The Study of Growth and Performance in Local Chicken Breeds and Varieties: A Review of Methods and Scientific Transference
Abstract
Simple Summary
Abstract
1. Introduction
2. Review of Data Collection and Analysis
2.1. Data Collection
2.2. Data Analysis
2.2.1. Assumption Testing
2.2.2. Statistical Approach Decision
3. Growth and Performance Modelling
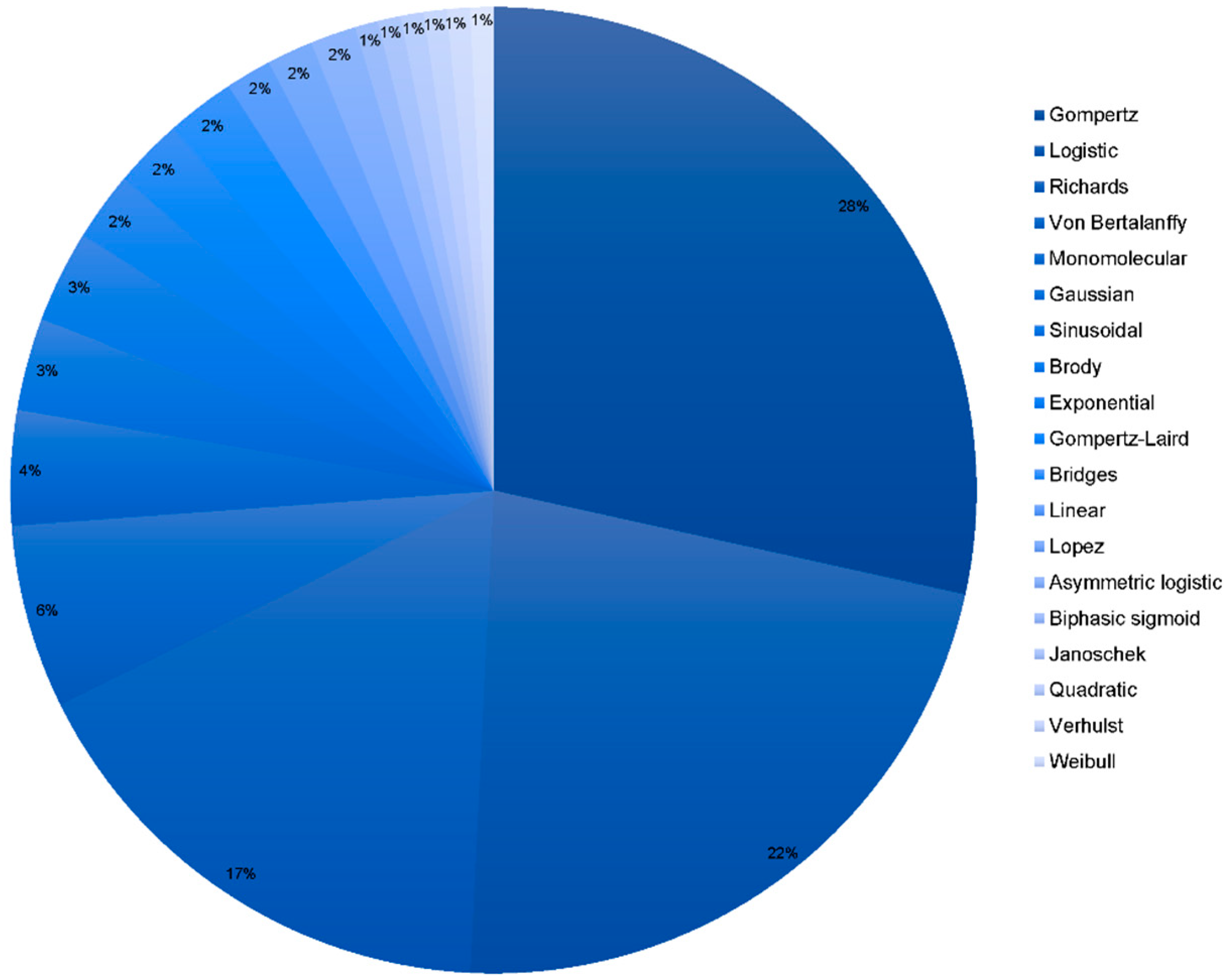
3.1. Models Used in the Literature to Fit for Growth and Performance
3.2. Goodness-of-Fit and Flexibility Criteria
3.3. Constraints and Particularities for Growth Modelling in Native Genotypes (Breeds and Varieties)
4. Scientific Transference
4.1. Year of Publication
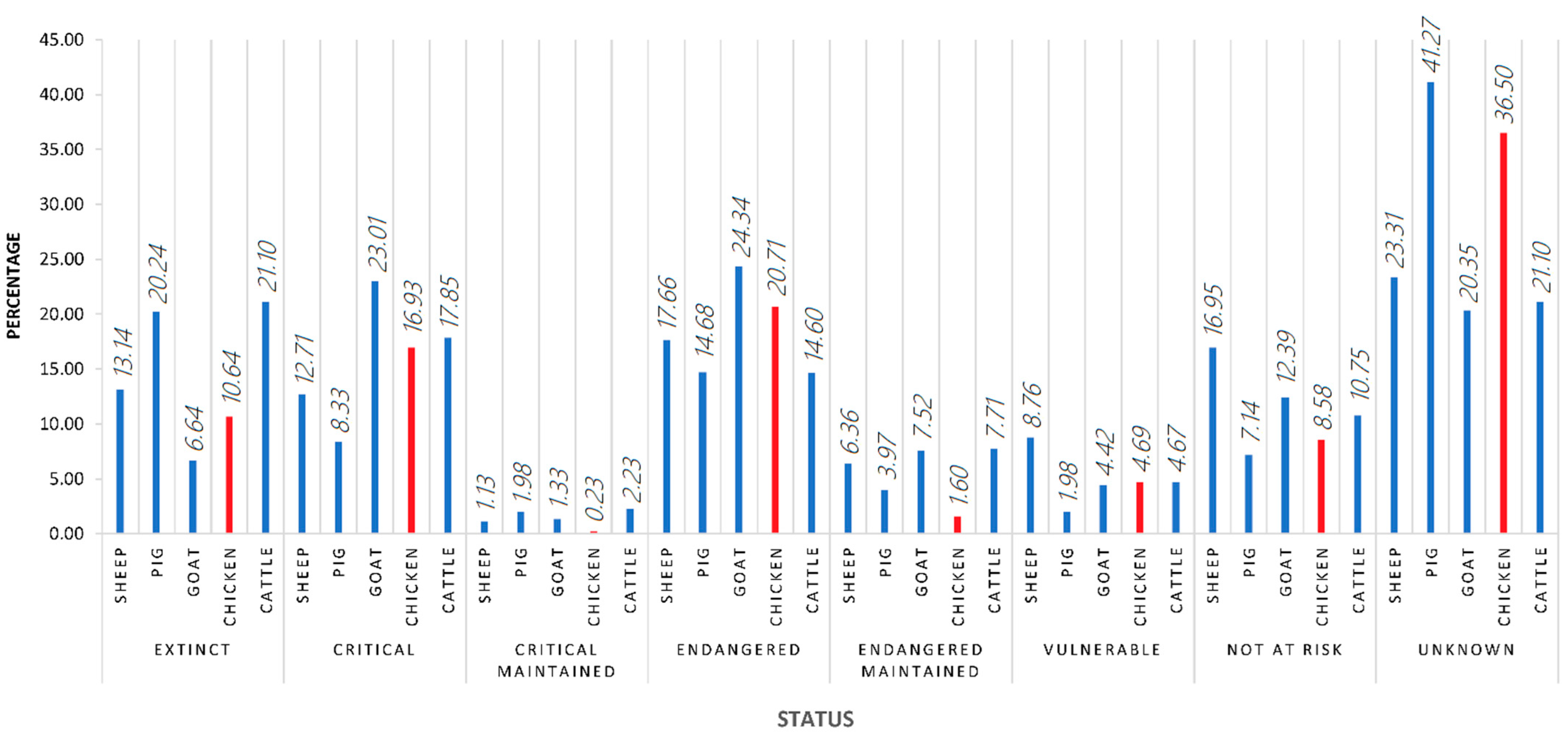
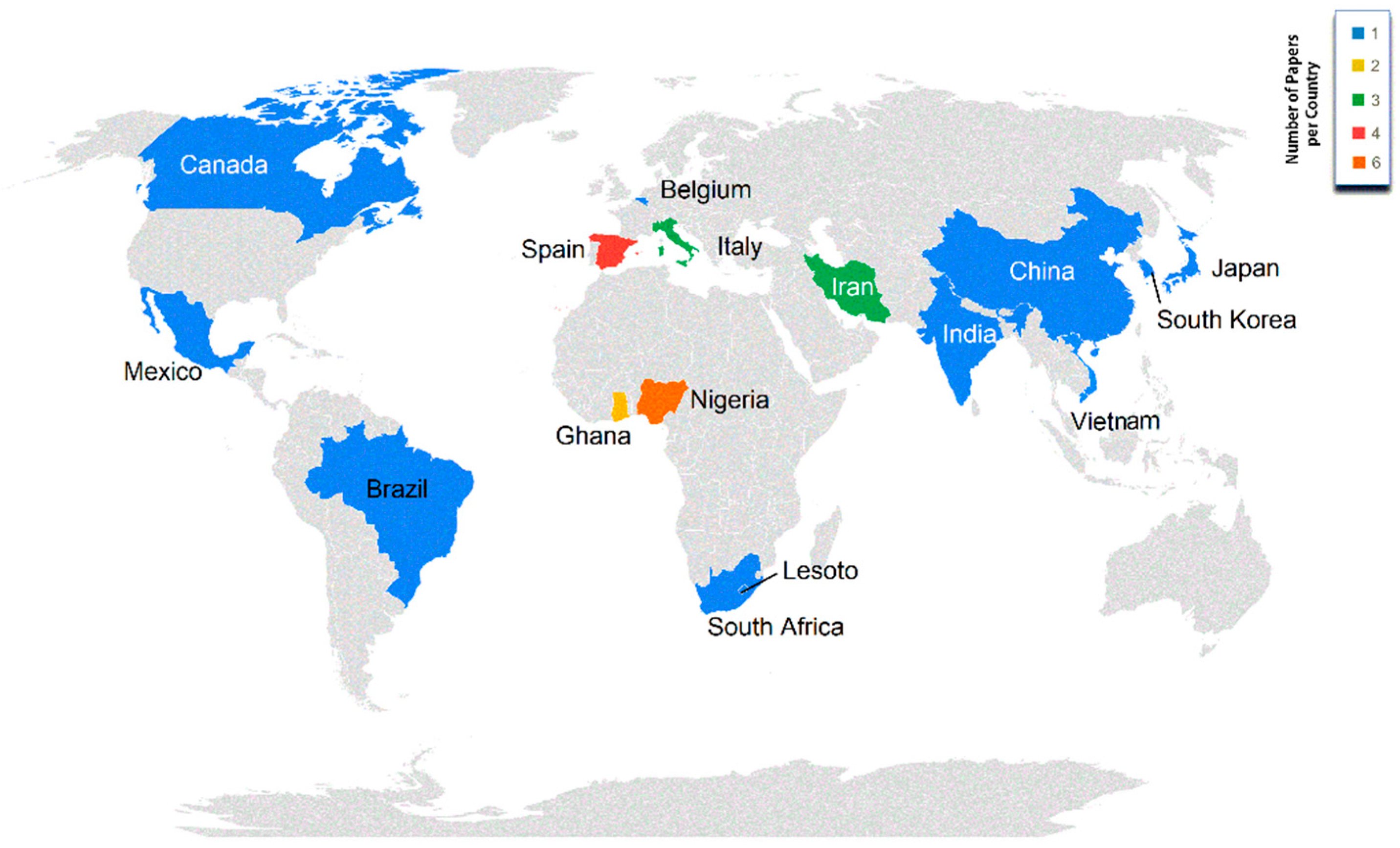
4.2. Study Georeferencing (Continents and Countries)
4.3. Method and Study Design-Related Research Impact Conditioning Factors
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. Domestic Animal Diversity Information System (DAD-IS): Data; FAO: Rome, Italy, 2021. [Google Scholar]
- FAO. Sustainable Development Goals: Goals; FAO: Rome, Italy, 2021. [Google Scholar]
- Hoffmann, I. Livestock genetic diversity and climate change adaptation. In Proceedings of the Livestock and Global Climate Change 2008, Hammamet, Tunisia, 17–20 May 2008; Volume 76. [Google Scholar]
