Detection of Anti-Erythrocyte Antibodies in Dogs with Inflammatory Bowel Disease (IBD)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hedin, C.R.H.; Vavricka, S.R.; Stagg, A.J.; Schoepfer, A.; Raine, T.; Puig, L.; Pleyer, U.; Navarini, A.; van der Meulen-de Jong, A.E.; Maul, J.; et al. The pathogenesis of extraintestinal manifestations: Implications for IBD research, diagnosis, and therapy. ECCOJC 2019, 13, 541–554. [Google Scholar] [CrossRef]
- Vavricka, S.R.; Schoepfer, A.; Scharl, M.; Lakatos, P.L.; Navarini, A.; Rogler, G. Extraintestinal manifestations of inflammatory bowel disease. Inflamm. Bowel Dis. 2015, 21, 1982–1992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zippi, M.; Corrado, C.; Pica, R.; Avallone, E.V.; Cassieri, C.; De Nitto, D.; Paoluzi, P.; Vernia, P. Extraintestinal manifestations in a large series of Italian inflammatory bowel disease patients. World J. Gastroenterol. 2014, 20, 17463–17467. [Google Scholar] [CrossRef]
- Greuter, T.; Vavricka, S.R. Extraintestinal manifestations in inflammatory bowel disease—Epidemiology, genetics, and pathogenesis. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 307–317. [Google Scholar] [CrossRef] [Green Version]
- Tian, T.; Wang, Z.; Zhang, J. Pathomechanisms of oxidative stress in inflammatory bowel disease and potential antioxidant therapies. Oxid. Med. Cell. Longev. 2017, 2017, 4535194. [Google Scholar] [CrossRef] [PubMed]
- Krzystek-Korpacka, M.; Kempiński, R.; Bromke, M.A.; Neubauer, K. Oxidative stress markers in inflammatory bowel diseases: Systematic review. Diagnostics 2020, 10, 601. [Google Scholar] [CrossRef]
- Uzzan, M.; Galicier, L.; Gornet, J.-M.; Oksenhendler, E.; Fieschi, C.; Allez, M.; Bouhnik, Y.; Kirchgesner, J.; Boutboul, D.; Treton, X.; et al. Autoimmune cytopenias associated with inflammatory bowel diseases: Insights from a multicenter retrospective cohort. Dig. Liver Dis. 2017, 49, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Gumaste, V.; Greenstein, A.J.; Meyers, R.; Sachar, D.B. Coombs-positive autoimmune hemolytic anemia in ulcerative colitis. Dig. Dis. Sci. 1989, 34, 1457–1461. [Google Scholar] [CrossRef] [PubMed]
- Giannadaki, E.; Potamianos, S.; Roussomoustakaki, M.; Kyriakou, D.; Fragkiadakis, N.; Manousos, O.N. Autoimmune hemolytic anemia and positive Coombs test associated with ulcerative colitis. Am. J. Gastroenterol. 1997, 92, 1872–1874. [Google Scholar] [PubMed]
- Yates, P.; Macht, L.M.; Williams, N.A.; Elson, C.J. Red cell autoantibody production by colonic mononuclear cells from a patient with ulcerative colitis and autoimmune haemolytic anaemia. Br. J. Haematol. 1992, 82, 753–756. [Google Scholar] [CrossRef]
- Hochman, J.A. Autoimmune hemolytic anemia associated with Crohn’s disease. Inflamm. Bowel Dis. 2002, 8, 98–100. [Google Scholar] [CrossRef]
