Co-Occurrence of Domestic Dogs and Gastropod Molluscs in Public Dog-Walking Spaces and Implications for Infection with Angiostrongylus vasorum: A Preliminary Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
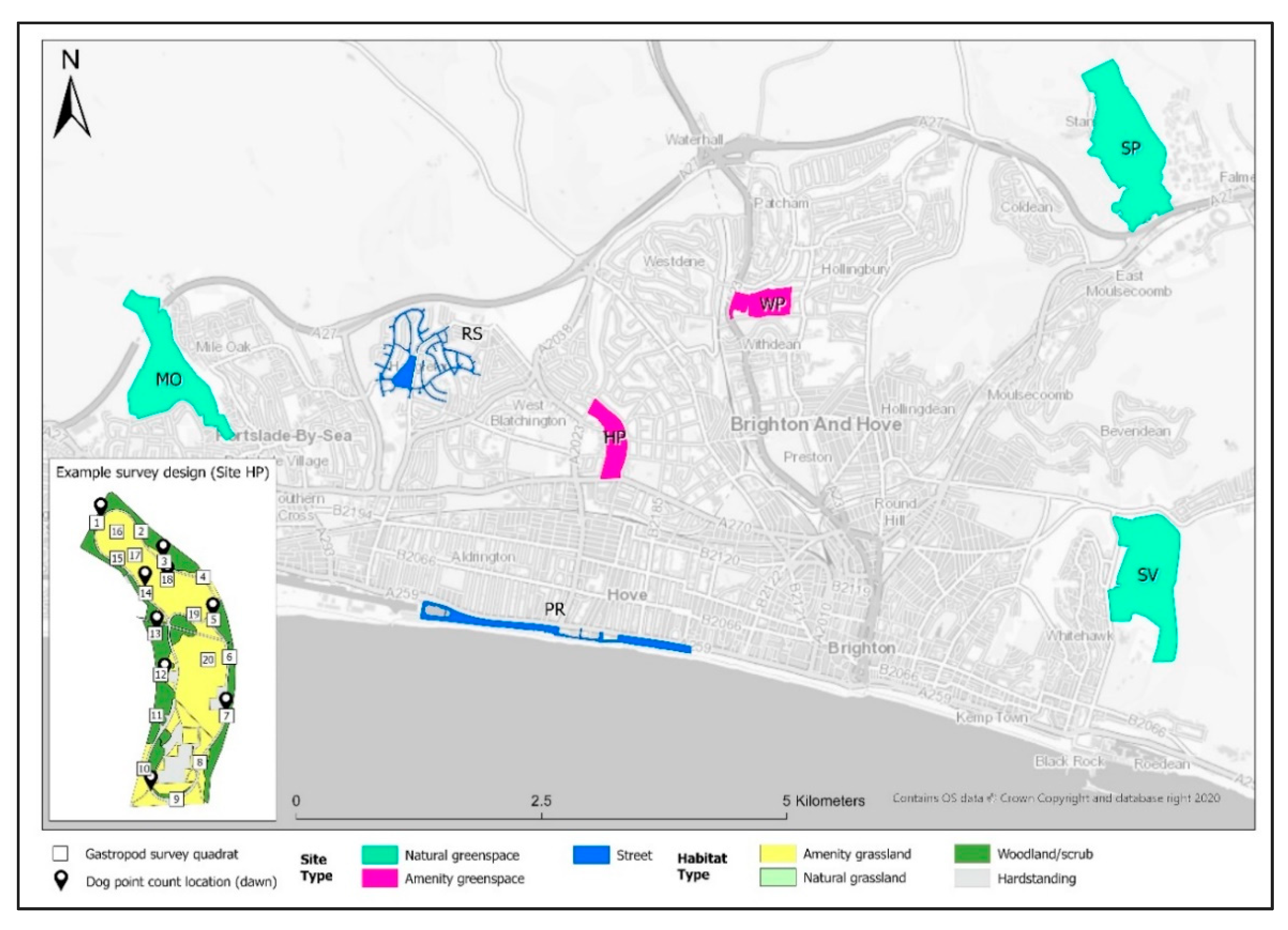
2.1. Study Area
2.2. Field Methods
2.3. Data Analysis
2.3.1. Gastropod Generalized Linear Models
2.3.2. Dog Generalized Linear Models
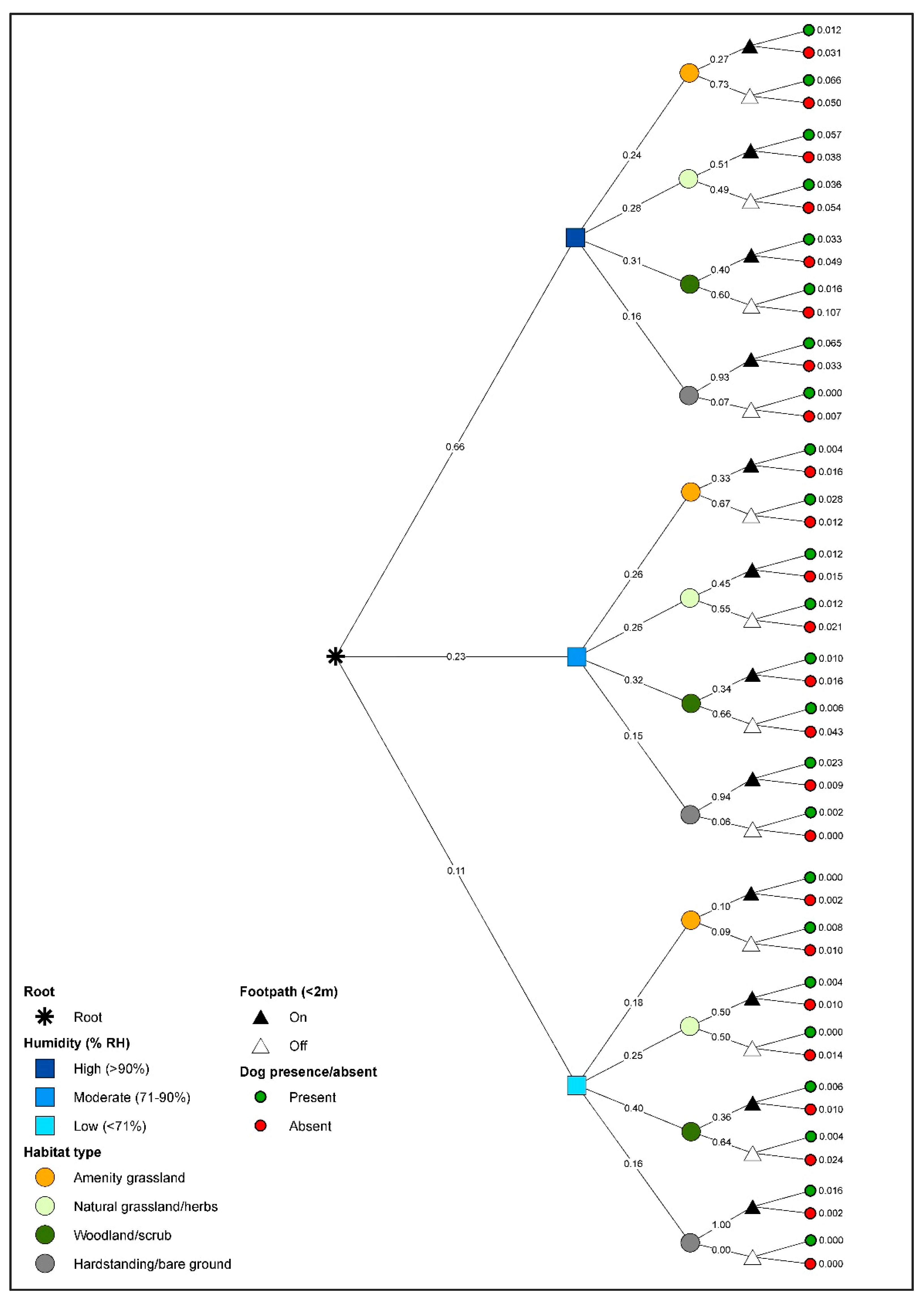
2.3.3. Conditional Probability Trees
3. Results
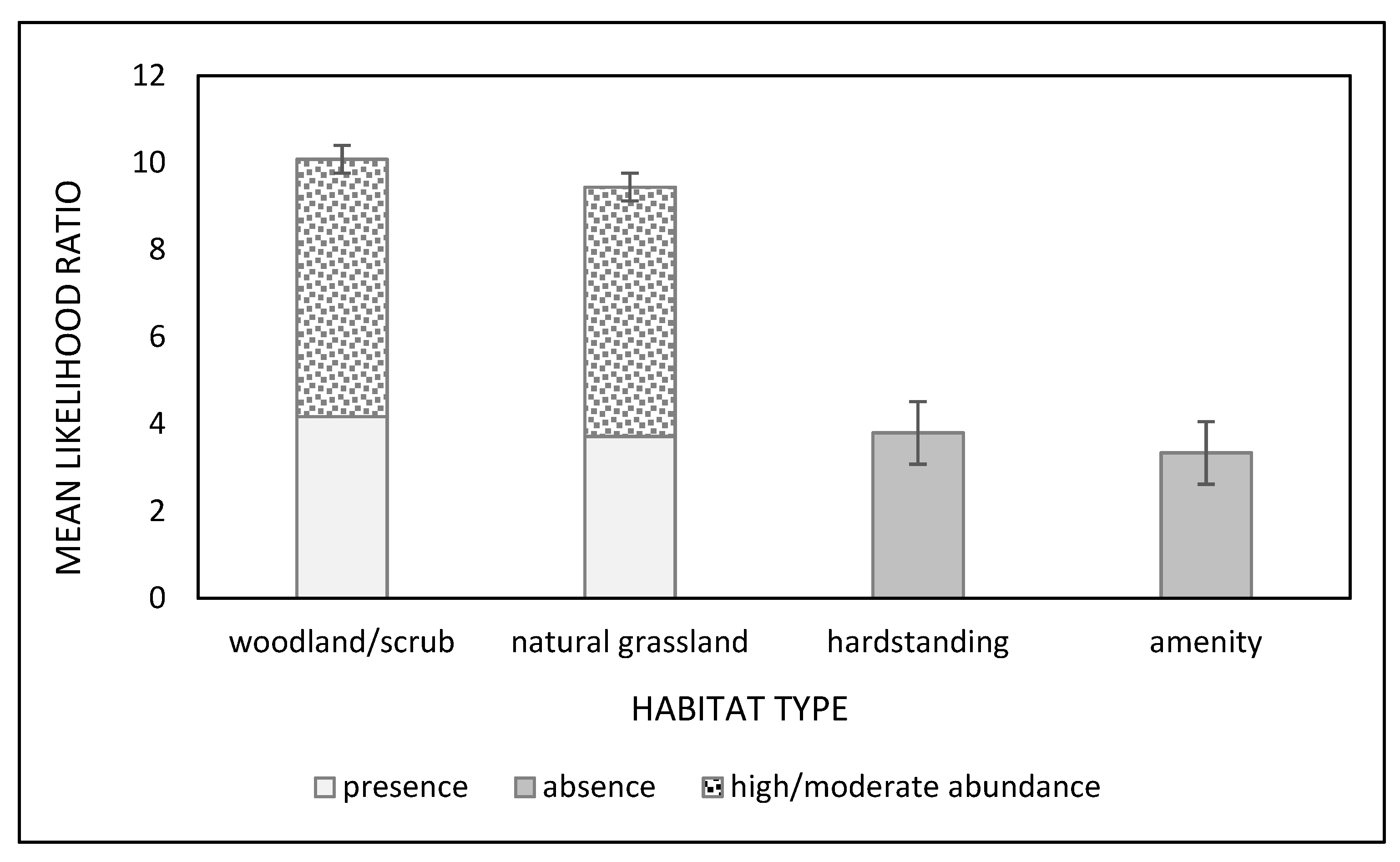
3.1. Gastropod GLMs
3.2. Dog GLMs
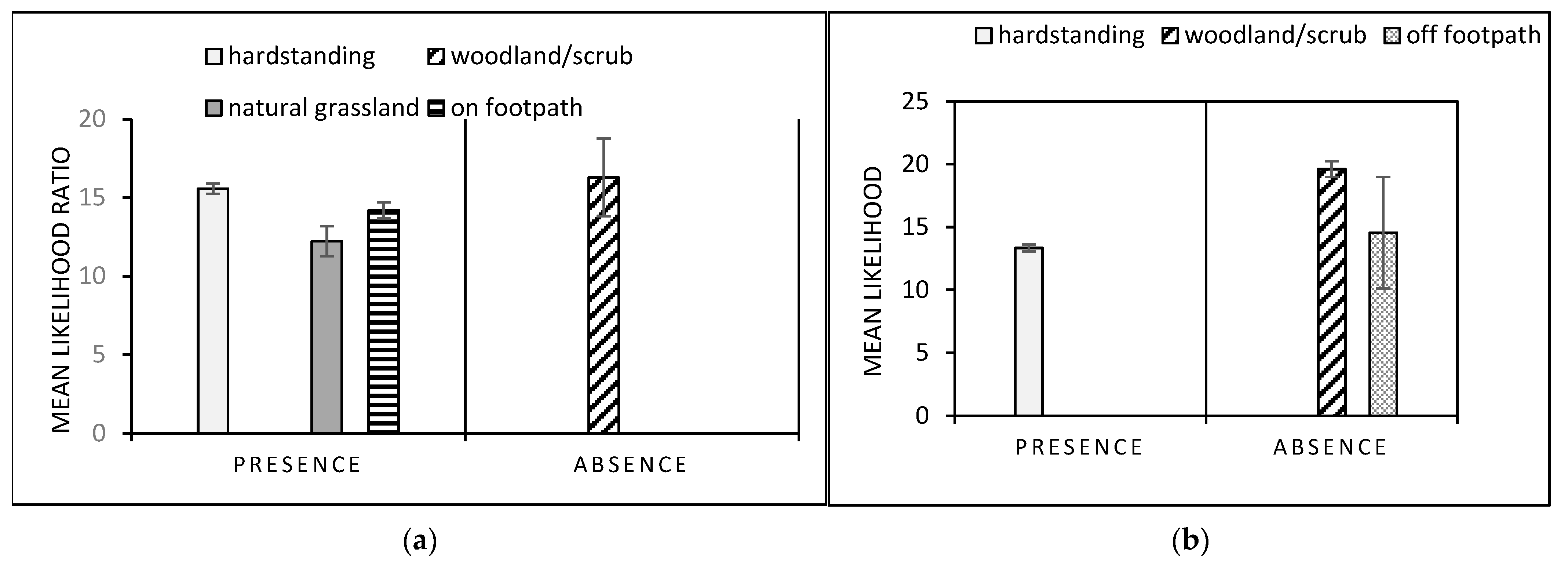
3.3. Gastropod Conditional Probability Trees
3.4. Dog Conditional Probability Trees
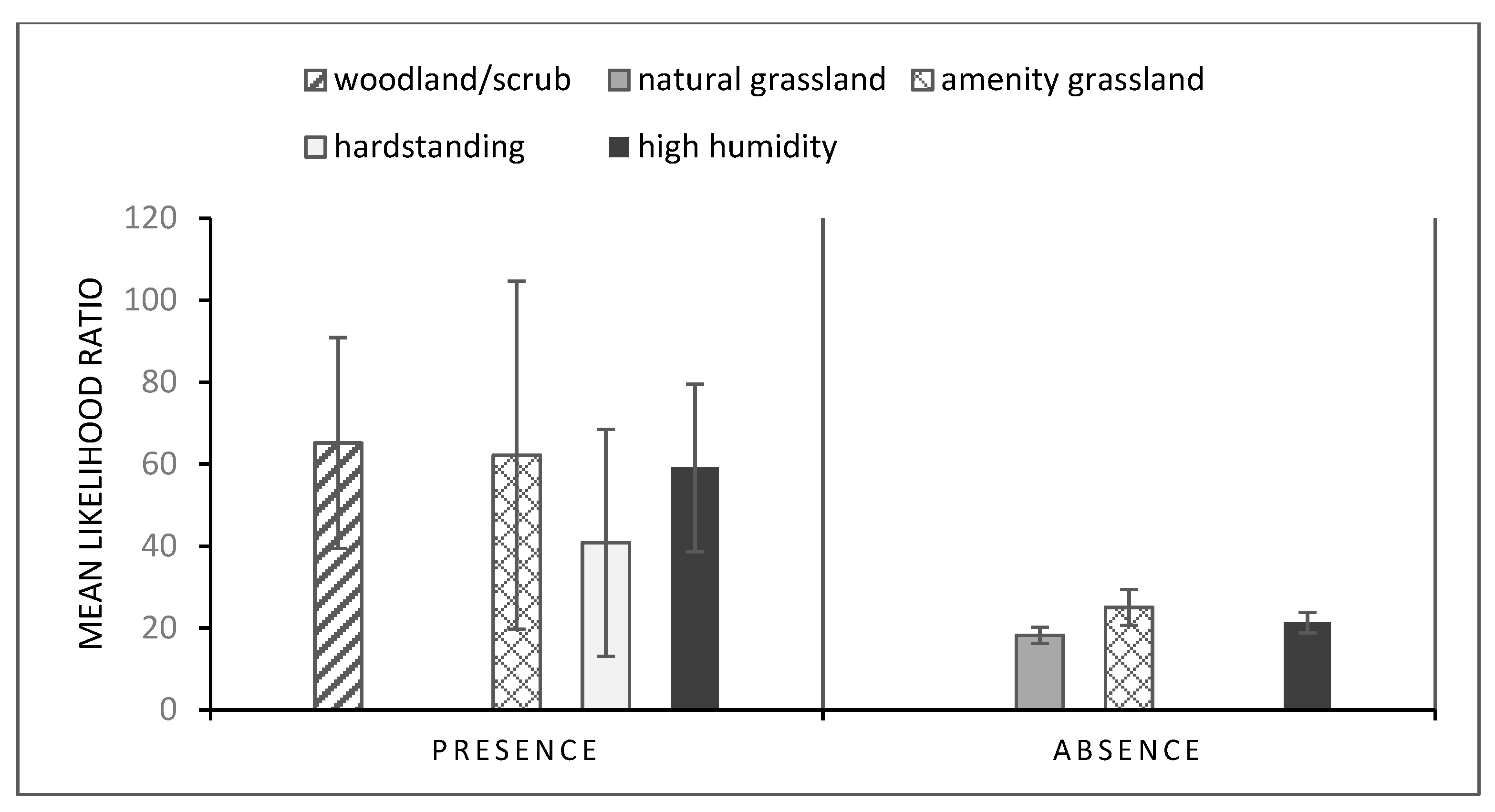
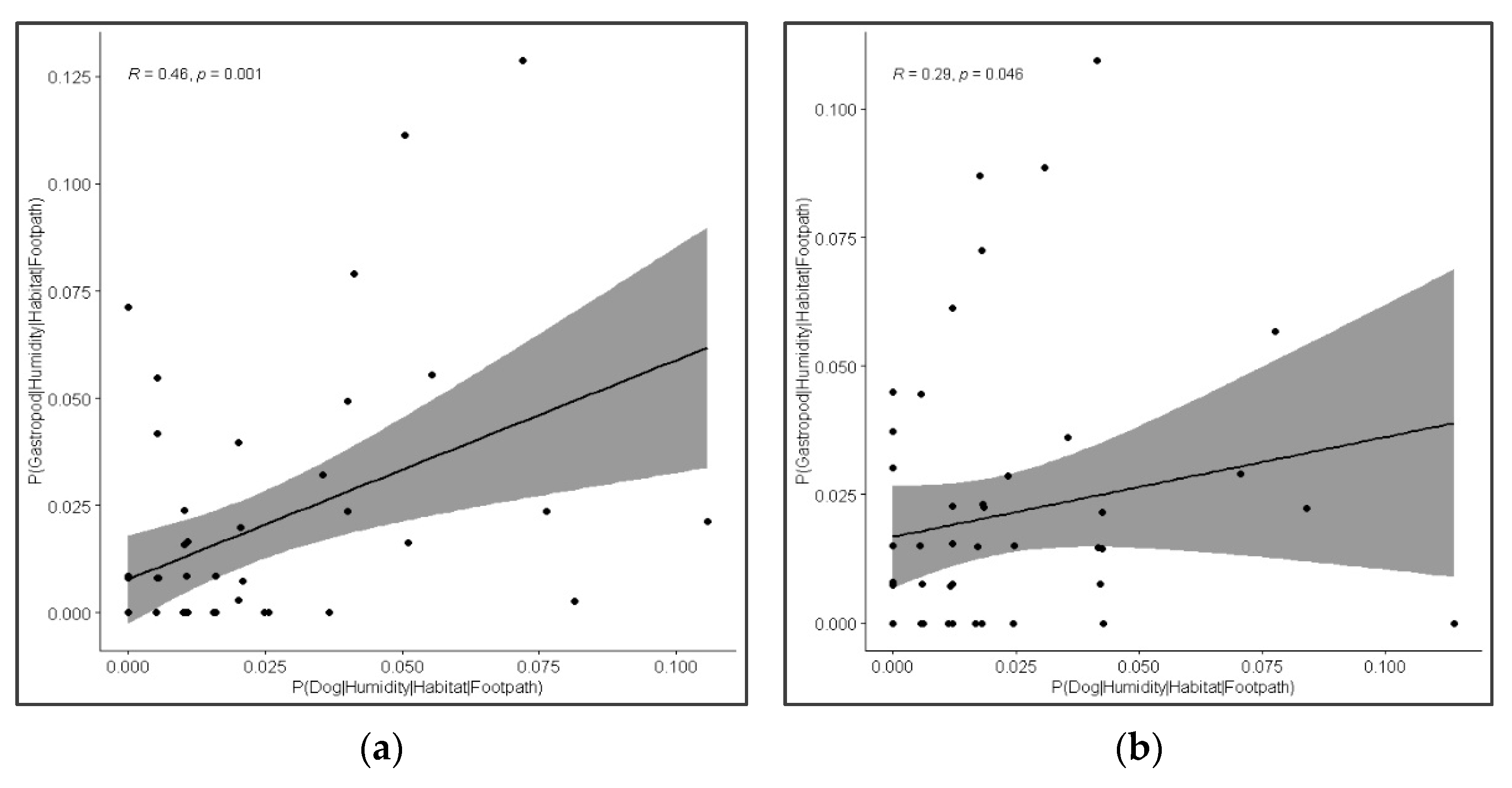
3.5. Co-Occurrence of Dogs and Gastropods
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Elsheikha, H.M.; Holmes, S.A.; Wright, I.; Morgan, E.R.; Lacher, D.W. Recent advances in the epidemiology, clinical and diagnostic features, and control of canine cardio-pulmonary angiostrongylosis. Vet. Rec. 2014, 45, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, C.S.; Garcia Gato, R.; Learmount, J.; Aziz, N.A.; Montgomery, C.; Rose, H.; Coulthwaite, C.L.; Mcgarry, J.W.; Forman, D.W.; Allen, S.; et al. Increased prevalence and geographic spread of the cardiopulmonary nematode Angiostrongylus vasorum in fox populations in Great Britain. Parasitology 2015, 142, 1190–1195. [Google Scholar] [CrossRef]