- Hu, Y.; Cheng, H.; Tao, S. Environmental and human health challenges of industrial livestock and poultry farming in China and their mitigation. Environ. Int. 2017, 107, 111–130. [Google Scholar] [CrossRef]
- Moges, F.; Mellesse, A.; Dessie, T. Assessment of village chicken production system and evaluation of the productive and reproductive performance of local chicken ecotype in Bure district, North West Ethiopia. Afr. J. Agric. Res. 2010, 5, 1739–1748. [Google Scholar]
- Delgado Bermejo, J.V.; Martínez Martínez, M.A.; Rodríguez Galván, G.; Stemmer, A.; Navas González, F.J.; Camacho Vallejo, M.E. Organization and management of conservation programs and research in domestic animal genetic resources. Diversity 2019, 11, 235. [Google Scholar] [CrossRef]
- Sponenberg, D.P.; Martin, A.; Couch, C.; Beranger, J. Conservation strategies for local breed biodiversity. Diversity 2019, 11, 177. [Google Scholar] [CrossRef]
- Gandini, G.; Hiemstra, S.J. Farm animal genetic resources and the COVID-19 pandemic. Anim. Front. 2021, 11, 54. [Google Scholar] [CrossRef] [PubMed]
- Leroy, G.; Baumung, R.; Notter, D.; Verrier, E.; Wurzinger, M.; Scherf, B. Stakeholder involvement and the management of animal genetic resources across the world. Livest. Sci. 2017, 198, 120–128. [Google Scholar] [CrossRef]
- González Ariza, A.; Navas González, F.J.; Arando Arbulu, A.; León Jurado, J.M.; Barba Capote, C.J.; Camacho Vallejo, M.E. Non-parametrical canonical analysis of quality-related characteristics of eggs of different varieties of native hens compared to laying lineage. Animals 2019, 9, 153. [Google Scholar] [CrossRef]
