Metabolic Profiling of Rumen Fluid and Milk in Lactating Dairy Cattle Influenced by Subclinical Ketosis Using Proton Nuclear Magnetic Resonance Spectroscopy
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Sampling
2.2. Prepared Proton Nuclear Magnetic Resonance Spectroscopy Analyses
2.3. Metabolites Identification, Quantification, and Statistical Analyses
3. Results
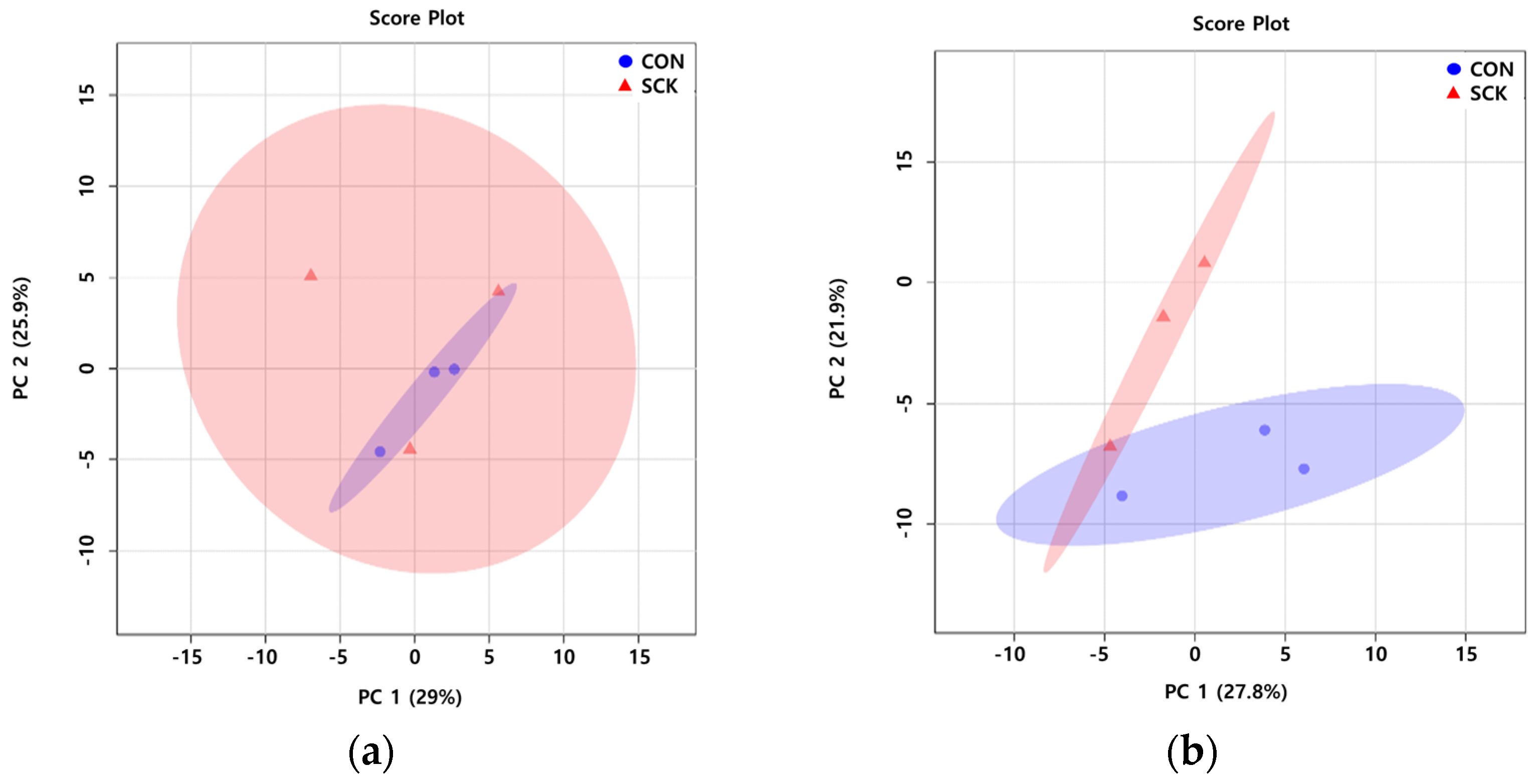
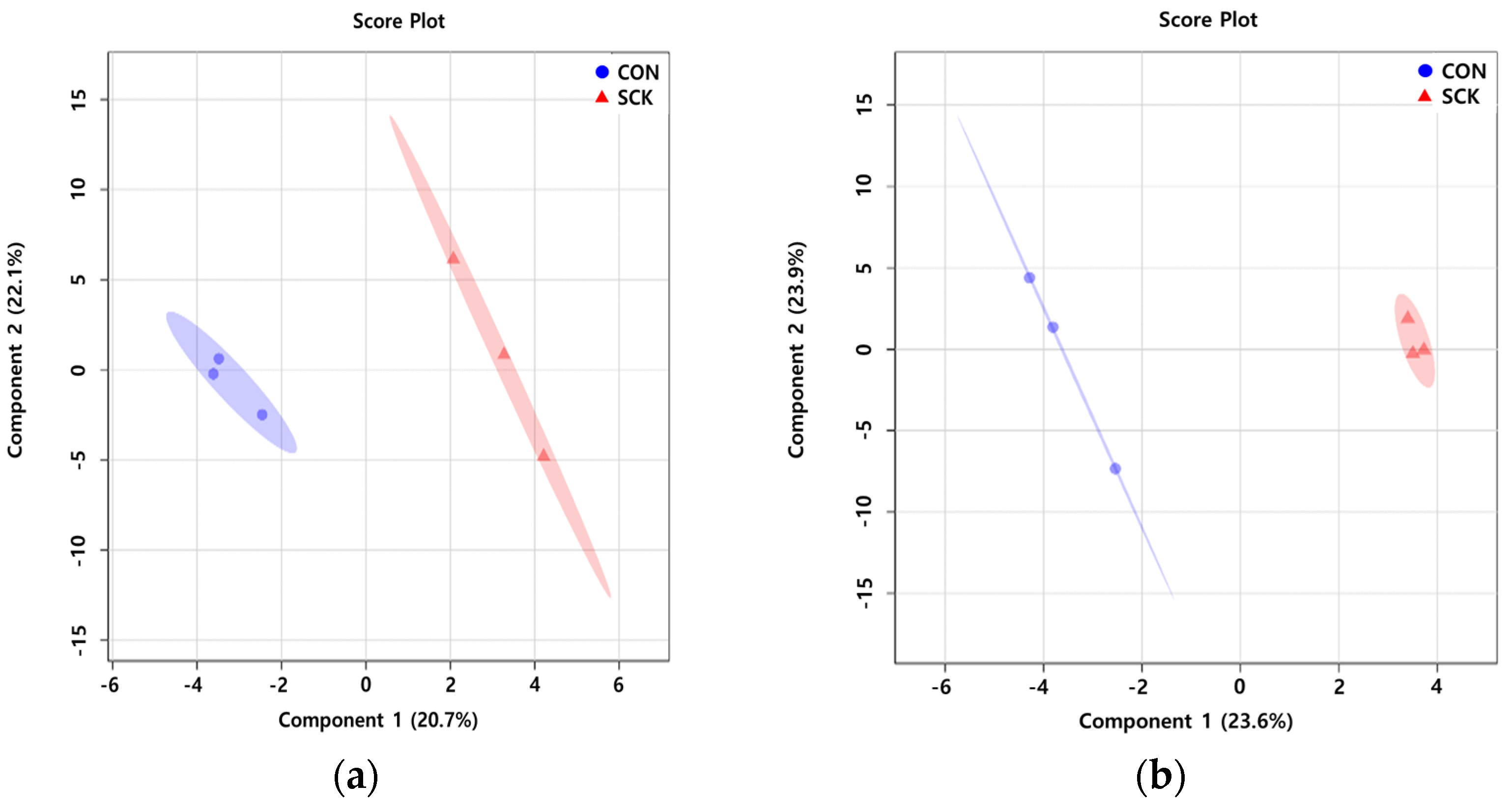
3.1. Multivariate Data Analysis
3.2. Detection and Quantification of Rumen Fluid and Milk Metabolites
3.3. Differences in Rumen Fluid and Milk Metabolites
3.4. Metabolic Pathway Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, G.; Ametaj, B.N. Ketosis an old story under a new approach. Dairy 2020, 1, 42–60. [Google Scholar] [CrossRef]
- Suthar, V.S.; Canelas-Raposo, J.; Deniz, A.; Heuwieser, W. Prevalence of subclinical ketosis and relationships with postpartum diseases in European dairy cows. J. Dairy Sci. 2013, 96, 2925–2938. [Google Scholar] [CrossRef] [PubMed]
- Duffield, T. Subclinical ketosis in lactating dairy cattle. Vet. Clin. North Am. Food Anim. Pract. 2000, 16, 231–253. [Google Scholar] [CrossRef]
- Bell, A.W. Regulation of organic nutrient metabolism during transition from late pregnancy to early lactation. J. Anim. Sci. 1995, 73, 2804–2819. [Google Scholar] [CrossRef] [PubMed]
- De Vries, M.J.; Veerkamp, R.F. Energy balance of dairy cattle in relation to milk production variables and fertility. J. Dairy Sci. 2000, 83, 62–69. [Google Scholar] [CrossRef]
- Grummer, R.R.; Mashek, D.G.; Hayirli, A. Dry matter intake and energy balance in the transition period. Vet. Clin. North Am.-Food Anim. Pract. 2004, 20, 447–470. [Google Scholar] [CrossRef] [PubMed]