- Blehaut, T.R.; Hardstaff, J.L.; Chapman, P.S.; Pfeiffer, D.U.; Boag, A.K.; Guitan, F.J. Spatial, Demographic and clinical patterns of Angiostrongylus vasorum infection in the dog population of southern England. Vet. Rec. 2014, 175, 148. [Google Scholar] [CrossRef] [PubMed]
- Helm, J.; Morgan, E.R. Canine and feline A. vasorum infections in the UK. Practice 2017, 39, 298–315. [Google Scholar] [CrossRef]
- Chapman, P.S.; Boag, A.K.; Guitian, J.; Boswood, A. Angiostrongylus vasorum infection in 23 dogs (1999–2002). J. Small. Anim. Pract. 2004, 45, 435–440. [Google Scholar] [CrossRef]
- Koch, J.; Willesen, J.L. Canine pulmonary angiostrongylosis: An update. Vet. J. 2009, 179, 348–359. [Google Scholar] [CrossRef]
- Schnyder, M.; Schaper, R.; Bilbrough, G.; Morgan, E.R.; Deplazes, P. Seroepidemiological survey for canine angiostrongylosis in dogs from Germany and the UK using combined detection of Angiostrongylus vasorum antigen and specific antibodies. Parasitology 2013, 140, 1442–1450. [Google Scholar] [CrossRef] [Green Version]
- Morgan, E.R.; Jefferies, R.; van Otterdijk, L.; McEniry, R.B.; Allen, F.; Bakewell, M.; Shaw, S.E. Angiostrongylus vasorum infection in dogs: Presentation and risk factors. Vet. Parasitol. 2010, 173, 255–261. [Google Scholar] [CrossRef]