- Alderson, G. Conservation of breeds and maintenance of biodiversity: Justification and methodology for the conservation of Animal Genetic Resources. Arch. Zootec. 2018, 67, 300–309. [Google Scholar] [CrossRef][Green Version]
- Taylor, P.S.; Hemsworth, P.H.; Groves, P.J.; Gebhardt-Henrich, S.G.; Rault, J.-L. Ranging behaviour of commercial free-range broiler chickens 1: Factors related to flock variability. Animals 2017, 7, 54. [Google Scholar] [CrossRef] [PubMed]
- González Ariza, A.; Arando Arbulu, A.; Navas González, F.J.; Ruíz Morales, F.d.A.; León Jurado, J.M.; Barba Capote, C.J.; Camacho Vallejo, M.E. Sensory preference and professional profile affinity definition of endangered native breed eggs compared to commercial laying lineages’ eggs. Animals 2019, 9, 920. [Google Scholar] [CrossRef] [PubMed]
- Narınc, D.; Aksoy, T.; Karaman, E.; Curek, D.I. Analysis of fitting growth models in medium growing chicken raised indoor system. Trends Anim. Vet. Sci. 2010, 1, 12–18. [Google Scholar]
- Shahbandeh, M. Projected Poultry Meat Consumption Worldwide from 2021 to 2030. Statista. July 2021. Available online: https://www.statista.com/statistics/739951/poultry-meat-consumption-worldwide/ (accessed on 16 July 2021).