- Duffield, T.F.; Lissemore, K.D.; McBride, B.W.; Leslie, K.E. Impact of hyperketonemia in early lactation dairy cows on health and production. J. Dairy Sci. 2009, 92, 571–580. [Google Scholar] [CrossRef] [PubMed]
- McArt, J.A.A.; Nydam, D.V.; Ospina, P.A.; Oetzel, G.R. A field trial on the effect of propylene glycol on milk yield and resolution of ketosis in fresh cows diagnosed with subclinical ketosis. J. Dairy Sci. 2011, 94, 6011–6020. [Google Scholar] [CrossRef]
- Ospina, P.A.; Nydam, D.V.; Stokol, T.; Overton, T.R. Evaluation of nonesterified fatty acids and β-hydroxybutyrate in transition dairy cattle in the northeastern United States: Critical thresholds for prediction of clinical diseases. J. Dairy Sci. 2010, 93, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Raboisson, D.; Mounié, M.; Maigné, E. Diseases, reproductive performance, and changes in milk production associated with subclinical ketosis in dairy cows: A meta-analysis and review. J. Dairy Sci. 2014, 97, 7547–7563. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Zhao, C.; Hu, P.; Chen, H.; Liu, Z.; Liu, G.; Wang, Z. Correlation between composition of the bacterial community and concentration of volatile fatty acids in the rumen during the transition period and ketosis in dairy cows. Appl. Environ. Microbiol. 2012, 78, 2386–2392. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Shiogama, Y. Acetone and isopropanol in ruminal fluid and feces of lactating dairy cows. J. Vet. Med. Sci. 2010, 72, 297–300. [Google Scholar] [CrossRef][Green Version]