- Helm, J.; Roberts, L.; Jefferies, R.; Shaw, S.E.; Morgan, E.R. Epidemiological survey of Angiostrongylus vasorum in dogs and slugs around a new endemic focus in Scotland. Vet. Rec. 2015, 177, 46. [Google Scholar] [CrossRef]
- Bolt, G.; Monrad, J.; Frandsen, F.; Heinriksen, P.; Dietz, H.H. The common frog (Rana temporaria) as a potential paratenic and intermediate host of Angiostrongylus vasorum. Parasitol. Res. 1994, 79, 428–430. [Google Scholar] [CrossRef]
- Mozzer, L.R.; Lima, W.S. Gallus gallus domesticus: Paratenic host of Angiostrongylus vasorum. Vet. Parasitol. 2015, 207, 81–84. [Google Scholar] [CrossRef]
- Roth, S.S.S.; Hatteland, B.A.; Solhoy, T. Some notes on reproductive biology and mating behaviour of Arion vulgaris Moquin-Tandon 1855 in Norway including a mating experiment with a hybrid of Arion rufus (Linnaeus 1758) × ater (Linnaeus 1758). J. Conchol. 2012, 41, 249–257. [Google Scholar]
- Patel, Z.; Christina, A.G.; Fox, M.T.; Hermosilla, C.; Backeljau, T.; Breugelmans, K.; Keevash, E.; McEwan, C.; Aghazadeh, M.; Elson-Riggins, J.G. Molecular identification of novel intermediate host species of Angiostrongylus vasorum in Greater London. Parasitol. Res. 2014, 113, 4363–4369. [Google Scholar] [CrossRef] [PubMed]