- Sariyel, V.; Aygun, A.; Keskin, I. Comparison of growth curve models in partridge. Poult. Sci 2017, 96, 1635–1640. [Google Scholar] [CrossRef] [PubMed]
- Lupi, T.; León, J.; Nogales, S.; Barba, C.; Delgado, J. Genetic parameters of traits associated with the growth curve in Segureña sheep. Animal 2016, 10, 729–735. [Google Scholar] [CrossRef] [PubMed]
- González Ariza, A.; Nogales Baena, S.; Lupi, T.M.; Arando Arbulu, A.; Navas González, F.J.; León Jurado, J.M.; Delgado Bermejo, J.V.; Camacho Vallejo, M.E. Characterisation of biological growth curves of different varieties of an endangered native hen breed kept under free range conditions. Ital. J. Anim. Sci. 2021, 20, 806–813. [Google Scholar] [CrossRef]
- Narinç, D.; Narinç, N.Ö.; Aygün, A. Growth curve analyses in poultry science. Worlds Poult. Sci. J. 2017, 73, 395–408. [Google Scholar] [CrossRef]
- McLean, A.K.; Navas Gonzalez, F.J. Can scientists influence donkey welfare? Historical perspective and a contemporary view. J. Equine Vet. Sci. 2018, 65, 25–32. [Google Scholar] [CrossRef]
- Iglesias Pastrana, C.; Navas González, F.J.; Ciani, E.; Barba Capote, C.J.; Delgado Bermejo, J.V. Effect of research impact on emerging camel husbandry, welfare and social-related awareness. Animals 2020, 10, 780. [Google Scholar] [CrossRef]
- Gehanno, J.-F.; Rollin, L.; Darmoni, S. Is the coverage of google scholar enough to be used alone for systematic reviews. BMC Med. Inform. Decis. Mak. 2013, 13, 7. [Google Scholar] [CrossRef]
- Schlosser, R.W.; Wendt, O.; Bhavnani, S.; Nail-Chiwetalu, B. Use of information-seeking strategies for developing systematic reviews and engaging in evidence-based practice: The application of traditional and comprehensive Pearl Growing. A review. Int. J. Lang. Commun. Disord. 2006, 41, 567–582. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Darmani Kuhi, H.; Kebreab, E.; Lopez, S.; France, J. An evaluation of different growth functions for describing the profile of live weight with time (age) in meat and egg strains of chicken. Poult. Sci. 2003, 82, 1536–1543. [Google Scholar] [CrossRef]
- Osei-Amponsah, R.; Kayang, B.; Naazie, A.; Barchia, I.; Arthur, P. Evaluation of models to describe temporal growth in local chickens of Ghana. Iran. J. Appl. Anim. Sci. 2014, 4, 855–861. [Google Scholar]
- Franco, D.; Rois, D.; Vázquez, J.A.; Lorenzo, J. Comparison of growth performance, carcass components, and meat quality between Mos rooster (Galician indigenous breed) and Sasso T-44 line slaughtered at 10 months. Poult. Sci. 2012, 91, 1227–1239. [Google Scholar] [CrossRef] [PubMed]
- Nguyen Hoang, T.; Do, H.T.; Bui, D.H.; Pham, D.K.; Hoang, T.A.; Do, D.N. Evaluation of non-linear growth curve models in the Vietnamese indigenous Mia chicken. Anim. Sci. J. 2021, 92, e13483. [Google Scholar] [CrossRef]
- Ogunshola, O.; Morenikeji, O.; Chineke, C. Modeling the Growth Curves of Selected Fulani Ecotype Chickens. Open Acc. J. Agri. Res. 2020, 2, 10028. [Google Scholar]
- Adenaike, A.; Akpan, U.; Udoh, J.; Wheto, M.; Durosaro, S.; Sanda, A.; Ikeobi, C. Comparative Evaluation of Growth Functions in Three Broiler Strains of Nigerian Chickens. Pertanika J. Trop. Agric. Sci. 2017, 40, 611–620. [Google Scholar]
- Yadav, S.; Sarkar, S.; Mahapatra, R.; Kannaki, T.; Dange, M.; Bhattacharya, T.; Chatterjee, R. Modeling growth curves for Indian native vs. exotic chicken breeds to assist in selection strategies. Indian J. Anim. Res. 2019, 89, 898–902. [Google Scholar]
- Olawoyin, O.O. Evaluation of the growth parameters of four strains of cockerels. Afr. J. Anim. Biomed. Sci 2007, 2, 17–25. [Google Scholar]
