Effect of Infant Presence on Social Networks of Sterilized and Intact Wild Female Balinese Macaques (Macaca fascicularis)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Study Groups
2.2. Data Collection
2.2.1. Determination of the Nursing Condition
2.2.2. Behavioral Data
2.2.3. Social Network Data
2.3. Social Network Analysis
Statistical Analysis
3. Results
3.1. Effect of Nursing Condition on Proximity and Grooming Networks
3.2. Effect of Sterilization on Grooming and Proximity Networks
4. Discussion
4.1. Females with Young Unweaned Infants Were Less Central than Expected
4.2. Sterilization Did neither Positively nor Neatively Impact the Female Social Networks in a Short Term
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Population | Ubud | |
| Number of social groups | 8 | |
| Population size | 1099 | |
| Home range size (ha) | 20.5 | |
| Density (ind/ha) | 54 | |
| Study groups | Michelin | Utara |
| Group size | 136 | 33 |
| Adult males | 16 | 7 |
| Adult females | 45 | 9 |
| Subadult males | 12 | 3 |
| Subadult females | 5 | 2 |
| Juveniles | 43 | 8 |
| Old infant | 8 | 3 |
| Young infant | 7 | 1 |
| Total females studied | 41 | 7 |
| Sterilized females | 13 | 1 |
| Intact females | 28 | 6 |
Appendix B
| Nursing Condition | ||||||||
|---|---|---|---|---|---|---|---|---|
| ID | Group | Age | Rank | Status | Time | Nursing YI | Nursing OI | Non-Nursing |
| Monroe | Utara | AF | 1.43 | Intact | - | No | No | Yes |
| Gumal | Utara | AF | 2.86 | Intact | - | Yes | Yes | Yes |
| H16 Tracy | Utara | AF | 4.29 | Sterilized | 4 | No | No | Yes |
| Littlefinger | Utara | AF | 5.71 | Intact | - | Yes | No | Yes |
| Cinderella | Utara | AF | 7.14 | Intact | - | Yes | Yes | Yes |
| Bigmama | Utara | AF | 8.57 | Intact | - | Yes | Yes | No |
| Amy | Utara | AF | 10 | Intact | - | Yes | No | Yes |
| H4 Nipples | Michelin | AF | 0.24 | Sterilized | 10 | No | No | Yes |
| Snick | Michelin | AF | 0.49 | Intact | - | Yes | No | Yes |
| Cleft | Michelin | AF | 0.73 | Intact | - | No | Yes | Yes |
| Asym | Michelin | AF | 0.98 | Intact | - | No | No | Yes |
| H11 Bosom | Michelin | AF | 1.22 | Sterilized | 10 | No | No | Yes |
| Young | Michelin | AF | 1.46 | Intact | - | Yes | Yes | No |
| Putih | Michelin | AF | 1.71 | Intact | - | Yes | Yes | No |
| Bat | Michelin | AF | 1.95 | Intact | - | Yes | No | Yes |
| You | Michelin | AF | 2.2 | Intact | - | Yes | Yes | No |
| BlackTits | Michelin | AF | 2.44 | Intact | - | Yes | No | Yes |
| Crunched | Michelin | AF | 2.68 | Intact | - | Yes | No | Yes |
| Spotty | Michelin | AF | 2.93 | Intact | - | No | No | Yes |
| Telinga | Michelin | AF | 3.17 | Intact | - | Yes | Yes | No |
| H7 Lauren | Michelin | AF | 3.41 | Sterilized | 10 | No | No | Yes |
| H8 Wink | Michelin | AF | 3.66 | Intact | - | Yes | Yes | No |
| Trivial | Michelin | AF | 3.9 | Intact | - | Yes | Yes | No |
| Excroissance | Michelin | AF | 4.15 | Intact | - | Yes | Yes | No |
| H13 Torticoli | Michelin | AF | 4.39 | Sterilized | 4 | No | No | Yes |
| Slash | Michelin | AF | 4.63 | Intact | - | Yes | No | Yes |
| H18 | Michelin | SF | 4.88 | Sterilized | 4 | No | No | Yes |
| Tip | Michelin | AF | 5.12 | Intact | - | No | No | Yes |
| White | Michelin | AF | 5.37 | Intact | - | Yes | Yes | Yes |
| Tugas | Michelin | AF | 5.61 | Intact | - | Yes | Yes | No |
| Tuft | Michelin | AF | 5.85 | Intact | - | Yes | Yes | Yes |
| Trident | Michelin | AF | 6.1 | Intact | - | Yes | No | Yes |
| H5 Pincang | Michelin | AF | 6.34 | Sterilized | 17 | No | No | Yes |
| H1 | Michelin | AF | 6.59 | Sterilized | 29 | No | No | Yes |
| H6 Terkulai | Michelin | AF | 6.83 | Sterilized | 10 | No | No | Yes |