- Bruss, M.L.; Lopez, M.J. Mixed ruminal microbes of cattle produce isopropanol in the presence of acetone but not 3-D-hydroxybutyrate. J. Dairy Sci. 2000, 83, 2580–2584. [Google Scholar] [CrossRef]
- Klein, M.S.; Buttchereit, N.; Miemczyk, S.P.; Immervoll, A.K.; Louis, C.; Wiedemann, S.; Junge, W.; Thaller, G.; Oefner, P.J.; Gronwald, W. NMR metabolomic analysis of dairy cows reveals milk glycerophosphocholine to phosphocholine ratio as prognostic biomarker for risk of ketosis. J. Proteome Res. 2012, 11, 1373–1381. [Google Scholar] [CrossRef]
- Klein, M.S.; Almstetter, M.F.; Nürnberger, N.; Sigl, G.; Gronwald, W.; Wiedemann, S.; Dettmer, K.; Oefner, P.J. Correlations between milk and plasma levels of amino and carboxylic acids in dairy cows. J. Proteome Res. 2013, 12, 5223–5232. [Google Scholar] [CrossRef]
- Collard, B.L.; Boettcher, P.J.; Dekkers, J.C.M.; Petitclerc, D.; Schaeffer, L.R. Relationships between energy balance and health traits of dairy cattle in early lactation. J. Dairy Sci. 2000, 83, 2683–2690. [Google Scholar] [CrossRef]
- Krogh, M.A.; Hostens, M.; Salavati, M.; Grelet, C.; Sorensen, M.T.; Wathes, D.C.; Ferris, C.P.; Marchitelli, C.; Signorelli, F.; Napolitano, F.; et al. Between- and within-herd variation in blood and milk biomarkers in Holstein cows in early lactation. Animal 2020, 14, 1067–1075. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Vervoort, J.; Saccenti, E.; van Hoeij, R.; Kemp, B.; van Knegsel, A. Milk metabolomics data reveal the energy balance of individual dairy cows in early lactation. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- KOSIS. Korean Statistical Information Service. Available online: kosis.kr (accessed on 2 May 2021).