- Naqvi, S.; Hasan, S.A.; Khalid, S.; Abbass, A.; Albors-Mora, M. A unique triad: Ulcerative colitis, primary sclerosing cholangitis, and autoimmune hemolytic anemia. Cureus 2018, 10, e2068. [Google Scholar] [CrossRef] [Green Version]
- Warman, S.; Hall, E.J.; Suchodolski, J.; Steiner, J.M. Canine pancreatic lipase immunoreactivity concentrations in dogs with IMHA. In Proceedings of the British Small Animal Veterinary Association, Birmingham, UK, 3–6 April 2008. [Google Scholar]
- Guadarrama-Olhovich, M.; Garcia Ortuno, L.E.; Ruiz Remolina, J.A.; Lopez Buitrago, C.; Ramirez Lezama, J.; Bouda, J. Acute pancreatitis, azotaemia, cholestasis and haemolytic anaemia in a dog: A case report. Vet. Med. 2013, 58, 44–49. [Google Scholar] [CrossRef] [Green Version]
- Gianesini, G.; Drigo, M.; Zoia, A. Association between immune-mediated hemolytic anemia (IMHA) and acute pancreatitis in dogs. In Proceedings of the 29th ECVIM-CA Congress, Milano, Italy, 19–21 September 2019. [Google Scholar]
- Zoia, A.; Drigo, M. Association between pancreatitis and immune-mediated haemolytic anaemia in Cats: A cross-sectional study. J. Comp. Pathol. 2017, 156, 384–388. [Google Scholar] [CrossRef]
- Dandrieux, J.R.S. Inflammatory bowel disease versus chronic enteropathy in dogs: Are they one and the same? J. Small Anim. Pract. 2016, 57, 589–599. [Google Scholar] [CrossRef]
- Dandrieux, J.R.S.; Mansfield, C.S. Chronic Enteropathy in Canines: Prevalence, Impact and Management Strategies. Vet. Med. 2019, 10, 203–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makielski, K.; Cullen, J.; O’Connor, A.; Jergens, A.E. Narrative review of therapies for chronic enteropathies in dogs and cats. J. Vet. Intern. Med. 2018, 33, 11–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allenspach, K.; Wieland, B.; Gröne, A.; Gaschen, F. Chronic enteropathies in dogs: Evaluation of risk factors for negative outcome. J. Vet. Intern. Med. 2007, 21, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Day, M.J.; Bilzer, T.; Mansell, J.; Wilcock, B.; Hall, E.J.; Jergens, A.; Minami, T.; Willard, M.; Washabau, R. World Small Animal Veterinary Association Gastrointestinal Standardization Group Histopathological standards for the diagnosis of gastrointestinal inflammation in endoscopic biopsy samples from the dog and cat: A report from the World Small Animal Veterinary Association Gastrointestinal Standardization Group. J. Comp. Pathol. 2008, 138 (Suppl. 1), S1–S43. [Google Scholar]
- Washabau, R.J.; Day, M.J.; Willard, M.D.; Hall, E.J.; Jergens, A.E.; Mansell, J.; Minami, T.; Bilzer, T.W.; WSAVA International Gastrointestinal Standardization Group. Endoscopic, biopsy, and histopathologic guidelines for the evaluation of gastrointestinal inflammation in companion animals. J. Vet. Intern. Med. 2010, 24, 10–26. [Google Scholar]
- Harvey, J.W. Hematology Procedures. In Veterinary Hematology: A Diagnostic Guide and Color Atlas, 1st ed.; Harvey, J.W., Ed.; Elsevier Saunders: St. Louis, MO, USA, 2012; pp. 11–33. [Google Scholar]