| Arcade | Michelin | AF | 7.07 | Intact | - | Yes | Yes | Yes |
| H10 Mimi | Michelin | AF | 7.32 | Sterilized | 10 | Yes | Yes | No |
| H9 Tumeur | Michelin | AF | 7.56 | Sterilized | 10 | No | No | Yes |
| Snag | Michelin | AF | 7.8 | Intact | - | No | No | Yes |
| H17 | Michelin | SF | 8.05 | Sterilized | 4 | No | No | Yes |
| H14 Pink | Michelin | AF | 8.29 | Sterilized | 4 | No | Yes | Yes |
| Helix | Michelin | AF | 8.54 | Intact | - | Yes | No | Yes |
| Gutter | Michelin | AF | 8.78 | Intact | - | Yes | Yes | Yes |
| Crack | Michelin | AF | 9.02 | Intact | - | Yes | No | Yes |
| Bang | Michelin | AF | 9.27 | Intact | - | Yes | Yes | No |
| Hole | Michelin | AF | 9.51 | Intact | - | Yes | Yes | No |
| Dua | Michelin | AF | 9.76 | Intact | - | Yes | Yes | No |
| H15 | Michelin | SF | 10 | Sterilized | 4 | No | No | Yes |
References
- Godde, S.; Côté, S.D.; Réale, D. Female mountain goats, Oreamnos americanus, associate according to kinship and reproductive status. Anim. Behav. 2015, 108, 101–107. [Google Scholar] [CrossRef] [Green Version]
- Maestripieri, D. Influence of infants on female social relationships in monkeys. Folia Primatol. 1994, 63, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Seyfarth, R.M. A model of social grooming among adult female monkeys. J. Theor. Biol. 1977, 65, 671–698. [Google Scholar] [CrossRef]
- Mann, J.; Smuts, B.B. Natal attraction: Allomaternal care and mother–infant separations in wild bottlenose dolphins. Anim. Behav. 1998, 55, 1097–1113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parsons, C.E.; Young, K.S.; Kumari, N.; Stein, A.; Kringelbach, M.L. The motivational salience of infant faces is similar for men and women. PLoS ONE 2011, 6, e20632. [Google Scholar] [CrossRef]
- Silk, J.B.; Rendall, D.; Cheney, D.L.; Seyfarth, R.M. Natal attraction in adult female baboons (Papio cynocephalus ursinus) in the Moremi reserve, Botswana. Ethology 2003, 109, 627–644. [Google Scholar] [CrossRef] [Green Version]
- Dunayer, E.S.; Berman, C.M. Infant Handling Among Primates. Int. J. Comp. Psychol. 2018, 31, 1–31. [Google Scholar] [CrossRef]
- Riedman, M.L. The evolution of alloparental care and adoption in mammals and birds. Q. Rev. Biol. 1982, 57, 405–435. [Google Scholar] [CrossRef]
- Silk, J.B. Social Components of Fitness in Primate Groups. Science 2007, 317, 1347–1352. [Google Scholar] [CrossRef] [Green Version]
- Armitage, K.B. Social Dynamics of Mammals: Reproductive Success, Kinship and Individual Fitness. Tree 1987, 2, 279–284. [Google Scholar] [CrossRef]
- Gumert, M.D. Grooming and infant handling interchange in Macaca fascicularis: The relationship between infant supply and grooming payment. Int. J. Primatol. 2007, 28, 1059–1074. [Google Scholar] [CrossRef]
- Noë, R.; Hammerstein, P. Biological markets: Supply and demand determine the effect of partner choice in cooperation, mutualism and mating. Behav. Ecol. Sociobiol. 1994, 35, 1–11. [Google Scholar] [CrossRef]
- Maestripieri, D. Social structure, infant handling, and mothering styles in group-living old world monkeys. Int. J. Primatol. 1994, 15, 531–553. [Google Scholar] [CrossRef]
- Thierry, B. Unity in diversity: Lessons from macaque societies. Evol. Anthropol. Issues News Rev. 2007, 16, 224–238. [Google Scholar] [CrossRef]
- Hiraiwa, M. Maternal and alloparental care in a troop of free-ranging Japanese monkeys. Primates 1981, 22, 309–329. [Google Scholar] [CrossRef]
- Small, M.F. A comparison of mother and nonmother behaviors during birth season in two species of captive macaques. Folia Primatol. 1982, 38, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Dunayer, E.S.; Berman, C.M. Infant handling enhances social bonds in free-ranging rhesus macaques (Macaca mulatta). Behaviour 2017, 154, 875–907. [Google Scholar] [CrossRef]