- Kim, S.; Cho, Y. Analysis of total mixed ration (TMR) nutrition and metabolic diseases in Korean dairy farm. Korean. J. Vet. Serv. 2019, 42, 67–71. [Google Scholar]
- Shohel Al Faruk, M.; Park, B.; Ha, S.; Lee, S.S.; Mamuad, L.L.; Cho, Y.I. Comparative study on different field tests of ketosis using blood, milk, and urine in dairy cattle. Vet. Med. 2020, 65, 199–206. [Google Scholar] [CrossRef]
- Mahboob, A.; Lee, S.; Choi, S.; Dang, C.; Mahboob, A.; Do, C. Genetic parameters of milk β-hydroxybutyrate acid, milk acetone, milk yield, and energy-corrected milk for Holstein dairy cattle in Korea. KDISS 2017, 28, 1349–1360. [Google Scholar]
- Eom, J.S.; Lee, S.J.; Kim, H.S.; Choi, Y.Y.; Kim, S.H.; Lee, Y.G.; Lee, S.S. Metabolomics comparison of hanwoo (Bos taurus coreanae) biofluids using proton nuclear magnetic resonance spectroscopy. Metabolites. 2020, 10, 333. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Kim, E.T.; Eom, J.S.; Choi, Y.Y.; Lee, S.J.; Lee, S.S.; Chung, C.D.; Lee, S.S. Exploration of metabolite profiles in the biofluids of dairy cows by proton nuclear magnetic resonance analysis. PLoS. ONE 2021, 16, 1–18. [Google Scholar]
- Eom, J.S.; Kim, E.T.; Kim, H.S.; Choi, Y.Y.; Lee, S.J.; Lee, S.S.; Kim, S.H.; Lee, S.S. Metabolomics comparison of rumen fluid and milk in dairy cattle using proton nuclear magnetic resonance spectroscopy. Anim. Biosci. 2021, 34, 213–222. [Google Scholar] [CrossRef]
- Rollin, E.; Berghaus, R.D.; Rapnicki, P.; Godden, S.M.; Overton, M.W. The effect of injectable butaphosphan and cyanocobalmin on postpartum serum β-hydroxybutyrate, calcium, and phosphorus concentrations in dairy cattle. J. Dairy Sci. 2010, 93, 978–987. [Google Scholar] [CrossRef]
- Iwersen, M.; Falkenberg, U.; Voigtsberger, R.; Forderung, D.; Heuwieser, W. Evaluation of an electronic cowside test to detect subclinical ketosis in dairy cows. J. Dairy Sci. 2009, 92, 2618–2624. [Google Scholar] [CrossRef]
- Duffield, T.F.; Sandals, D.; Leslie, K.E.; Lissemore, K.; McBride, B.W.; Lumsden, J.H.; Dick, P.; Bagg, R. Efficacy of monensin for the prevention of subclinical ketosis in lactating dairy cows. J. Dairy Sci. 1998, 81, 2866–2873. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists; AOAC International: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Foroutan, A.; Fitzsimmons, C.; Mandal, R.; Piri-moghadam, H.; Zheng, J.; Guo, A.; Li, C.; Guan, L.L.; Wishart, D.S. The bovine metabolome. Metabolites 2020, 10, 233. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, T.F.; Vázquez-Fresno, R.; Serra-Cayuela, A.; Dong, E.; Mandal, R.; Hennessy, D.; McAuliffe, S.; Dillon, P.; Wishart, D.S.; Stanton, C.; et al. Pasture feeding changes the bovine rumen and milk metabolome. Metabolites 2018, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Lamanna, R.; Braca, A.; Paolo, D.; Imparato, G. Identification of milk mixtures by 1H NMR profiling. Magn. Reson. Chem. 2012, 49, S22–S26. [Google Scholar] [CrossRef]
- MacLEOD, N.A.; ØRSKOV, E.R. Absorption and utilization of volatile fatty acids in ruminants. Can. J. Anim. Sci. 1984, 64, 354–355. [Google Scholar] [CrossRef]