- Faraji Arough, H.; Rokouei, M.; Maghsoudi, A.; Mehri, M. Evaluation of non-linear growth curves models for native slow-growing Khazak Chickens. Poult. Sci. 2019, 7, 25–32. [Google Scholar]
- Moula, N.; Antoine-Moussiaux, N.; Farnir, F.; Leroy, P. Evaluation of the production performances of an endangered local poultry breed, the Famennoise. Int. J. Poult. Sci. 2009, 8, 389–396. [Google Scholar] [CrossRef][Green Version]
- Moharrery, A.; Mirzaei, M. Growth characteristics of commercial broiler and native chickens as predicted by different growth functions. J. Anim. Feed Sci. 2014, 23, 82–89. [Google Scholar] [CrossRef][Green Version]
- Rizzi, C.; Contiero, B.; Cassandro, M. Growth patterns of Italian local chicken populations. Poult. Sci. 2013, 92, 2226–2235. [Google Scholar] [CrossRef]
- Soglia, D.; Sartore, S.; Maione, S.; Schiavone, A.; Dabbou, S.; Nery, J.; Zaniboni, L.; Marelli, S.; Sacchi, P.; Rasero, R. Growth performance analysis of two Italian slow-growing chicken breeds: Bianca di Saluzzo and Bionda Piemontese. Animals 2020, 10, 969. [Google Scholar] [CrossRef]
- Selvaggi, M.; Laudadio, V.; Dario, C.; Tufarelli, V. Modelling growth curves in a nondescript Italian chicken breed: An opportunity to improve genetic and feeding strategies. J. Poult. Sci. 2015, 52, 288–294. [Google Scholar] [CrossRef]
- Peters, S.; Ikeobi, C.; Ozoje, M.; Adebambo, O. Modelling growth in seven chicken genotypes. Niger. J. Anim. Prod. 2005, 32, 28–38. [Google Scholar] [CrossRef]
- Ibiapina, V.; Barbosa, F.J.V.; Campelo, J.E.G.; Sarmento, J.L.R. Non-linear mixed models in the study of growth of naturalized chickens. Rev. Bras. Zootec. 2020, 49, e20190201. [Google Scholar] [CrossRef]
- Abbaszadeh, S.; Pirany, N.; Ahmadipour, B. Studying growth pattern and body weight gain of Isfahan native chickens. Iran J. Appl. Anim. Sci. 2019, 11, 97–110. [Google Scholar]
- Ridho, M.; Putra, W.; Sola-Ojo, F. The growth curve of Gompertz and Logistic models in body weight of Ecotype Fulani Chickens (Gallus domesticus). IOP Conf. Ser. Earth Environ. Sci. 2021, 637, 012098. [Google Scholar] [CrossRef]
- Norris, D.; Ngambi, J.; Benyi, K.; Makgahlele, M.; Shimelis, H.; Nesamvuni, E. Analysis of growth curves of indigenous male Venda and Naked Neck chickens. S. Afr. J. Anim. Sci. 2007, 37, 21–26. [Google Scholar] [CrossRef]
- Bashiru, H.A.; Oseni, S.O.; Omadime, L.A. Assessment of spline functions and non-linear models for estimating growth curve parameters of Funaab-alpha chickens. Slovak J. Anim. Sci. 2020, 53, 19–31. [Google Scholar]
- Zhao, Z.; Li, S.; Huang, H.; Li, C.; Wang, Q.; Xue, L. Comparative study on growth and developmental model of indigenous chicken breeds in China. Open J. Anim. Sci. 2015, 5, 219. [Google Scholar] [CrossRef]
- Manjula, P.; Park, H.-B.; Yoo, J.; Wickramasuriya, S.; Seo, D.-W.; Choi, N.-R.; Kim, C.D.; Kang, B.-S.; Oh, K.-S.; Sohn, S.-H. Comparative study on growth patterns of 25 commercial strains of Korean native chicken. Korean J. Poult. Sci. 2016, 43, 1–14. [Google Scholar] [CrossRef]
- Goto, T.; Goto, N.; Shiraishi, J.-i.; Bungo, T.; Tsudzuki, M. Comparison of growth curves of four breeds of Japanese native chicken, Onaga-dori, Tosa-jidori, Ukokkei and Hinai-dori. J. Anim. Vet. Adv. 2010, 9, 1362–1365. [Google Scholar] [CrossRef]
- Aggrey, S. Comparison of three nonlinear and spline regression models for describing chicken growth curves. Poult. Sci. 2002, 81, 1782–1788. [Google Scholar] [CrossRef] [PubMed]
- Miguel, J.; Asenjo, B.; Ciria, J.; Calvo, J. Growth and lay modelling in a population of Castellana Negra native Spanish hens. Br. Poult. Sci. 2007, 48, 651–654. [Google Scholar] [CrossRef] [PubMed]