- Villiers, E. Disorders of erythrocytes. In BSAVA Manual of Canine and Feline Clinical Pathology, 3rd ed.; Villiers, E., Ristić, J., Eds.; British Small Animal Veterinary Association: Gloucester, UK, 2016; pp. 38–66. [Google Scholar]
- Carli, E.; Tasca, S.; Trotta, M.; Furlanello, T.; Caldin, M.; Solano-Gallego, L. Detection of erythrocyte binding IgM and IgG by flow cytometry in sick dogs with Babesia canis canis or Babesia canis vogeli infection. Vet. Parasitol. 2009, 162, 51–57. [Google Scholar] [CrossRef]
- Morley, P.; Mathes, M.; Guth, A.; Dow, S. Anti-erythrocyte antibodies and disease associations in anemic and nonanemic dogs. J. Vet. Intern. Med. 2008, 22, 886–892. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, K.; Hiyoshi, S.; Ohno, K.; Uchida, K.; Goto-Koshino, Y.; Maeda, S.; Mizutani, N.; Takeuchi, A.; Tsujimoto, H. Prognostic factors in dogs with protein-losing enteropathy. Vet. J. 2015, 205, 28–32. [Google Scholar] [CrossRef]
- Patel, D.; Trivedi, C.; Khan, N. Management of anemia in patients with inflammatory bowel disease (IBD). Curr. Treat. Opt. Gastroenterol. 2018, 16, 112–128. [Google Scholar] [CrossRef] [PubMed]
- Wilkerson, M.J.; Davis, E.; Shuman, W.; Harkin, K.; Cox, J.; Rush, B. Isotype-specific antibodies in horses and dogs with immune-mediated hemolytic anemia. J. Vet. Intern. Med. 2000, 14, 190–196. [Google Scholar] [CrossRef]
- Quigley, K.A.; Chelack, B.J.; Haines, D.M.; Jackson, M.L. Application of a direct flow cytometric erythrocyte immunofluorescence assay in dogs with immune-mediated hemolytic anemia and comparison to the direct antiglobulin test. J. Vet. Diagn. Investig. 2001, 13, 297–300. [Google Scholar] [CrossRef]
- Members of the Veterinary and Comparative Clinical Immunology Society Diagnostic Task, Force; MacNeill, A.L.; Dandrieux, J.; Lubas, G.; Seelig, D.; Szladovits, B. The utility of diagnostic tests for immune-mediated hemolytic anemia. Vet. Clin. Pathol. 2019, 48 (Suppl. 1), 7–16. [Google Scholar] [PubMed]
- Fujii, J.; Kurahashi, T.; Konno, T.; Homma, T.; Iuchi, Y. Oxidative stress as a potential causal factor for autoimmune hemolytic anemia and systemic lupus erythematosus. World J. Nephrol. 2015, 4, 213. [Google Scholar] [CrossRef] [PubMed]
- Garden, O.A.; Kidd, L.; Mexas, A.M.; Chang, Y.-M.; Jeffery, U.; Blois, S.L.; Fogle, J.E.; MacNeill, A.L.; Lubas, G.; Birkenheuer, A.; et al. ACVIM consensus statement on the diagnosis of immune-mediated hemolytic anemia in dogs and cats. J. Vet. Intern. Med. 2019, 33, 313–334. [Google Scholar] [CrossRef]
- Kendall, A.; Woolcock, A.; Brooks, A.; Moore, G.E. Glutathione peroxidase activity, plasma total antioxidant capacity, and urinary F2-isoprostanes as markers of oxidative stress in anemic dogs. J. Vet. Intern. Med. 2017, 31, 1700–1707. [Google Scholar] [CrossRef]