- Doreau, M.; Ollier, A.; Michalet Doreau, B. An atypical case of ruminal fermentations leading to ketosis in early lactating cows [rumen pH, ruminal volatile fatty acids]. Rev. Med. Vet. 2001, 152, 301–306. [Google Scholar]
- Tveit, B.; Lingaas, F.; Svendsen, M.; Sjaastad, Ø.V. Etiology of acetonemia in Norwegian cattle. 1. Effect of ketogenic silage, season, energy level, and genetic factors. J. Dairy Sci. 1992, 75, 2421–2432. [Google Scholar] [CrossRef]
- Robertson, A.; Thin, C.A. Study of starvation ketosis in the ruminant. Br. J. Nutr. 1953, 7, 181–195. [Google Scholar] [CrossRef] [PubMed]
- Thin, C.; Robertson, A. Biochemical aspects of ruminant ketosis. J. Comp. Pathol. 1953, 63, 184–194. [Google Scholar] [CrossRef]
- Phillips, R.W. Religious revelations and bovine ketosis (a nonsacred cow). Perspect. Biol. Med. 1978, 21, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Mellado, M.; Dávila, A.; Gaytán, L.; Macías-Cruz, U.; Avendaño-Reyes, L.; García, E. Risk factors for clinical ketosis and association with milk production and reproduction variables in dairy cows in a hot environment. Trop. Anim. Health. Prod. 2018, 50, 1611–1616. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Min, L.; Zheng, N.; Wang, J. Effect of heat stress on bacterial composition and metabolism in the rumen of lactating dairy cows. Animals 2019, 9, 925. [Google Scholar] [CrossRef]
- Song, S.; Jiang, M.; Zhou, J.; Zhao, F.; Hou, X.; Lin, Y. Nutrigenomic role of acetate and β-hydroxybutyrate in bovine mammary epithelial cells. DNA Cell Biol. 2020, 39, 389–397. [Google Scholar] [CrossRef]
- Van Gylswyk, N. Succiniclasticum ruminis gen. nov., sp. nov., a ruminal bacterium converting succinate and propionate as the sole energy-yielding mechanism. Int. J. Syst. Evol. Microbiol. 1995, 45, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Clemmons, B.A.; Powers, J.B.; Campagna, S.R.; Seay, T.B.; Embree, M.M.; Myer, P.R. Rumen fluid metabolomics of beef steers differing in feed efficinecy. Metabolomics 2020, 16, 23. [Google Scholar] [CrossRef]
- Stein, D.R.; Allen, D.T.; Perry, E.B.; Bruner, J.C.; Gates, K.W.; Rehberger, T.G.; Mertz, K.; Jones, D.; Spicer, L.J. Effects of feeding propionibacteria to dairy cows on milk yield, milk components, and reproduction. J. Dairy Sci. 2006, 89, 111–125. [Google Scholar] [CrossRef]
- McArt, J.A.A.; Nydam, D.V.; Oetzel, G.R. Epidemiology of subclinical ketosis in early lactation dairy cattle. J. Dairy Sci. 2012, 95, 5056–5066. [Google Scholar] [CrossRef] [PubMed]
- Mansor, R. Proteomic and Metabolomic Studies on Milk during Bovine Mastitis. Ph.D. Thesis, University of Glasgow, Glasgow, UK, 2012. [Google Scholar]
- Van Gastelen, S.; Antunes-Fernandes, E.C.; Hettinga, K.A.; Dijkstra, J. The relationship between milk metabolome and methane emission of Holstein Friesian dairy cows: Metabolic interpretation and prediction potential. J. Dairy. Sci. 2018, 101, 2110–2126. [Google Scholar] [CrossRef]
- Wu, G.; Wu, Z.; Dai, Z.; Yang, Y.; Yang, W.; Liu, C.; Wang, B.; Wang, J.; Yin, Y. Dietary requirements of “nutritionally non-essential amino acids” by animals and human. Amino. Acid. 2013, 44, 1107–1113. [Google Scholar] [CrossRef]