- Mata-Estrada, A.; González-Cerón, F.; Pro-Martínez, A.; Torres-Hernández, G.; Bautista-Ortega, J.; Becerril-Pérez, C.M.; Vargas-Galicia, A.J.; Sosa-Montes, E. Comparison of four nonlinear growth models in Creole chickens of Mexico. Poult. Sci. 2020, 99, 1995–2000. [Google Scholar] [CrossRef]
- Miguel, J.; Ciria, J.; Asenjo, B.; Calvo, J. Effect of caponisation on growth and on carcass and meat characteristics in Castellana Negra native Spanish chickens. Animal 2008, 2, 305–311. [Google Scholar] [CrossRef]
- Nthimo, A.; Neser, F.; Odenya, W.; Fair, M. A comparison of the growth performance of exotic and indigenous chickens in Lesotho. S. Afr. J. Anim. Sci. 2006, 36, 86–89. [Google Scholar]
- Osei-Amponsah, R.; Kayang, B.B.; Naazie, A.; Arthur, P.F.; Barchia, I.M. Characterisation of local Ghanaian chickens: Growth performance evaluation based on Richards growth model and genetic size scaling. Trop. Anim. Health Prod. 2011, 43, 1195–1201. [Google Scholar] [CrossRef]
- Koushandeh, A.; Chamani, M.; Yaghobfar, A.; Sadeghi, A.; Baneh, H. Comparison of the Accuracy of Nonlinear Models and Artificial Neural Network in the Performance Prediction of Ross 308 Broiler Chickens. Poult. Sci. 2019, 7, 151–161. [Google Scholar]
- Krawczyk, B. Learning from imbalanced data: Open challenges and future directions. Prog. Artif. Intell. 2016, 5, 221–232. [Google Scholar] [CrossRef]
- González Ariza, A.; Arando Arbulu, A.; León Jurado, J.M.; Navas González, F.J.; Delgado Bermejo, J.V.; Camacho Vallejo, M.E. Discriminant Canonical Tool for Differential Biometric Characterization of Multivariety Endangered Hen Breeds. Animals 2021, 11, 2211. [Google Scholar] [CrossRef]
- Spalona, A.; Ranvig, H.; Cywa-Benko, K.; Zanon, A.; Sabbioni, A.; Szalay, I.; Benková, J.; Baumgartner, J.; Szwaczkowski, T. Population size in conservation of local chicken breeds in chosen European countries. Arch. Geflügelkd. Sonderh. 2007, 71, 49–55. [Google Scholar]
- Colin Cameron, A.; Windmeijer, F.A.G. An R-squared measure of goodness of fit for some common nonlinear regression models. J. Econom. 1997, 77, 329–342. [Google Scholar] [CrossRef]
- Buchanan, R.L.; Whiting, R.C.; Damert, W.C. When is simple good enough: A comparison of the Gompertz, Baranyi, and three-phase linear models for fitting bacterial growth curves. Food Microbiol. 1997, 14, 313–326. [Google Scholar] [CrossRef]
- Emiliano, P.C.; Vivanco, M.J.F.; de Menezes, F.S. Information criteria: How do they behave in different models? Comput. Stat. Data Anal. 2014, 69, 141–153. [Google Scholar] [CrossRef]
- Yang, Y. Can the strengths of AIC and BIC be shared? A conflict between model indentification and regression estimation. Biometrika 2005, 92, 937–950. [Google Scholar] [CrossRef]
- Van Vleck, L.D. Selection Index and Introduction to Mixed Model Methods for Genetic Improvement of Animals: The Green Book; CRC Press: Boca Ratón, FL, USA, 1993. [Google Scholar]
- Afrouziyeh, M.; Kwakkel, R.P.; Zuidhof, M.J. Improving a nonlinear Gompertz growth model using bird-specific random coefficients in two heritage chicken lines. Poult. Sci. 2021, 100, 101059. [Google Scholar] [CrossRef]
- El Halimi, R. Nonlinear Mixed Effects Models and Non Parametric Inference. A Method Based on Bootstrap for the Analysis of Non-normal Repeated, Department of Statistics, Autonomus University of Barcelona. Ph.D. Thesis, Abdelmalek Essaâdi University, Tétouan, Morocco, 2005. [Google Scholar]
- Harrison, X.A.; Donaldson, L.; Correa-Cano, M.E.; Evans, J.; Fisher, D.N.; Goodwin, C.E.; Robinson, B.S.; Hodgson, D.J.; Inger, R. A brief introduction to mixed effects modelling and multi-model inference in ecology. PeerJ 2018, 6, e4794. [Google Scholar] [CrossRef]