- Aziz, N.A.; Daly, E.; Allen, S.; Rowson, B.; Greig, C.; Forman, D.; Morgan, E.R. Distribution of Angiostrongylus vasorum and its gastropod intermediate hosts along the rural–urban gradient in two cities in the United Kingdom, using real time PCR. Parasites Vectors 2016, 9, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jefferies, R.; Morgan, E.R.; Shaw, S.E. A SYBR-green real-time PCR assay for the detection of the nematode Angiostrongylus vasorum in the definitive and intermediate hosts. Vet. Parasitol. 2009, 166, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Hinklenton, L.; Betson, M. Molecular detection of Angiostrongylus vasorum in gastropods in Surrey, UK. Parasitol. Res. 2019, 118, 1051–1054. [Google Scholar] [CrossRef] [Green Version]
- Conboy, G.; Guselle, N.; Schaper, R. Spontaneous shedding of metastrongyloid third-stage larvae by experimentally infected Limax maximus. Parasitol. Res. 2017, 116, S41–S54. [Google Scholar] [CrossRef]
- Barcante, T.A.; Barcante, J.M.P.; Dias, S.R.C.; Lima, W.S. Angiostrongylus vasorum (Baillet, 1866) Kamensky, 1905: Emergence of third-stage larvae from infected Biomphalaria glabrata snails. Parasitol. Res. 2003, 91, 471–475. [Google Scholar] [CrossRef]
- Lebon, W.; Tielemans, E.; Rehbein, S.; Dumont, P.; Yoon, S.; Beugnet, F.; Jeannin, P.; Larsen, D.; Halos, L. Monthly administrations of milbemycin oxime plus afoxolaner chewable tablets to prevent Angiostrongylus vasorum infection in dogs. Parasites Vectors 2016, 9, 485. [Google Scholar] [CrossRef] [Green Version]