- Sundekilde, U.K.; Frederiksen, P.D.; Clausen, M.R.; Larsen, L.B.; Bertram, H.C. Relationship between the metabolite profile and technological properties of bovine milk from two dairy breeds elucidated by NMR-based metabolomics. J. Agric. Food Chem. 2011, 59, 7360–7367. [Google Scholar] [CrossRef]
- Sundekilde, U.K.; Larsen, L.B.; Bertram, H.C. NMR-based milk metabolomics. Metabolites 2013, 3, 204–222. [Google Scholar] [CrossRef]
- Sundekilde, U.K.; Poulsen, N.A.; Larsen, L.B.; Bertram, H.C. Nuclear magnetic resonance metabonomics reveals strong association between milk metabolites and somatic cell count in bovine milk. J. Dairy Sci. 2013, 96, 290–299. [Google Scholar] [CrossRef]
- Bauman, D.E.; Brown, R.E.; Davis, C.L. Pathways of fatty acid synthesis and reducing equivalent generation in mammary gland of rat, sow, and cow. Arch. Biochem. Biophys. 1970, 140, 237–244. [Google Scholar] [CrossRef]
- Rhie, S.G.; Lee, E.K. Milk and dairy intake and acceptability in fifth- and sixth-graders in Hwaseong, Korea. Korean. J. Community. Living. Sci. 2015, 26, 499–509. [Google Scholar] [CrossRef]
- Cerbulis, J.; Farrell, H.M., Jr. Xanthine oxidase activity in dairy products. J. Dairy. Sci. 1977, 60, 170–176. [Google Scholar] [CrossRef]
- Spitsberg, V.L.; Gorewit, R.C. Solubilization and purification of xanthine oxidase from bovine milk fat globule membrane. Protein. Expres. Purif. 1998, 13, 229–234. [Google Scholar] [CrossRef]
- Ozturk, G.; Shah, I.M.; Mills, D.A.; Bruce German, J.; de Moura Bell, J.M.L.N. The antimicrobial activity of bovine milk xanthine oxidase. Int. Dairy. J. 2020, 102, 104581. [Google Scholar] [CrossRef] [PubMed]
- Zavaleta, N.; Kvistgaard, A.S.; Graverholt, G.; Respicio, G.; Guija, H.; Valencia, N.; Lönnerdal, B. Efficay of an MFGM-enriched complementary food in diarrhea, anemia, and micronutrient status in infants. J. Pediatr. Gastroenterol. Nutr. 2011, 53, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Zavaleta, N.; Chen, S.Y.; Lönnerdal, B.; Slupsky, C. Effect of bovine milk fat globule memberances as a complementary food on the serum metabolome and immune markers of 6–11-month-old Peruvian infants. NPJ. Sci. Food. 2018, 2, 1–9. [Google Scholar] [CrossRef] [PubMed]


| Items | Amount |
|---|---|
| Ingredients composition, % of DM | |
| Concentrate | 15.30 |
| Soybean meal | 2.40 |
| Corn silage | 47.20 |
| Alfalfa hay | 7.10 |
| Tall fescue | 9.40 |
| Timothy | 5.90 |
| Energy booster 1 | 7.10 |
| Cash Gold 1 | 4.50 |
| Lyzin-Plus 2 | 0.20 |
| Limestone 3 | 0.20 |
| Zin Care 1 | 0.10 |
| Supex-F 1 | 0.50 |
| Trace minerals 4 | 0.05 |
| Vitamins premix 5 | 0.05 |
| Chemical composition (% of DM basis) | |
| Dry matter (DM), % | 53.2 |
| CP | 10.0 |
| NDF | 28.2 |
| ADF | 16.9 |
| Ca | 0.40 |
| P | 0.15 |
| General Information | Healthy | Subclinical Ketosis |
|---|---|---|
| Rumen fluid | ||
| Detected metabolites (n ≥ 1) | 145 | 171 |
| Quantified metabolites (n = 3) | 48 | 45 |
| Classified chemical classes | 13 | 13 |