- Gelman, A.; Hill, J. Data Analysis Using Regression and Hierarchical/Multilevel Models; Cambridge University Press: New York, NY, USA; Cambridge, UK, 2007. [Google Scholar]
- Harrison, X.A. A comparison of observation-level random effect and Beta-Binomial models for modelling overdispersion in Binomial data in ecology & evolution. PeerJ 2015, 3, e1114. [Google Scholar]
- Silk, M.J.; Harrison, X.A.; Hodgson, D.J. Perils and pitfalls of mixed-effects regression models in biology. PeerJ 2020, 8, e9522. [Google Scholar] [CrossRef]
- Chan, J.S.; Kuk, A.Y. Maximum likelihood estimation for probit-linear mixed models with correlated random effects. Biometrics 1997, 53, 86–97. [Google Scholar] [CrossRef]
- Barr, D.; Levy, R.; Scheepers, C.; Tily, H. Random effects structure for confirmatory hypothesis testing: Keep it maximal. J. Mem. Lang. 2013, 68, 197–202. [Google Scholar] [CrossRef]
- Pizarro Inostroza, M.G.; Navas González, F.J.; Landi, V.; León Jurado, J.M.; Delgado Bermejo, J.V.; Fernández Álvarez, J.; Martínez Martínez, M.d.A. Software-Automatized Individual Lactation Model Fitting, Peak and Persistence and Bayesian Criteria Comparison for Milk Yield Genetic Studies in Murciano-Granadina Goats. Mathematics 2020, 8, 1505. [Google Scholar] [CrossRef]
- Pizarro Inostroza, M.G.; Navas González, F.J.; Landi, V.; León Jurado, J.M.; Delgado Bermejo, J.V.; Fernández Álvarez, J.; Martínez Martínez, M.d.A. Goat Milk Nutritional Quality Software-Automatized Individual Curve Model Fitting, Shape Parameters Calculation and Bayesian Flexibility Criteria Comparison. Animals 2020, 10, 1693. [Google Scholar] [CrossRef]
- Kéry, M. Introduction to WinBUGS for Ecologists: Bayesian Approach to Regression, ANOVA, Mixed Models and Related Analyses; Academic Press: London, UK, 2010. [Google Scholar]
- Nogales, S.; Bressan, M.C.; Delgado, J.V.; Telo da Gama, L.; Barba, C.; Camacho, M.E. Fatty acid profile of feral cattle meat. Ital. J. Anim. Sci. 2017, 16, 172–184. [Google Scholar] [CrossRef]
- Bathaei, S.S.; Leroy, P.L. Genetic and phenotypic aspects of the growth curve characteristics in Mehraban Iranian fat-tailed sheep. Small Rumin. Res. 1998, 29, 261–269. [Google Scholar] [CrossRef]
- Arando Arbulu, A.; González Ariza, A.; Lupi, T.M.; Nogales Baena, S.; Jurado, J.M.L.; Navas González, F.J.; Delgado Bermejo, J.V.; Camacho Vallejo, M.E. Comparison of non-linear models to describe the growth in the Andalusian turkey breed. Ital. J. Anim. Sci. 2021, 20, 1156–1167. [Google Scholar] [CrossRef]
- Castillo, A.; Gariglio, M.; Franzoni, A.; Soglia, D.; Sartore, S.; Buccioni, A.; Mannelli, F.; Cassandro, M.; Cendron, F.; Castellini, C.; et al. Overview of Native Chicken Breeds in Italy: Conservation Status and Rearing Systems in Use. Animals 2021, 11, 490. [Google Scholar] [CrossRef]
- Mosca, F.; Madeddu, M.; Mangiagalli, M.G.; Colombo, E.; Cozzi, M.C.; Zaniboni, L.; Cerolini, S. Bird density, stress markers and growth performance in the Italian chicken breed Milanino. J. Appl. Poult. Res. 2015, 24, 529–535. [Google Scholar] [CrossRef]
- Guo, Y.; Ou, J.-H.; Zan, Y.; Wang, Y.; Li, H.; Zhu, C.; Chen, K.; Zhou, X.; Hu, X.; Carlborg, Ö. Researching on the fine structure and admixture of the worldwide chicken population reveal connections between populations and important events in breeding history. Evol. Appl. 2021. [Google Scholar] [CrossRef]
- Lupi, T.; Nogales, S.; León, J.; Barba, C.; Delgado, J. Characterization of commercial and biological growth curves in the Segureña sheep breed. Animal 2015, 9, 1341–1348. [Google Scholar] [CrossRef] [PubMed]
| Variable | Variable Set | Type | Levels (Maximum–Minimum) |