- Berman, C.M. The ontogeny of social relationships with group companions among free-ranging infant rhesus monkeys II. Differentiation and attractiveness. Anim. Behav. 1982, 30, 163–170. [Google Scholar] [CrossRef]
- De Lima, V.C.C.; Ferreira, R.G. Social network changes during the development of immature capuchin monkeys (Sapajus spp.). Primates 2021, 110. [Google Scholar] [CrossRef]
- Liao, Z.; Sosa, S.; Wu, C.; Zhang, P. The influence of age on wild rhesus macaques’ affiliative social interactions. Am. J. Primatol. 2018, 80, e22733. [Google Scholar] [CrossRef] [PubMed]
- Fedurek, P.; Lehmann, J. The effect of excluding juveniles on apparent adult olive baboons (Papio anubis) social networks. PLoS ONE 2017, 12, e0173146. [Google Scholar] [CrossRef]
- Sueur, C.; Jacobs, A.; Amblard, F.; Petit, O.; King, A.J. How can social network analysis improve the study of primate behavior? Am. J. Primatol. 2011, 73, 703–719. [Google Scholar] [CrossRef] [PubMed]
- Sih, A.; Hanser, S.F.; McHugh, K.A. Social network theory: New insights and issues for behavioral ecologists. Behav. Ecol. Sociobiol. 2009, 63, 975–988. [Google Scholar] [CrossRef] [Green Version]
- Krause, J.; Croft, D.P.; James, R. Social network theory in the behavioural sciences: Potential applications. Behav. Ecol. Sociobiol. 2007, 62, 15–27. [Google Scholar] [CrossRef]
- Farine, D.R. When to choose dynamic vs. static social network analysis. J. Anim. Ecol. 2018, 87, 128–138. [Google Scholar] [CrossRef] [Green Version]
- Farine, D.R.; Whitehead, H. Constructing, conducting and interpreting animal social network analysis. J. Anim. Ecol. 2015, 84, 1144–1163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farine, D.R. Measuring phenotypic assortment in animal social networks: Weighted associations are more robust than binary edges. Anim. Behav. 2014, 89, 141–153. [Google Scholar] [CrossRef]
- Wasserman, S.; Faust, K. Social Network Analysis: Methods and Applications; Cambridge University Press: Cambridge, UK, 1994. [Google Scholar]
- Sosa, S.; Sueur, C.; Puga-Gonzalez, I. Network measures in animal social network analysis: Their strengths, limits, interpretations and uses. Methods Ecol. Evol. 2020, 12, 10–21. [Google Scholar] [CrossRef]
- Hanneman, R.A.; Riddle, M. Introduction to Social Network Methods; University of California: Riverside, CA, USA, 2005. [Google Scholar]
- Brakes, P. Sociality and Wild Animal Welfare: Future Directions. Front. Vet. Sci. 2019, 6, 1–7. [Google Scholar] [CrossRef]
- Botreau, R.; Veissier, I.; Butterworth, A.; Bracke, M.B.M.; Keeling, L.J. Definition of criteria for overall assessment of animal welfare. Anim. Welf. 2007, 16, 225–228. [Google Scholar]
- Kirkwood, J.K. Wild Animal Welfare. Anim. Welf. 2013, 22, 147–148. [Google Scholar] [CrossRef]
- Wrangham, R.W. An ecological model of female-bonded primate groups. Behaviour 1980, 75, 262–300. [Google Scholar] [CrossRef]
- Ohl, F.; Putman, R.J. Animal Welfare at the Group Level: More Than the Sum of Individual Welfare? Acta Biotheor. 2014, 62, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.J.; Van De Kerk, M.; Pine, W.E.; Hostetler, M.E.; Heard, D.J.; Johnson, S.A. Population estimate and management options for introduced rhesus macaques. J. Wildl. Manag. 2019, 83, 295–303. [Google Scholar] [CrossRef]
- Reddy, A.R.M.; Chander, J. Human-monkey conflict in India: Some available solutions for conflict mitigation with special reference to Himachal Pradesh. Indian For. 2016, 142, 941–949. [Google Scholar]