- Schnyder, M.; Fahrion, A.; Ossent, P.; Kohler, L.; Webster, P.; Heine, J.; Deplazes, P. Larvicidal effect of imidacloprid/moxidectin spot-on solution in dogs experimentally inoculated with Angiostrongylus vasorum. Vet. Parasitol. 2009, 166, 326–332. [Google Scholar] [CrossRef] [Green Version]
- Ferdushy, T.; Kapel, C.M.O.; Webster, P.; Al-Sabi, M.N.S.; Grønvold, J. The occurrence of Angiostrongylus vasorum in terrestrial slugs from forests and parks in the Copenhagen area, Denmark. J. Helminthol. 2009, 83, 379–383. [Google Scholar] [CrossRef]
- Willis, J.C.; Bohan, D.A.; Choi, Y.H.; Conrad, K.F.; Semenov, M.A. Use of an individual-based model to forecast the effect of climate change on the dynamics, abundance and geographical range of the pest slug Deroceras reticulatum in the UK. Glob. Chang. Biol. 2006, 12, 1643–1657. [Google Scholar] [CrossRef]
- Wilson, M.J.; Digweed, A.J.; Brown, J.; Ivanonva, E.S.; Hapca, S.H. Invasive slug pests and their parasites-temperature responses and potential implications of climate change. Biol. Fertil. Soils 2015, 51, 739–748. [Google Scholar] [CrossRef]
- Crawford-Sidebotham, T.J. The influence of weather upon the activity of slugs. Oecologia 1972, 9, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Young, A.G.; Port, G.R.; Green, D.B. Development of a forecast of slug activity—Validation of models to predict slug activity from meteorological conditions. Crop Prot. 1993, 12, 232–236. [Google Scholar] [CrossRef]