| Range of metabolites concentration | 0.87~23,207.30 μM | 7.30~22,139.17 μM |
| Milk | ||
| Detected metabolites (n ≥ 1) | 163 | 162 |
| Quantified metabolites (n = 3) | 69 | 84 |
| Classified chemical classes | 14 | 13 |
| Range of metabolites concentration | 1.00~98,861.57 μM | 1.53~77,940.70 μM |
| Metabolites | Classification | CON/SCK 1 | p-Value | VIP Score 2 | Fold Change 3 |
|---|---|---|---|---|---|
| Rumen Fluid | |||||
| Butyrate | Organic acids | SCK | 6.74 × 10−4 | 0.94 | −0.14 |
| N,N-dimethylformamide | Carboxylic acids | CON | 1.15 × 10−3 | 2.17 | 0.78 |
| Acetate | Organic acids | CON | 3.06 × 10−3 | 0.72 | 0.09 |
| Sucrose | Carbohydrates | SCK | 1.18 × 10−2 | 1.81 | −0.57 |
| Glucose | Carbohydrates | CON | 1.44 × 10−2 | 1.21 | 0.25 |
| Propionate | Organic acids | CON | 1.50 × 10−2 | 0.83 | 0.25 |
| 3-hydroxybutyrate | Lipids | SCK | 2.20 × 10−2 | 1.87 | −0.57 |
| Maltose | Carbohydrates | SCK | 3.36 × 10−2 | 1.51 | −0.46 |
| Valerate | Organic acids | SCK | 3.83 × 10−2 | 0.81 | −0.12 |
| Methylamine | Amines | SCK | 7.36 × 10−2 | 1.67 | −0.44 |
| Methionine | Amino acids | SCK | 7.39 × 10−2 | 2.06 | −0.82 |
| Isopropanol | Alcohols | SCK | 9.56 × 10−2 | 1.55 | −0.64 |
| Milk | |||||
| Galactitol | Carbohydrates | CON | 1.81 × 10−4 | 1.92 | 0.68 |
| 1,3-dihydroxyacetone | Carbohydrates | CON | 3.75 × 10−4 | 4.43 | 0.56 |
| Maleate | Carboxylic acids | SCK | 6.18 × 10−4 | 2.32 | −1.05 |
| γ-glutamylphenylalanine | Amino acids | CON | 7.73 × 10−4 | 2.01 | 0.77 |
| 3-hydroxybutyrate | Lipids | SCK | 5.28 × 10−3 | 1.87 | −0.70 |
| Acetoacetate | Carbohydrates | SCK | 1.80 × 10−2 | 1.23 | −0.32 |
| 5-aminolevulinate | Carboxylic acids | CON | 2.11 × 10−2 | 1.91 | 0.78 |
| Acetate | Organic acids | CON | 2.98 × 10−2 | 1.53 | 0.46 |
| Galactonate | Carbohydrates | SCK | 4.15 × 10−2 | 0.92 | −0.19 |
| 3-hydroxykynurenine | Organic acids | SCK | 4.54 × 10−2 | 1.73 | −0.54 |
| Methylamine | Amines | CON | 4.90 × 10−2 | 2.17 | 1.12 |
| Acetone | Others | SCK | 5.73 × 10−2 | 1.01 | −0.25 |
| Guanidoacetate | Carboxylic acids | SCK | 7.68 × 10−2 | 1.01 | −0.22 |
| 2-oxoisocaproate | Organic acids | SCK | 7.94 × 10−2 | 1.56 | −0.70 |
| Xanthine | Nucleosides, Nucleotides | SCK | 8.15 × 10−2 | 1.61 | −0.77 |
| Riboflavin | Others | CON | 8.55 × 10−2 | 0.68 | 0.23 |
| Choline | Lipids | CON | 9.24 × 10−2 | 1.76 | 0.74 |
| Trehalose | Carbohydrates | SCK | 9.63 × 10−2 | 2.16 | −2.00 |
| Metabolic Pathway | Total Cmpd 1 | Hits 2 | p-Value | −Log (p-Value) | FDR 3 | Impact 4 |
|---|---|---|---|---|---|---|
| Rumen fluid | ||||||
| Starch and sucrose metabolism | 18 | 2 | 1.98 × 10−4 | 3.70 | 1.98 × 10−3 | 0.12 |
| Pyruvate metabolism | 22 | 1 | 3.07 × 10−3 | 2.51 | 1.02 × 10−2 | 0.06 |
| Glyoxylate and dicarboxylate metabolism | 32 | 1 | 3.07 × 10−3 | 2.51 | 1.02 × 10−2 | 0.00 |
| Glycolysis and gluconeogenesis | 26 | 2 | 8.43 × 10−3 | 2.07 | 1.69 × 10−2 | 0.03 |
| Butanoate metabolism | 15 | 2 | 1.04 × 10−2 | 1.98 | 1.69 × 10−2 | 0.00 |
| Galactose metabolism | 27 | 1 | 1.13 × 10−2 | 1.95 | 1.69 × 10−2 | 0.04 |
| Propanoate metabolism | 23 | 1 | 1.18 × 10−2 | 1.93 | 1.69 × 10−2 | 0.00 |