- Buyukmihci, N.C. Castration for Population Control of Macaques in a Sanctuary Setting; University of California: Davis, CA, USA, 2017; pp. 1–4. [Google Scholar]
- Ramsey, D. Effects of fertility control on behavior and disease transmission in brushtail possums. J. Wildl. Manag. 2007, 71, 109–116. [Google Scholar] [CrossRef]
- Biquand, S.; Boug, A.; Biquand-Guyot, V.; Gauthier, J.-P. Management of commensal baboons in Saudi Arabia. Rev. d’Ecologie Terre Vie 1994, 49, 213–222. [Google Scholar]
- Cheng, W.W. A review of the management measures of feral macaques in Hong Kong. Master’s Thesis, The Chinese University of Hong Kong, Hong Kong, China, 2014. [Google Scholar]
- Martelli, P.; Krishnasamy, K.; Kwan, A.; Wong, A. Permanent contraception by laparoscopic tubectomy with ovarian conservation in Hong Kong macaques. Jpn. J. Vet. Res. 2020, 68, 209–215. [Google Scholar] [CrossRef]
- Deleuze, S.; Brotcorne, F.; Polet, R.; Soma, G.; Rigaux, G.; Giraud, G.; Cloutier, F.; Poncin, P.; Wandia, I.N.; Huynen, M.-C. Modified endoscopic tubectomy of pregnant and non-pregnant female Balinese macaques (Macaca fascicularis) with postoperative monitoring. Front. Vet. Sci. 2021. [Google Scholar] [CrossRef]
- Kalbitzer, U.; Chapman, C.A. Primate responses to changing environments in the Anthropocene. In Primate Life Histories, Sex Roles, and Adaptability. Developments in Primatology: Progress and Prospects; Kalbitzer, U., Jack, K., Eds.; Springer: Cham, Switzerland, 2018; pp. 283–310. [Google Scholar]
- Yu, P.-H.; Weng, C.-C.; Kuo, H.-C.; Chi, C.-H. Evaluation of endoscopic salpingectomy for sterilization of female Formosan macaques (Macaca cyclopis). Am. J. Primatol. 2015, 77, 359–367. [Google Scholar] [CrossRef]
- Phoenix, C.H. Sexual behavior in rhesus monkeys after vasectomy. Science 1973, 179, 493–494. [Google Scholar] [CrossRef]
- Zhao, J.R.; Wing, R.; Hulka, J.F. Ovarian function in monkeys after bilateral salpingectomy. Int. J. Fertil. 1984, 29, 118–121. [Google Scholar]
- Dede, F.S.; Dilbaz, B.; Akyuz, O.; Caliskan, E.; Kurtaran, V.; Dilbaz, S. Changes in menstrual pattern and ovarian function following bipolar electrocauterization of the fallopian tubes for voluntary surgical contraception. Contraception 2006, 73, 88–91. [Google Scholar] [CrossRef]
- Ross, C. Life history patterns and ecology of macaque species. Primates 1992, 33, 207–215. [Google Scholar] [CrossRef]
- Whitten, T.; Soeriaatmadja, R.E.; Afiff, S.A. The Ecology of Java and Bali (Vol. 2); Oxford University Press: Singapore, 1996. [Google Scholar]
- Fuentes, A. Naturalcultural encounters in Bali: Monkeys, temples, tourists, and ethnoprimatology. Cult. Anthropol. 2010, 25, 600–624. [Google Scholar] [CrossRef]
- Brotcorne, F.; Fuentes, A.; Wandia, I.N.; Beudels-Jamar, R.C.; Huynen, M.-C. Changes in activity patterns and intergroup relationships after a significant mortality event in commensal long-tailed macaques (Macaca fascicularis) in Bali, Indonesia. Int. J. Primatol. 2015, 36, 548–566. [Google Scholar] [CrossRef]
- Caro, T.M.; Roper, R.; Young, M.; Dank, G.R. Inter-observer reliability. Behaviour 1979, 69, 303–315. [Google Scholar] [CrossRef]
- Brotcorne, F. Behavioral Ecology and Commensal Long-Tailed Macaque (Macaca fascicularis) Populations in Bali, Indonesia: Impact of Anthropic Factors. Ph.D. Thesis, University of Liège, Liège, Belgium, 2014. [Google Scholar]
- Jewett, D.A.; Dukelow, W.R. Cyclicity and gestation length of Macaca fascicularis. Primates 1972, 13, 327–332. [Google Scholar] [CrossRef]
- Van Noordwijk, M.A. From Maternal Investment to Lifetime Maternal Care. In The Evolution of Primate Societies; Mitani, J.C., Call, J., Kappeler, P.M., Palombit, R.A., Silk, J.B., Eds.; University of Chicago Press: Chicago, IL, USA, 2012; pp. 321–342. [Google Scholar]