- Slotsbo, S.; Fisker, K.V.; Hansen, L.M.; Holmstrup, M. Drought tolerance in eggs and juveniles of the Iberian slug, Arion lusitanicus. J. Comp. Physiol. B. 2011, 181, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Dörler, D.; Kropf, M.; Laaha, G.; Zaller, J.G. Occurrence of the invasive Spanish slug in gardens: Can a citizen science approach help deciphering underlying factors. BMC Ecol. 2018, 18, 23. [Google Scholar] [CrossRef] [Green Version]
- Slotsbo, S.; Hansen, L.M.; Holmstrup, M. Low temperature survival in different life stages of the Iberian slug, Arion lusitanicus. Cryobiology 2011, 62, 68–73. [Google Scholar] [CrossRef]
- Slotsbo, S.; Damgaard, C.; Hansen, L.M.; Holmstrup, M. The influence of temperature on life history traits in the Iberian slug, Arion lusitanicus. Ann. Appl. Biol. 2013, 162, 80–88. [Google Scholar] [CrossRef]
- Knop, E.; Reusser, N. Jack-of-all-trades: Phenotypic plasticity facilitates the invasion of an alien slug species. Proc. R. Soc. B. Biol. Sci. 2012, 279, 4668–4676. [Google Scholar] [CrossRef] [Green Version]
- Grimm, B.; Schaumberger, K. Daily activity of the pest slug A. lusitanicus under laboratory conditions. Ann. Appl. Biol. 2002, 141, 31–44. [Google Scholar] [CrossRef]