- Craven, M.; Simpson, J.W.; Ridyard, A.E.; Chandler, M.L. Canine inflammatory bowel disease: Retrospective analysis of diagnosis and outcome in 80 cases (1995–2002). J. Small Anim. Pract. 2004, 45, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, V.; Lubas, G.; Lombardo, A.; Corazza, M.; Guidi, G.; Cardini, G. Evaluation of erythrocytes, platelets, and serum iron profile in dogs with chronic enteropathy. Vet. Med. Int. 2010, 2010, 716040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paltrinieri, S. The diagnostic approach to anaemia in the dog and cat. J. Hell. Vet. Med. Soc. 2014, 65, 149–164. [Google Scholar] [CrossRef] [Green Version]
- eClinPath. Available online: https://eclinpath.com/hematology/anemia/causes-of-anemia/ (accessed on 1 May 2021).
- Akhtar, S.; Mahure, S. Nuance of nucleated rbcs (normoblastemia) in peripheral blood film. Panacea J. Med. Sci. 2015, 5, 7–13. [Google Scholar]
- Katsaros, M.; Paschos, P.; Giouleme, O. Red cell distribution width as a marker of activity in inflammatory bowel disease: A narrative review. Ann. Gastroenterol. 2020, 33, 348–354. [Google Scholar] [CrossRef] [PubMed]
| Parameter | All Dogs | Anti-RBC ab ⊖ | Anti-RBC ab ⊕ | Reference Range |
|---|---|---|---|---|
| RBC (×106/μL) | 6 (3.8–7.8) | 6.6 (5.5–7.8) | 6 (3.8–7.8) | 5.65–8.8 |
| HCT (%) | 42.5 (23.6–57.5) | 47.3 (37.3–54) | 39.9 (23.6–57.5) | 37.3–61.7 |
| HGB (g/dL) | 14.4 (8.5–20) | 15.4 (13.5–19) | 13.5 (8.5–20) | 13.1–20.5 |
| MCV (fL) | 68.6 (57–77.1) | 69 (67.9–75.2) | 67.7 (57–77.1) | 61–73 |
| MCHC (g/dL) | 34.6 (31.6–37.2) | 33.9 (31.6–36.2) | 34.8 (32–37.2) | 32–38 |
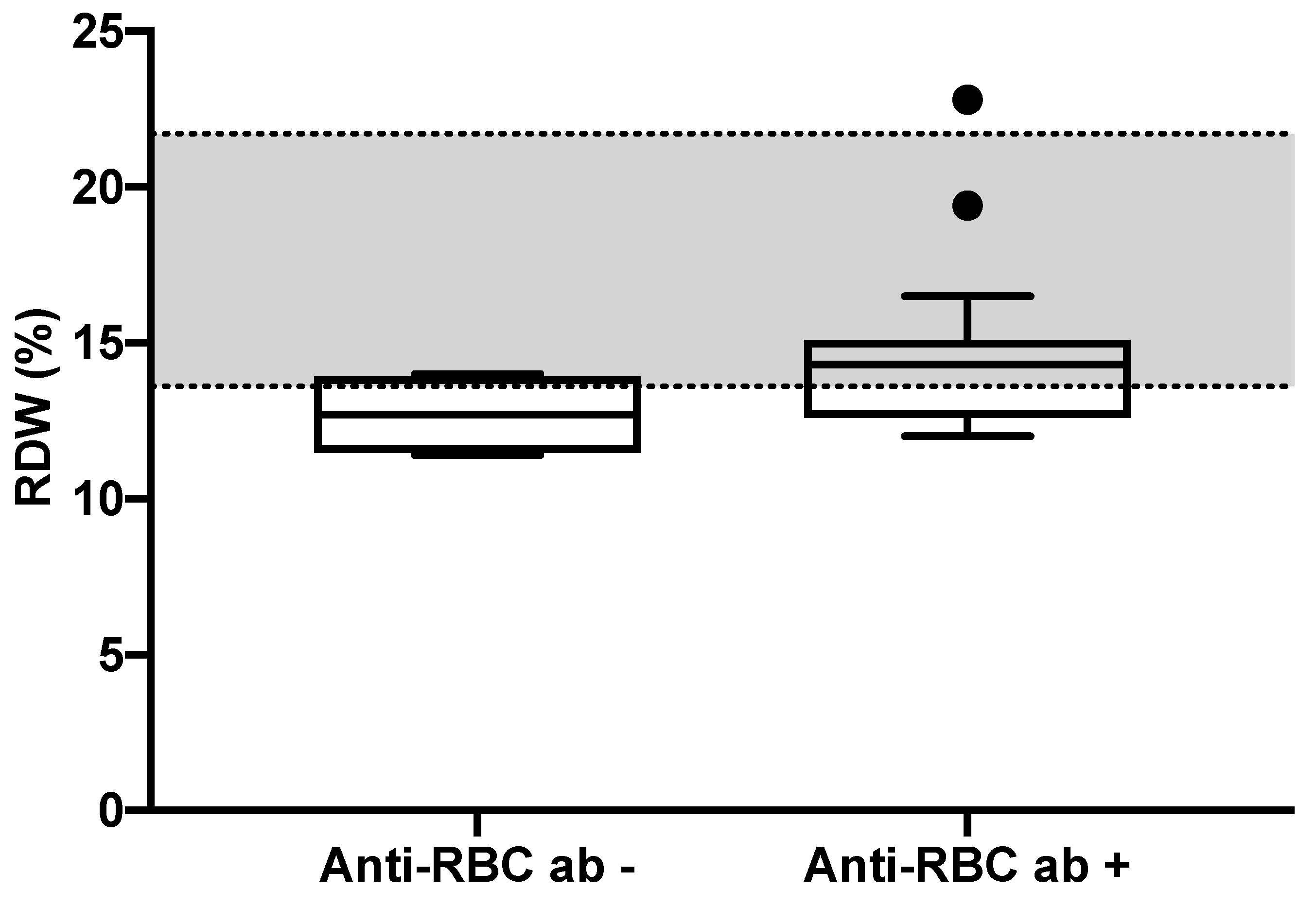
| RDW (%) | 14 (11.4–22.8) | 12.7 (11.4–14) | 14.3 (12–22.8) 1 | 13.6–21.7 |
| Retic (×103/μL) | 35 (13–361) | 32 (13–61) | 40 (14–361) | 10–110 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gori, E.; Pierini, A.; Nesci, M.; Benvenuti, E.; Tasca, S.; Lubas, G.; Marchetti, V. Detection of Anti-Erythrocyte Antibodies in Dogs with Inflammatory Bowel Disease (IBD). Animals 2021, 11, 2580. https://doi.org/10.3390/ani11092580
Gori E, Pierini A, Nesci M, Benvenuti E, Tasca S, Lubas G, Marchetti V. Detection of Anti-Erythrocyte Antibodies in Dogs with Inflammatory Bowel Disease (IBD). Animals. 2021; 11(9):2580. https://doi.org/10.3390/ani11092580
Chicago/Turabian StyleGori, Eleonora, Alessio Pierini, Martina Nesci, Elena Benvenuti, Silvia Tasca, George Lubas, and Veronica Marchetti. 2021. "Detection of Anti-Erythrocyte Antibodies in Dogs with Inflammatory Bowel Disease (IBD)" Animals 11, no. 9: 2580. https://doi.org/10.3390/ani11092580
APA StyleGori, E., Pierini, A., Nesci, M., Benvenuti, E., Tasca, S., Lubas, G., & Marchetti, V. (2021). Detection of Anti-Erythrocyte Antibodies in Dogs with Inflammatory Bowel Disease (IBD). Animals, 11(9), 2580. https://doi.org/10.3390/ani11092580