- Poirier, F.E.; Smith, E.O. The crab-eating macaques (Macaca fascicularis) of Angaur Island, Palau, Micronesia. Folia Primatol. 1974, 22, 258–306. [Google Scholar] [CrossRef] [PubMed]
- Altmann, J. Observational study of behavior: Sampling methods. Behaviour 1974, 49, 227–266. [Google Scholar] [CrossRef] [Green Version]
- Newton-Fisher, N.E. Animal Behaviour Pro: 1.4.5. Apple iTunes Download. 2012. [Google Scholar]
- Sosa, S. The influence of gender, age, matriline and hierarchical rank on individual social position, role and interactional patterns in Macaca sylvanus at ‘La Forêt des Singes’: A multilevel social network approach. Front. Psychol. 2016, 7, 529. [Google Scholar] [CrossRef]
- Mishra, P.S.; Pal, A.; Velankar, A.D.; Kumara, H.N.; Singh, M.; Cooper, M. Does rank rule? Rank-related grooming patterns in Nicobar long-tailed macaques Macaca fascicularis umbrosus. Primates 2020, 61, 443–453. [Google Scholar] [CrossRef]
- Veenema, H.; Spruijt, B.; Gispen, W.; van Hooff, J.A.R.A.M. Aging, dominance history, and social behavior in Java-monkeys (Macaca fascicularis). Neurobiol. Aging 1997, 18, 509–515. [Google Scholar] [CrossRef]
- David, H.A. Ranking from unbalanced paired-comparison data. Biometrika 1987, 74, 432–436. [Google Scholar] [CrossRef]
- de Vries, H. Finding a dominance order most consistent with a linear hierarchy: A new procedure and review. Anim. Behav. 1998, 55, 827–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Vries, H.; Stevens, J.M.G.; Vervaecke, H. Measuring and testing the steepness of dominance hierarchies. Anim. Behav. 2006, 71, 585–592. [Google Scholar] [CrossRef] [Green Version]
- Silk, M.J.; Jackson, A.L.; Croft, D.P.; Colhoun, K.; Bearhop, S. The consequences of unidentifiable individuals for the analysis of an animal social network. Anim. Behav. 2015, 104, 1–11. [Google Scholar] [CrossRef]
- Newman, M.E.J. Mixing patterns in networks. Phys. Rev. E 2003, 67, 026126. [Google Scholar] [CrossRef] [Green Version]
- Barthélemy, M.; Barrat, A.; Pastor-Satorras, R.; Vespignani, A. Characterization and modeling of weighted networks. Phys. A Stat. Mech. Appl. 2005, 346, 34–43. [Google Scholar] [CrossRef] [Green Version]
- Qi, X.; Duval, R.D.; Christensen, K.; Fuller, E.; Spahiu, A.; Wu, Q.; Wu, Y.; Tang, W.; Zhang, C. Terrorist networks, network energy and node removal: A new measure of centrality based on Laplacian energy. Soc. Netw. 2013, 2, 19–31. [Google Scholar] [CrossRef] [Green Version]
- Sosa, S.; Puga-Gonzalez, I.; Hu, F.; Pansanel, J.; Xie, X.; Sueur, C. A multilevel statistical toolkit to study animal social networks: The Animal Network Toolkit Software (ANTs) R package. Sci. Rep. 2020, 10, 12507. [Google Scholar] [CrossRef]
- Borgatti, S.; Everett, M.; Freeman, L. UCINET 6 For Windows: Software for Social Network Analysis; Analytic Technologies: Harvard, MA, USA, 2002. [Google Scholar]
- R Development Core Team. R: A language and Environment for Statistical Computing. 2013. Available online: https://www.r-project.org/ (accessed on 29 August 2021).
- Krause, J.; Lusseau, D.; James, R. Animal social networks: An introduction. Behav. Ecol. Sociobiol. 2009, 63, 967–973. [Google Scholar] [CrossRef]
- Whitehead, H. Analyzing Animal Societies: Quantitative Methods for Vertebrate Social Analysis; University of Chicago Press: Chicago, IL, USA, 2008. [Google Scholar]
- Croft, D.P.; Madden, J.R.; Franks, D.W.; James, R. Hypothesis testing in animal social networks. Trends Ecol. Evol. 2011, 26, 502–507. [Google Scholar] [CrossRef]
- Puga-Gonzalez, I.; Sueur, C.; Sosa, S. Null models for animal social network analysis and data collected via focal sampling: Pre-network or node network permutation? Methods Ecol. Evol. 2021, 12, 22–32. [Google Scholar] [CrossRef]