- Morgan, E.R.; Tomlinson, A.; Hunter, S.; Nichols, T.; Roberts, E.; Fox, M.T.; Taylor, M.A. Angiostrongylus vasorum and Eucoleus aerophilus in foxes (Vulpes vulpes) in Great Britain. Vet. Parasitol. 2008, 14, 48–57. [Google Scholar] [CrossRef]
- Gillis-Germitsch, N.; Tritten, L.; Hegglin, D.; Deplazes, P.; Schnyder, M. Conquering Switzerland: The emergence of Angiostrongylus vasorum in foxes over three decades and its rapid regional increase in prevalence contrast with the stable occurrence of lungworms. Parasitology 2020, 147, 1071–1079. [Google Scholar] [CrossRef]
- Lemming, L.; Jorgensen, A.C.; Nielsen, L.B.; Nielsen, S.T.; Mejer, H.; Chriel, M.; Petersen, H.H. Cardiopulmonary nematodes of wild carnivores from Denmark: Do they serve as reservoir hosts for infections in domestic animals? Int. J. Parasitol. Parasites Wildl. 2020, 13, 90–97. [Google Scholar] [CrossRef]
- Westgarth, C.; Chistley, R.M.; Christian, H.E. How might we increase physical activity through dog walking? A comprehensive review of dog walking correlates. Int. J. Behav. 2014, 11, 83. Available online: http://www.ijbnpa.org/content/11/1/83 (accessed on 20 June 2021). [CrossRef] [PubMed] [Green Version]
- Brighton and Hove City Council (BHCC)/PMP. Open Space, Sport and Recreation Study—Brighton & Hove. 2008. Available online: https://ww3.brighton-hove.gov.uk/sites/brighton-hove.gov.uk/files/downloads/ldf/Open_Space__Sport_and_Recreation_Study_-_Final_Report_Mar_2009_3.pdf (accessed on 20 June 2021).
