 (0.73–0.56) | 0.06 |
| 18 DIM | 1.05 (0.85–1.30) | 0.66 | 18 DIM | 0.77 (0.56–1.04) | 0.09 |
| 78 DIM | 1.05 (0.79–1.40) | 0.71 | 78 DIM | 0.90 (0.60–1.35) | 0.62 |
| 138 DIM | 1.25 (0.90–1.73) | 0.18 | 138 DIM | 1.17 (0.73–1.87) | 0.52 |
| <30 × 103 Cell/mL | 30–100 × 103 Cells/mL | ≥200 × 103 Cells/mL | ||||
|---|---|---|---|---|---|---|
| Herd 1 (H1) | ||||||
| Treatment | WD | WFD | WD | WFD | WD | WFD |
| 4 DIM | 0.49 | 0.53 | 0.31 | 0.30 | 0.13 | 0.10 |
| 18 DIM | 0.79 | 0.80 | 0.11 | 0.10 | 0.07 | 0.07 |
| 78 DIM | 0.86 | 0.87 | 0.04 | 0.02 | 0.07 | 0.08 |
| 138 DIM | 0.80 | 0.83 | 0.03 | 0.01 | 0.13 | 0.12 |
| Herd 2 (H2) | ||||||
| Treatment | NP | FD | NP | FD | NP | FD |
| 4 DIM | 0.52 | 0.58 | 0.24 | 0.26 | 0.16 | 0.12 |
| 18 DIM | 0.80 | 0.82 | 0.08 | 0.09 | 0.06 | 0.06 |
| 78 DIM | 0.84 | 0.83 | 0.03 | 0.03 | 0.08 | 0.06 |
| 138 DIM | 0.86 | 0.86 | 0.01 | 0.01 | 0.10 | 0.09 |
| Treatment (Total No. of Samples) | ||||
|---|---|---|---|---|
| Organism | WD (573) | WFD (575) | NP (250) | FD (236) |
| Herd 1 | Herd 2 | |||
| Staphylococcus aureus (aB) | 114 (0.20) | 142 (0.25) | 48 (0.19) | 43 (0.18) |
| Streptococcus uberis | 9 (0.02) | 7 (0.01) | 0 (0) | 0 (0) |
| Streptococcus dysagalactiae | 1 (0.002) | 2 (0.003) | 1 (0.004) | 2 (0.008) |
| Escherichia coli (NH) | 1 (0.002) | 0 (0) | 1 (0.004) | 0 (0) |
| Escherichia coli (H) | 1 (0.002) | 0 (0) | 0 (0) | 0 (0) |
| Unclassified Gram (-) Cocci | 20 (0.04) | 20 (0.05) | 5 (0.02) | 5 (0.02) |
| No growth | 431 (0.75) | 415 (0.72) | 195 (0.78) | 195 (0.83) |
| Total | 573 | 575 | 250 | 236 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fitzpatrick, S.R.; Garvey, M.; Flynn, J.; O’Brien, B.; Gleeson, D. Effect of Pre-Milking Teat Foam Disinfection on the Prevention of New Mastitis Rates in Early Lactation. Animals 2021, 11, 2582. https://doi.org/10.3390/ani11092582
Fitzpatrick SR, Garvey M, Flynn J, O’Brien B, Gleeson D. Effect of Pre-Milking Teat Foam Disinfection on the Prevention of New Mastitis Rates in Early Lactation. Animals. 2021; 11(9):2582. https://doi.org/10.3390/ani11092582
Chicago/Turabian StyleFitzpatrick, Sarah Rose, Mary Garvey, Jim Flynn, Bernadette O’Brien, and David Gleeson. 2021. "Effect of Pre-Milking Teat Foam Disinfection on the Prevention of New Mastitis Rates in Early Lactation" Animals 11, no. 9: 2582. https://doi.org/10.3390/ani11092582
APA StyleFitzpatrick, S. R., Garvey, M., Flynn, J., O’Brien, B., & Gleeson, D. (2021). Effect of Pre-Milking Teat Foam Disinfection on the Prevention of New Mastitis Rates in Early Lactation. Animals, 11(9), 2582. https://doi.org/10.3390/ani11092582