- Lenth, R.; Lenth, M.R. Package R ‘lsmeans’. Am. Stat. 2018, 34, 216–221. [Google Scholar]
- Overduin-de Vries, A.M.; Olesen, C.U.; de Vries, H.; Spruijt, B.M.; Sterck, E.H.M. Sneak copulations in long-tailed macaques (Macaca fascicularis): No evidence for tactical deception. Behav. Ecol. Sociobiol. 2013, 67, 101–111. [Google Scholar] [CrossRef]
- van Noordwijk, M.A.; van Schaik, C.P. Competition among female long-tailed macaques, Macaca fascicularis. Anim. Behav. 1987, 35, 577–589. [Google Scholar] [CrossRef]
- Asquith, P.J. Provisioning and the study of free-ranging primates: History, effects, and prospects. Am. J. Phys. Anthropol. 1989, 32, 129–158. [Google Scholar] [CrossRef]
- McFarland, R.; Murphy, D.; Lusseau, D.; Henzi, S.P.; Parker, J.L.; Pollet, T.V.; Barrett, L. The ‘strength of weak ties’ among female baboons: Fitness-related benefits of social bonds. Anim. Behav. 2017, 126, 101–106. [Google Scholar] [CrossRef] [Green Version]
- Silk, J.B.; Beehner, J.C.; Bergman, T.J.; Crockford, C.; Engh, A.L.; Moscovice, L.R.; Wittig, R.M.; Seyfarth, R.M.; Cheney, D.L. The benefits of social capital: Close social bonds among female baboons enhance offspring survival. Proc. R. Soc. B Biol. Sci. 2009, 276, 3099–3104. [Google Scholar] [CrossRef] [Green Version]
- Berman, C.M.; Rasmussen, K.L.R.; Suomi, S.J. Group size, infant development and social networks in free-ranging rhesus monkeys. Anim. Behav. 1997, 53, 405–421. [Google Scholar] [CrossRef]
- Brent, L.J.N.; MacLarnon, A.; Platt, M.L.; Semple, S. Seasonal changes in the structure of rhesus macaque social networks. Behav. Ecol. Sociobiol. 2013, 67, 349–359. [Google Scholar] [CrossRef] [Green Version]
- Chapais, B.; Berman, C.M. Kinship and Behavior in Primates; Oxford University Press: New York, NY, USA, 2004. [Google Scholar]
- Maestripieri, D. Intraspecific variability in parenting styles of rhesus macaques (Macaca mulatta): The role of the social environment. Ethology 2001, 107, 237–248. [Google Scholar] [CrossRef]
- Giraud, G. Relation between social tension and demographic density of commensal long-tailed macaques (Macaca fascicularis) in Bali (Indonesia). Master’s Thesis, Université de Liège, Liège, Belgium, 2015. [Google Scholar]
- Brotcorne, F.; Wandia, I.N.; Rompis, A.L.; Soma, I.G.; Suartha, I.N.; Huynen, M.C. Box 6.1 Recent demographic and behavioral data of Macaca fascicularis at Padangtegal, Bali, Indonesia. In Monkeys on the Edge Ecology and Management of Long-Tailed Macaques and Their Interface with Humans; Gumert, M.D., Fuentes, A., Jones-Engel, L., Eds.; Cambridge University Press: Cambridge, UK, 2011; p. 180. [Google Scholar]
- Chism, J. Allocare patterns among Cercopithecines. Folia Primatol. 2000, 71, 55–66. [Google Scholar] [CrossRef]
- Quenette, P.-Y. Functions of vigilance behaviour in mammals: A review. Acta Oecologica 1990, 11, 801–818. [Google Scholar]
- Le Bohec, C.; Gauthier-Clerc, M.; Le Maho, Y. The adaptive significance of crèches in the king penguin. Anim. Behav. 2005, 70, 527–538. [Google Scholar] [CrossRef]
- Giraud, G.; Tibesar, F.; Huynen, M.-C.; Broens, D.; Delooz, S.; Cloutier, F.; Wandia, I.N.; Poncin, P.; Brotcorne, F. Preliminary results on behavioural variation following tubectomy sterilisations in free-ranging female long-tailed macaques (Macaca fascicularis) in Bali, Indonesia. In 8th European Federation for Primatology Meeting. Folia Primatol. 2020, 91, 341. [Google Scholar] [CrossRef] [Green Version]
- Huchard, E.; Cowlishaw, G. Female–female aggression around mating: An extra cost of sociality in a multimale primate society. Behav. Ecol. 2011, 22, 1003–1011. [Google Scholar] [CrossRef]