- Westgarth, C.; Chistley, R.M.; Jewell, C.; German, A.J.; Boddy, L.M.; Christian, H.E. Dog owners are more likely to meet physical activity guidelines than those without a dog: An investigation of the association between dog ownership and physical activity levels in a UK community. Sci. Rep. 2019, 9, 5704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuur, A.F.; Ieno, E.N.; Walker, N.; Saveliev, A.A.; Smith, G.M. Chapter 11—Zero-Truncated and Zero Inflated Models for Count Data. In Mixed Effects Models and Extensions in Ecology with R; Springer: New York, NY, USA, 2009. [Google Scholar]
- Lange, M.K.; Penagos-Tabares, F.; Hirzmann, J.; Failing, K.; Schaper, R.; Van Bourgonie, J.R.; Backeljau, T.; Hermosilla, C.; Taubert, A. Prevalence of Angiostrongylus vasorum, Aelurostrongylus abstrusus and Crenosoma vulpis larvae in native slug populations in Germany. Vet. Parasitol. 2018, 254, 120–130. [Google Scholar] [CrossRef] [PubMed]
- McKee, A.; Voltzow, J.; Pernet, B. Substrate Attributes Determine Gait in a Terrestrial Gastropod. Biol. Bull. 2013, 224, 53–61. [Google Scholar] [CrossRef]
- Ng, T.P.T.; Saltin, S.H.; Davies, M.S.; Johannesson, K.; Gray, R.S.; Williams, A. Snails and their trails: The multiple functions of trail-following in gastropods. Biol. Rev. 2013, 88, 683–700. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J.; Petrovskaya, N.; Forbes, E.; Walters, K.F.A.; Petrovskii, S. Movement patterns of the grey field slug (Deroceras reticulatum) in an arable field. Sci. Rep. 2020, 10, 17970. [Google Scholar] [CrossRef]
- Morgan, E.R.; Shaw, S.E. Angiostrongylus vasorum infection in dogs: Continuing spread and developments in diagnosis and treatment. J. Small Animal Practice. 2010, 51, 616–621. [Google Scholar] [CrossRef] [PubMed]
| Variable Name | Variable Description | Variable Type | Levels |
|---|---|---|---|
| Sample Day | 1–21 progressive sampling days | Continuous | n/a |
| Mintemp | minimum temperature (°C) measured in a central location at the site, recorded at 15-minute intervals | Continuous | n/a |
| Minrh | Minimum relative humidity (%) in central location recorded at 15-minute intervals | Continuous | n/a |
| Time | Time of day between 06.00 and 23.00 | Nominal | AM (06.30–10.30) DAY (12.30–16.30) PM (18.30–23.30) |
| Habitat | Classification of quadrat habitat type | Nominal | hardstanding (bare ground and artificial substrates, e.g., paved) amenity grassland natural grassland woodland/scrub |
| Footpath | Location of quadrat on or off (<2 m from) a footpath | Nominal | off footpath on footpath |
| Model Term | Deviance | df | % Deviance ([Term Deviance/Total Deviance] × 100) | p |
|---|---|---|---|---|
| Habitat | 73.35 | 396 | 79 | <0.001 *** |
| Time | 12.14 | 396 | 13 | <0.01 ** |
| Minrh | 7.76 | 397 | 8 | <0.05 * |
| Model Term | Deviance | df | % Deviance ([Term Deviance/Total Deviance] × 100) | p |
|---|---|---|---|---|
| Time | 67.279 | 501 | 54 | <0.001 *** |
| Habitat | 38.563 | 503 | 31 | <0.001 *** |
| Footpath | 14.153 | 501 | 11 | <0.001 *** |
| Minrh | 3.569 | 500 | 3 | 0.059 |
| Model | LR Difference | df | ꭕ2 | p |
|---|---|---|---|---|
| Time | −756.15 vs. 786.01 | 4 | 59.717 | <0.001 *** |
| Time + Habitat | −739.25 vs. −756.15 | 6 | 33.791 | <0.001 *** |
| Time + Habitat + Footpath | −735.55 vs. −739.25 | 2 | 7.402 | <0.05 * |
| Time + Habitat + Footpath + Minrh | −730.68 vs. −739.25 | 2 | 9.750 | <0.01 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tolhurst, B.A.; Overall, A.D.J.; King, P.J.; Morgan, E.R.; Baker, R.J. Co-Occurrence of Domestic Dogs and Gastropod Molluscs in Public Dog-Walking Spaces and Implications for Infection with Angiostrongylus vasorum: A Preliminary Study. Animals 2021, 11, 2577. https://doi.org/10.3390/ani11092577
Tolhurst BA, Overall ADJ, King PJ, Morgan ER, Baker RJ. Co-Occurrence of Domestic Dogs and Gastropod Molluscs in Public Dog-Walking Spaces and Implications for Infection with Angiostrongylus vasorum: A Preliminary Study. Animals. 2021; 11(9):2577. https://doi.org/10.3390/ani11092577
Chicago/Turabian StyleTolhurst, Bryony A., Andrew D. J. Overall, Peter J. King, Eric R. Morgan, and Rowenna J. Baker. 2021. "Co-Occurrence of Domestic Dogs and Gastropod Molluscs in Public Dog-Walking Spaces and Implications for Infection with Angiostrongylus vasorum: A Preliminary Study" Animals 11, no. 9: 2577. https://doi.org/10.3390/ani11092577
APA StyleTolhurst, B. A., Overall, A. D. J., King, P. J., Morgan, E. R., & Baker, R. J. (2021). Co-Occurrence of Domestic Dogs and Gastropod Molluscs in Public Dog-Walking Spaces and Implications for Infection with Angiostrongylus vasorum: A Preliminary Study. Animals, 11(9), 2577. https://doi.org/10.3390/ani11092577