| Synthesis and degradation of ketone bodies | 5 | 1 | 2.01 × 10−2 | 1.70 | 2.51 × 10−2 | 0.00 |
| Cysteine and methionine metabolism | 33 | 1 | 7.77 × 10−2 | 1.11 | 7.77 × 10−2 | 0.10 |
| Aminoacyl-tRNA biosynthesis | 48 | 1 | 7.77 × 10−2 | 1.11 | 7.77 × 10−2 | 0.00 |
| Milk | ||||||
| Galactose metabolism | 27 | 1 | 3.99 × 10−4 | 3.40 | 3.43 × 10−3 | 0.00 |
| Glycerolipid metabolism | 16 | 1 | 4.03 × 10−4 | 3.39 | 3.43 × 10−3 | 0.00 |
| Glycine, serine and threonine metabolism | 34 | 3 | 7.64 × 10−4 | 3.12 | 4.33 × 10−3 | 0.02 |
| Synthesis and degradation of ketone bodies | 5 | 2 | 3.24 × 10−3 | 2.49 | 1.10 × 10−2 | 0.60 |
| Butanoate metabolism | 15 | 2 | 3.24 × 10−3 | 2.49 | 1.10 × 10−2 | 0.11 |
| Tyrosine metabolism | 42 | 1 | 4.18 × 10−3 | 2.38 | 1.18 × 10−2 | 0.00 |
| Arginine and proline metabolism | 38 | 1 | 5.96 × 10−3 | 2.22 | 1.45 × 10−2 | 0.02 |
| Valine, leucine and isoleucine degradation | 40 | 2 | 1.01 × 10−2 | 2.00 | 2.14 × 10−2 | 0.01 |
| Riboflavin metabolism | 4 | 1 | 1.15 × 10−2 | 1.94 | 2.17 × 10−2 | 0.50 |
| Pyruvate metabolism | 22 | 1 | 4.27 × 10−2 | 1.37 | 5.56 × 10−2 | 0.06 |
| Glycolysis and gluconeogenesis | 26 | 1 | 4.27 × 10−2 | 1.37 | 5.56 × 10−2 | 0.03 |
| Glyoxylate and dicarboxylate metabolism | 32 | 1 | 4.27 × 10−2 | 1.37 | 5.56 × 10−2 | 0.00 |
| Porphyrin and chlorophyll metabolism | 30 | 1 | 4.36 × 10−2 | 1.36 | 5.56 × 10−2 | 0.03 |
| Valine, leucine and isoleucine biosynthesis | 8 | 1 | 4.58 × 10−2 | 1.34 | 5.56 × 10−2 | 0.00 |
| Glycerophospholipid metabolism | 36 | 1 | 5.82 × 10−2 | 1.23 | 6.60 × 10−2 | 0.03 |
| Purine metabolism | 66 | 1 | 6.23 × 10−2 | 1.21 | 6.62 × 10−2 | 0.03 |
| Starch and sucrose metabolism | 18 | 1 | 7.36 × 10−2 | 1.13 | 7.36 × 10−2 | 0.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eom, J.-S.; Kim, H.-S.; Lee, S.-J.; Choi, Y.-Y.; Jo, S.-U.; Kim, J.; Lee, S.-S.; Kim, E.-T.; Lee, S.-S. Metabolic Profiling of Rumen Fluid and Milk in Lactating Dairy Cattle Influenced by Subclinical Ketosis Using Proton Nuclear Magnetic Resonance Spectroscopy. Animals 2021, 11, 2526. https://doi.org/10.3390/ani11092526
Eom J-S, Kim H-S, Lee S-J, Choi Y-Y, Jo S-U, Kim J, Lee S-S, Kim E-T, Lee S-S. Metabolic Profiling of Rumen Fluid and Milk in Lactating Dairy Cattle Influenced by Subclinical Ketosis Using Proton Nuclear Magnetic Resonance Spectroscopy. Animals. 2021; 11(9):2526. https://doi.org/10.3390/ani11092526
Chicago/Turabian StyleEom, Jun-Sik, Hyun-Sang Kim, Shin-Ja Lee, You-Young Choi, Seong-Uk Jo, Jaemin Kim, Sang-Suk Lee, Eun-Tae Kim, and Sung-Sill Lee. 2021. "Metabolic Profiling of Rumen Fluid and Milk in Lactating Dairy Cattle Influenced by Subclinical Ketosis Using Proton Nuclear Magnetic Resonance Spectroscopy" Animals 11, no. 9: 2526. https://doi.org/10.3390/ani11092526
APA StyleEom, J.-S., Kim, H.-S., Lee, S.-J., Choi, Y.-Y., Jo, S.-U., Kim, J., Lee, S.-S., Kim, E.-T., & Lee, S.-S. (2021). Metabolic Profiling of Rumen Fluid and Milk in Lactating Dairy Cattle Influenced by Subclinical Ketosis Using Proton Nuclear Magnetic Resonance Spectroscopy. Animals, 11(9), 2526. https://doi.org/10.3390/ani11092526