- Baniel, A.; Cowlishaw, G.; Huchard, E. Context dependence of female reproductive competition in wild chacma baboons. Anim. Behav. 2018, 139, 37–49. [Google Scholar] [CrossRef]
- Ehardt, C.L. Birth-season interactions of adult female Japanese Macaques (Macaca fuscata) without newborn infants. Int. J. Primatol. 1987, 8, 245–259. [Google Scholar] [CrossRef]
- Engelhardt, A.; Hodges, J.K.; Heistermann, M. Post-conception mating in wild long-tailed macaques (Macaca fascicularis): Characterization, endocrine correlates and functional significance. Horm. Behav. 2007, 51, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Snijders, L.; Blumstein, D.T.; Stanley, C.R.; Franks, D.W. Animal Social Network Theory Can Help Wildlife Conservation. Trends Ecol. Evol. 2017, 32, 567–577. [Google Scholar] [CrossRef] [PubMed]
- McCowan, B.; Anderson, K.; Heagarty, A.; Cameron, A. Utility of social network analysis for primate behavioral management and well-being. Appl. Anim. Behav. Sci. 2008, 109, 396–405. [Google Scholar] [CrossRef]
- Beisner, B.A.; McCowan, B. Social networks and animal welfare. In Animal Social Networks; Krause, J., James, R., Franks, D., Croft, D., Eds.; Oxford University Press: Oxford, UK, 2014; pp. 111–122. [Google Scholar]
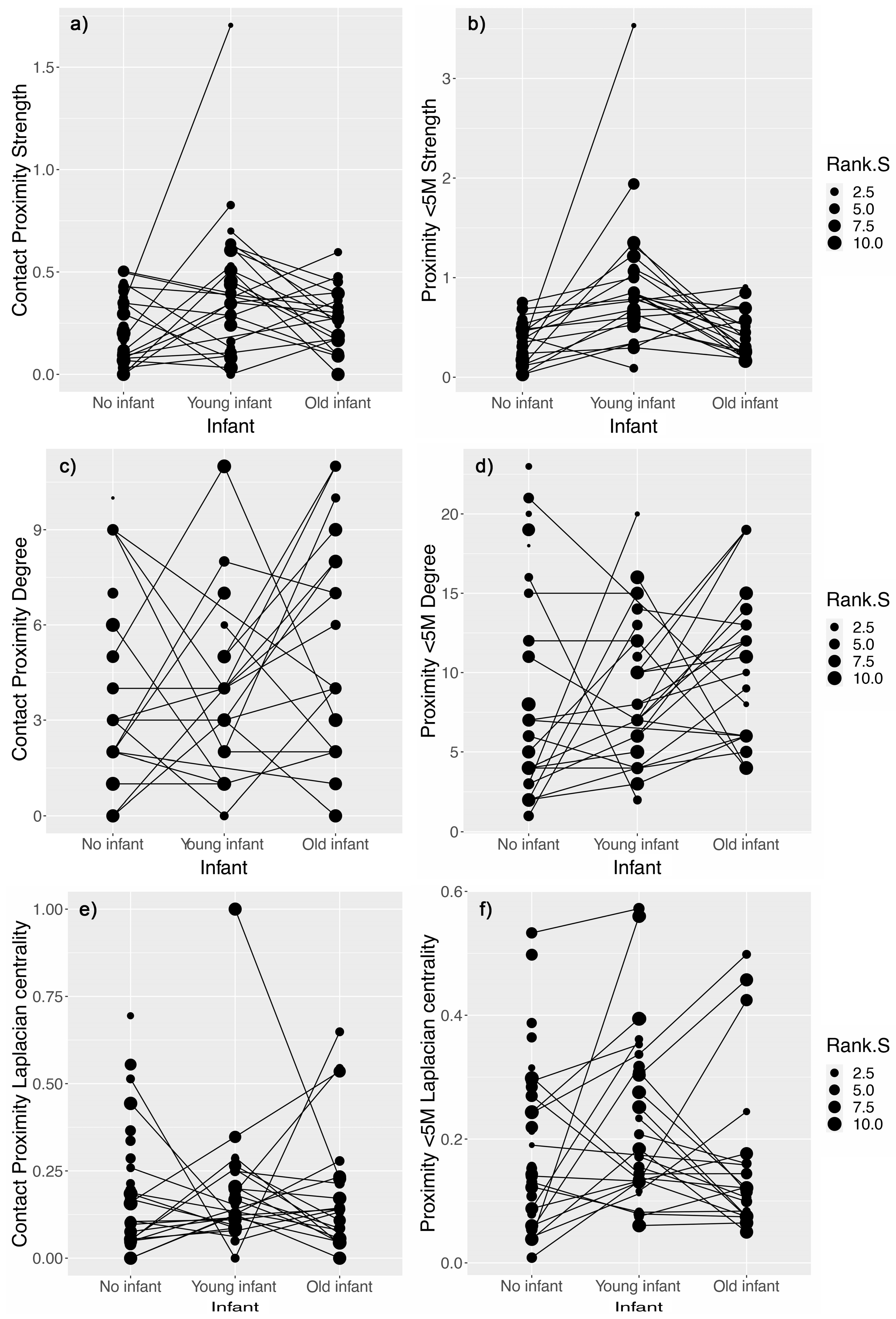
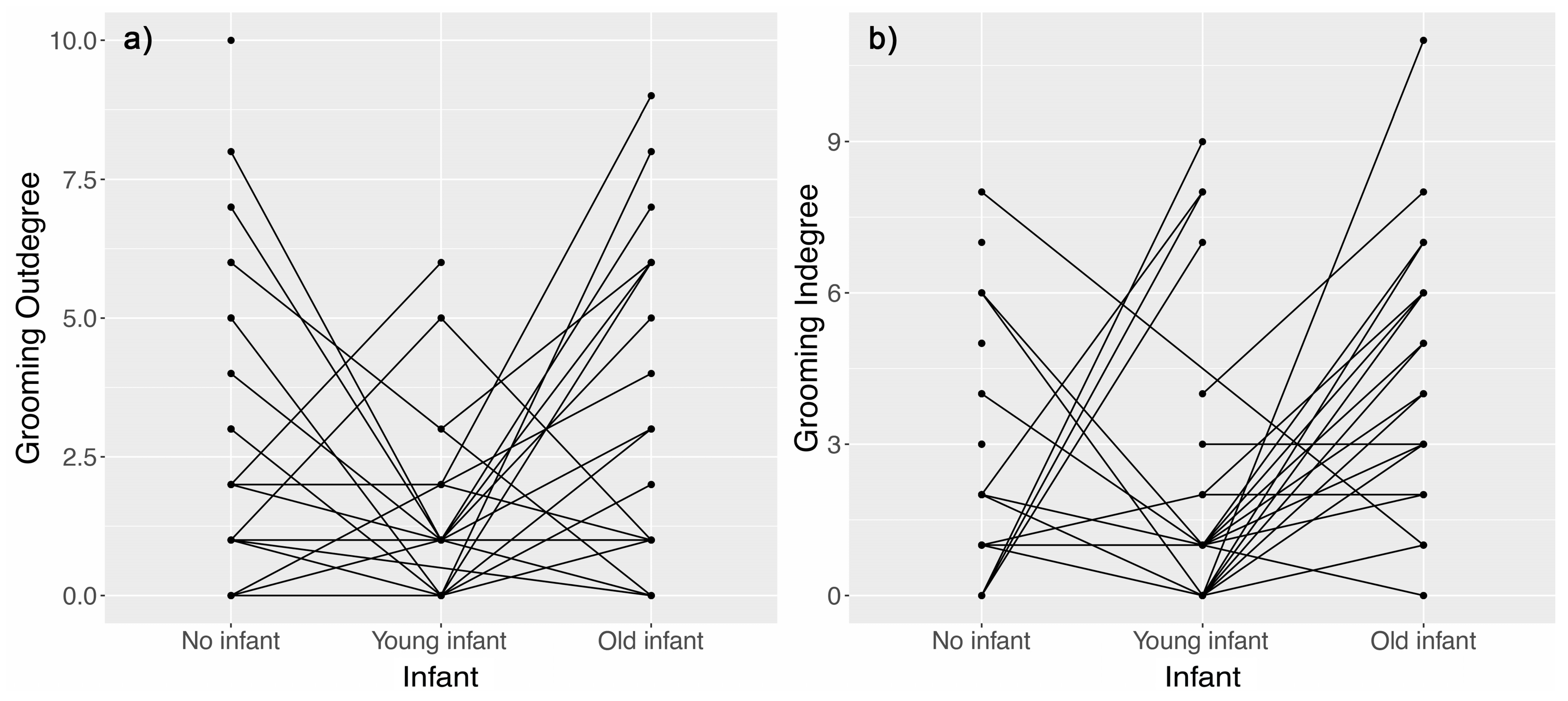
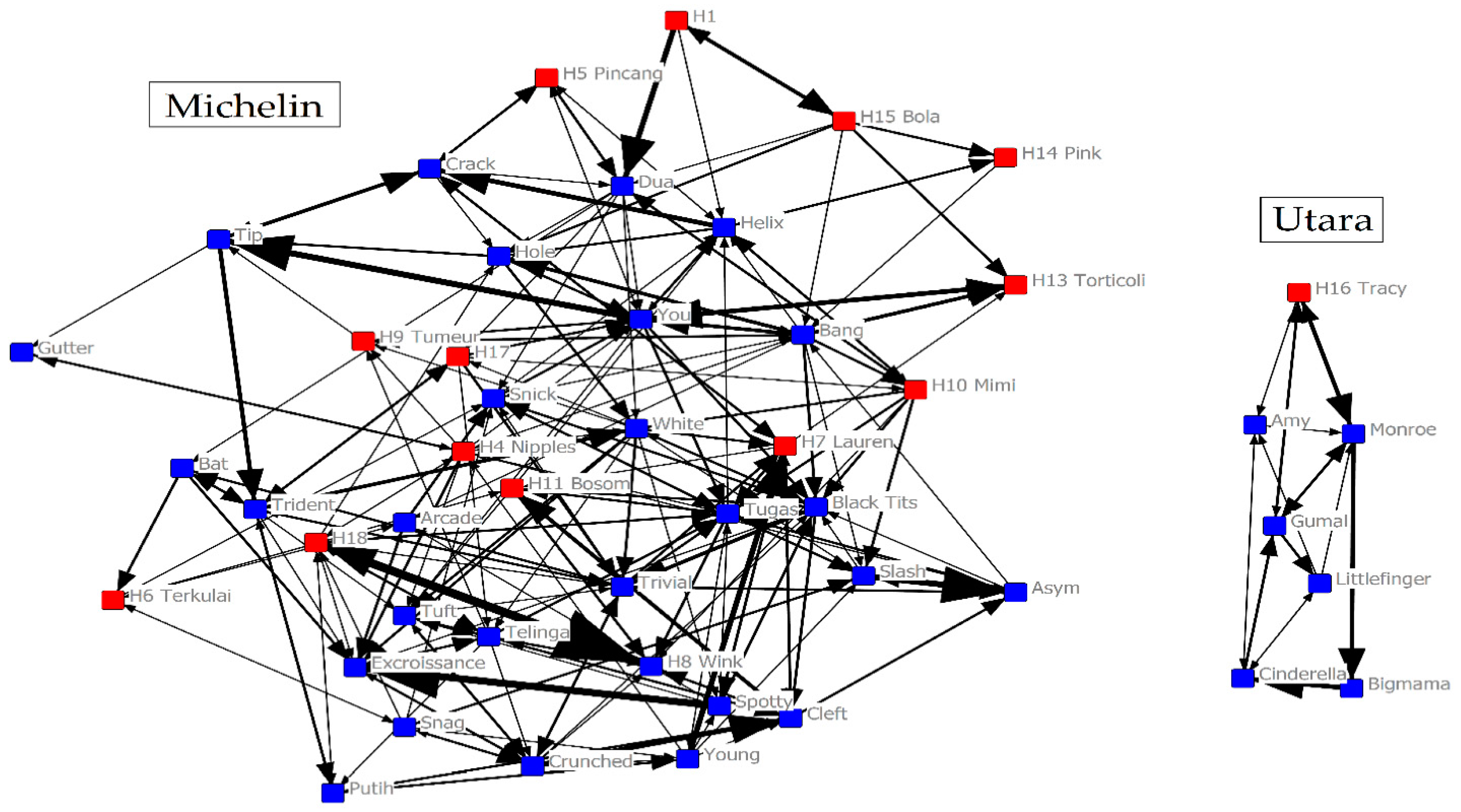
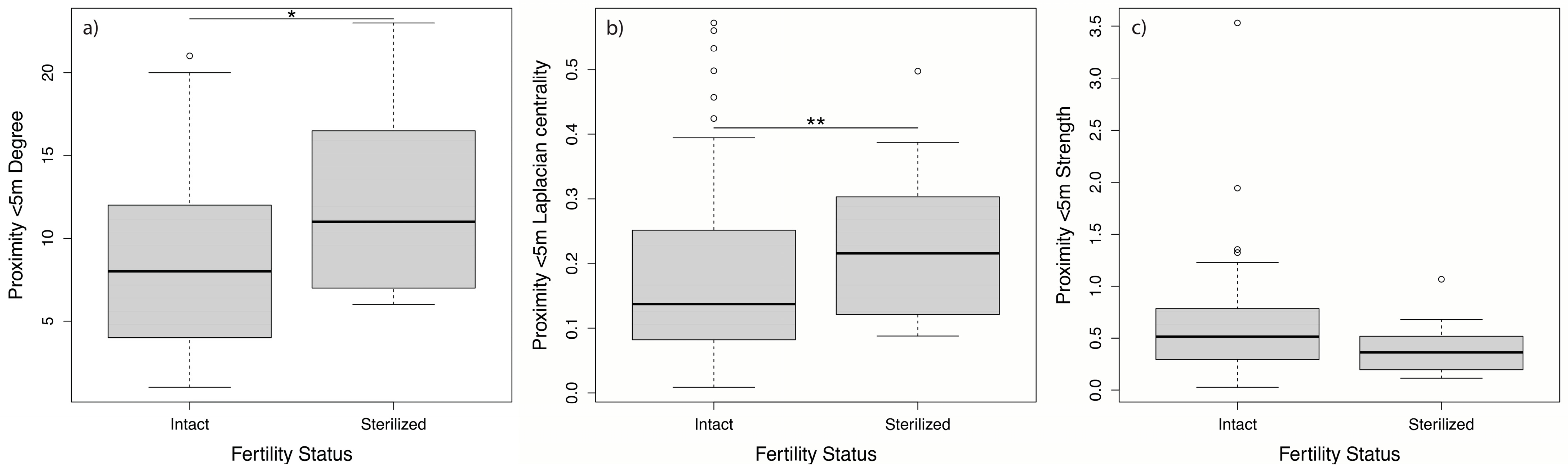
| Response Variable | Proximity | Fixed Effects | Estimate ± SE | z/t Values | p-Values |
|---|---|---|---|---|---|
| Degree | Contact | Intercept | −0.41 ± 0.33 | −1.23 | 0.11 |
| Nursing condition | |||||
| YI-NN | 0.06 ± 0.15 | 0.42 | 0.73 | ||
| YI-OI | 0.39 ± 0.14 | 2.75 | <0.05 | ||
| OI-NN | −0.33 ± 0.15 | −2.19 | 0.11 | ||
| Status a | 0.08 ± 0.17 | 0.47 | 0.69 | ||
| Rank | −0.07 ± 0.02 | −3.04 | <0.01 | ||
| Group size | −0.03 ± 0.01 | −4.24 | 0.11 | ||
| LRT: deviance = 396.74, Df = 5, p < 0.0001 | |||||
| ≤5 m | Intercept | 1.62 ± 0.37 | 4.39 | <0.05 | |
| Nursing condition | |||||
| YI-NN | −0.07 ± 0.13 | −0.53 | 0.76 | ||
| YI-OI | 0.28 ± 0.12 | 2.42 | 0.23 | ||
| OI-NN | −0.35 ± 0.14 | −2.51 | 0.16 | ||
| Status a | 0.45 ± 0.18 | 2.52 | <0.05 | ||
| Rank | −0.09 ± 0.03 | −3.50 | <0.01 | ||
| Group size | −0.06 ± 0.01 | −6.94 | <0.05 | ||
| LRT: deviance = 518.75, Df = 5, p < 0.0001 | |||||
| Strength | Contact | Intercept | 0.35 ± 0.09 | 4.01 | <0.001 |
| Nursing condition | |||||
| YI-NN | −0.20 ± 0.06 | −3.66 | <0.001 | ||
| YI-OI | −0.15 ± 0.06 | −2.59 | <0.05 | ||
| OI-NN | 0.06 ± 0.06 | −0.91 | 0.07 | ||
| Status a | −0.03 ± 0.06 | −0.49 | 0.72 | ||
| Rank | −0.02 ± 0.01 | −3.10 | <0.01 | ||
| Group size | 0.006 ± 0.002 | 3.18 | 0.32 | ||
| LRT: deviance = −37.24, Df = 5, p < 0.0001 | |||||
| ≤5 m | Intercept | 1.15 ± 0.09 | 12.42 | <0.001 | |
| Nursing condition | |||||
| YI-NN | −0.37 ± 0.06 | −6.32 | <0.001 | ||
| YI-OI | −0.28 ± 0.06 | −4.53 | <0.001 | ||
| OI-NN | −0.09 ± NA | NA | 0.25 | ||
| Status a | 0.05 ± 0.07 | 0.71 | 0.58 | ||
| Rank | −0.02 ± 0.01 | −3.03 | <0.05 | ||
| Group size | −0.003 ± 0.002 | −1.59 | 0.16 | ||
| LRT: deviance = −28.22, Df = 5, p < 0.0001 | |||||
| Laplacian centrality | Contact | Intercept | 0.64 ± 0.08 | 8.53 | 0.15 |
| Nursing condition | |||||
| YI-NN | −0.051 ± 0.047 | −1.09 | 0.28 | ||
| YI-OI | −0.01 ± 0.05 | −0.14 | 0.90 | ||
| OI-NN | −0.04 ± 0.05 | −0.91 | 0.37 | ||
| Status a | 0.052 ± 0.054 | 0.98 | 0.29 | ||
| Rank | −0.009 ± 0.007 | −1.27 | 0.21 | ||
| Group size | −0.006 ± 0.002 | −3.75 | 0.19 | ||
| LRT: deviance = −62.93, Df = 5, p < 0.05 | |||||
| ≤5 m | Intercept | 0.43 ± 0.05 | 8.86 | 0.27 | |
| Nursing condition | |||||
| YI-NN | −0.06 ± 0.03 | −2.06 | 0.053 | ||
| YI-OI | −0.05 ± 0.03 | −1.79 | 0.09 | ||
| OI-NN | −0.01 ± 0.03 | −0.17 | 0.87 | ||
| Status a | 0.10 ± 0.03 | 2.82 | <0.01 | ||
| Rank | −0.0004 ± 0.004 | −0.10 | 0.93 | ||
| Group size | −0.006 ± 0.001 | −6.07 | 0.07 | ||
| LRT: deviance = −141.96, Df = 5, p < 0.0001 | |||||
| Response Variable | Fixed Effects | Estimate ± SE | z/t Values | p Values |
|---|---|---|---|---|
| In-degree | Intercept | −1.00 ± 0.40 | −2.51 | 0.81 |
| Nursing condition | ||||
| YI-NN | 0.39 ± 0.20 | 2.00 | 0.19 | |
| YI-OI | 0.89 ± 0.19 | 4.69 | <0.01 | |
| OI-NN | −0.49 ± 0.19 | −2.57 | 0.13 | |
| Status a | 0.05 ± 0.22 | 0.25 | 0.87 | |
| Rank | −0.07 ± 0.03 | −2.40 | 0.10 | |
| Group size | −0.04 ± 0.01 | −4.52 | 0.43 | |
| LRT: deviance = 377.22, Df = 5, p < 0.0001 | ||||
| Out-degree | Intercept | −1.47 ± 0.43 | −3.42 | 0.21 |
| Nursing condition | ||||
| YI-NN | 0.92 ± 0.21 | 4.40 | <0.01 | |
| YI-OI | 1.02 ± 0.21 | 4.92 | <0.001 | |
| OI-NN | −0.10 ± 0.19 | −0.50 | 0.79 | |
| Status a | 0.23 ± 0.22 | 1.04 | 0.52 | |
| Rank | −0.05 ± 0.03 | −1.42 | 0.33 | |
| Group size | −0.04 ± 0.01 | −4.23 | 0.36 | |
| LRT: deviance = 349.58, Df = 5, p < 0.0001 | ||||
| In-strength | Intercept | 0.012 ± 0.013 | 0.93 | 0.36 |
| Nursing condition | ||||
| YI-NN | 0.004 ± 0.01 | 0.39 | 0.71 | |
| YI-OI | 0.02 ± 0.01 | 1.91 | 0.07 | |
| OI-NN | −0.02 ± 0.01 | −1.49 | 0.14 | |
| Status a | −0.013 ± 0.011 | −1.15 | 0.26 | |
| Group size | 0.0005 ± 0.0003 | 1.84 | 0.51 | |
| LRT: deviance = −325.93, Df = 4, p = 0.06 | ||||
| Out-strength | Intercept | 0.03 ± 0.02 | 1.56 | 0.98 |
| Nursing condition | ||||
| YI-NN | 0.015 ± 0.010 | 1.48 | 0.16 | |
| YI-OI | 0.0101 ± 0.0102 | 1.00 | 0.34 | |
| OI-NN | 0.004 ± 0.01 | 0.40 | 0.70 | |
| Status a | −0.008 ± 0.01 | −0.66 | 0.50 | |
| Rank | −0.001 ± 0.002 | −0.82 | 0.40 | |
| Group size | 0.00032 ± 0.00035 | 0.94 | 0.92 | |
| LRT: deviance = −325.66, Df = 5, p = 0.49 | ||||
| Laplacian centrality | Intercept | 0.56 ± 0.15 | 3.82 | 0.20 |
| Nursing condition | ||||
| YI-NN | 0.19 ± 0.08 | 2.27 | <0.05 | |
| YI-OI | 0.10 ± 0.09 | 1.11 | 0.29 | |
| OI-NN | 0.091 ± 0.093 | 0.98 | 0.34 | |
| Status a | −0.04 ± 0.10 | −0.39 | 0.70 | |
| Rank | 0.002 ± 0.01 | 0.17 | 0.88 | |
| Group size | −0.002 ± 0.003 | −0.60 | 0.56 | |
| LRT: deviance = 29.76, Df = 5, p = 0.29 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giraud, G.; Sosa, S.; Hambuckers, A.; Deleuze, S.; Wandia, I.N.; Huynen, M.-C.; Poncin, P.; Brotcorne, F. Effect of Infant Presence on Social Networks of Sterilized and Intact Wild Female Balinese Macaques (Macaca fascicularis). Animals 2021, 11, 2538. https://doi.org/10.3390/ani11092538
Giraud G, Sosa S, Hambuckers A, Deleuze S, Wandia IN, Huynen M-C, Poncin P, Brotcorne F. Effect of Infant Presence on Social Networks of Sterilized and Intact Wild Female Balinese Macaques (Macaca fascicularis). Animals. 2021; 11(9):2538. https://doi.org/10.3390/ani11092538
Chicago/Turabian StyleGiraud, Gwennan, Sebastian Sosa, Alain Hambuckers, Stefan Deleuze, I Nengah Wandia, Marie-Claude Huynen, Pascal Poncin, and Fany Brotcorne. 2021. "Effect of Infant Presence on Social Networks of Sterilized and Intact Wild Female Balinese Macaques (Macaca fascicularis)" Animals 11, no. 9: 2538. https://doi.org/10.3390/ani11092538
APA StyleGiraud, G., Sosa, S., Hambuckers, A., Deleuze, S., Wandia, I. N., Huynen, M.-C., Poncin, P., & Brotcorne, F. (2021). Effect of Infant Presence on Social Networks of Sterilized and Intact Wild Female Balinese Macaques (Macaca fascicularis). Animals, 11(9), 2538. https://doi.org/10.3390/ani11092538