|---|---|---|---|
| Breed | Population | Nominal | 41 breeds |
| Variety | Nominal | 69 varieties | |
| Country | Study Georeferencing | Nominal | 16 countries |
| Continent | Nominal | Africa, Asia, Europe, America, and Australasia | |
| Growth model | Method | Nominal | 20 models (see Table 2 for model definition) |
| Number of model parameters | Numeric | 2 to 6 model parameters | |
| Male/Female sample | Study design | Numeric | 11 to 749 males/12 to 1255 females |
| Total sample | Numeric | 17 to 2004 individuals | |
| Total male/female observations | Numeric | 85 to 16,000 males/80 to 31,808 females | |
| Total observations | Numeric | 170 to 47,808 observations | |
| R2 (variance explicative potential) | Goodness of fit and flexibility criteria | Numeric | 0.01 to 1 for males/0.16 to 1 for females |
| MSE (model accuracy) | Numeric | 1443 to 37,596,433 for males/1107 to 39,687 for females | |
| RMSE (model accuracy) | Numeric | 0.03 to 128 for males and 7.17 to 106 for females | |
| RSD (deviation from the theoretical model) | Numeric | 11,47 to 197 for males/10.41 to 191 for females | |
| AIC (observative ability) | Numeric | 49.42 to 74,719 for males/44.21 to 21,142 for females | |
| BIC (predictive ability) | Numeric | 60.12 to 74,739 for males/54.15 to 94,595 for females | |
| Year of publication | Scientific impact | Ordinal | 2002 to 2020 |
| Journal | Nominal | 24 journals | |
| Indexed | Nominal | Yes, no, not at the moment of data collection | |
| Impact factor | Numeric | 0.14 to 2.217 | |
| Quartile | Ordinal | Q1, Q2, Q3, Q4 | |
| Data Base | Nominal | Not indexed, JRC, SJR, Scopus |
| Interpretation | No Effect | Effect Is Not Presumed but Can Be Detected with Additional Laboratory Techniques | Effect Is Presumed and Can Be Detected but Additional Laboratory Techniques Are Needed | Effect Can Be Detected with the Naked Eye |
|---|---|---|---|---|
| Degress of Freedom (df) | Negligible | Small | Medium | Large |
| 1 | 0.00 < 0.10 | 0.10 < 0.30 | 0.30 < 0.50 | 0.50 or more |
| 2 | 0.00 < 0.07 | 0.07 < 0.21 | 0.21 < 0.35 | 0.35 or more |
| 3 | 0.00 < 0.06 | 0.06 < 0.17 | 0.17 < 0.29 | 0.29 or more |
| 4 | 0.00 < 0.05 | 0.05 < 0.15 | 0.15 < 0.25 | 0.25 or more |
| 5 or more | 0.00 < 0.05 | 0.05 < 0.13 | 0.13 < 0.22 | 0.22 or more |
| Model | SPSS Model Syntax | References |
|---|---|---|
| Asymmetric logistic | b0/((1 + b1*EXP(-b2*t))**(1/b3)) | [26] |
| Biphasic sigmoid | b0/1 + EXP(b1*(b2-t)) + (b3/(1 + EXP(b4*(b5-t))) | [27] |
| Bridges | b0 + b1*(1-EXP(-(b2*t **b3))) | [28,29] |
| Brody | b0*(1-b1*EXP(-b2*t)) | [18,29,30] |
| Exponential | b0*(1 + b1)*t | [31] |
| Gaussian | b0*(1-b2*EXP(-b1*t**2)) | [32] |
| Gompertz | b0*EXP(-b1*EXP(-b2*t)) | [18,26,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48] |
| Gompertz–Laird | b0*EXP((b1/b2)*(1-EXP(-b2*t))) | [49,50,51] |
| Janoschek | b0-(b0-b1)*EXP(-b2*(t**b3)) | [29] |
| Linear | b0 + b1*t | [36,52] |
| Logistic | b0*(1 + EXP(-b2*t))**(-b3) | [18,26,28,29,30,31,32,33,35,36,38,40,41,42,43,44,45,46,48,50] |
| Lopez | (b0*b1*b2 + b3*t*b2)/(b1*b2 + t*b2) | [33,35] |
| Monomolecular | b0*(1-b1*EXP(-b2*t)) | [31,39] |
| Quadratic | b0 + b1*t + b2*t**2 + b3 | [52] |
| Richards | b0*(1-b1*EXP(-b2*t))**b3 | [26,28,29,30,32,33,35,36,38,39,41,43,44,48,50,53] |
| Sinusoidal | b0*(1-b1*COS(b2*t + b3)) | [32] |
| Verhulst | b0/(1 + b1*EXP(-b2*t)) | [18] |
| Von Bertalanffy | b0*(1-b1*EXP(-b2*t))**3 | [18,30,33,40,44,45,46,50] |
| Weibull | b0-(b1*(EXP(-b2*(t**b3)))) | [35] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González Ariza, A.; Arando Arbulu, A.; Navas González, F.J.; Nogales Baena, S.; Delgado Bermejo, J.V.; Camacho Vallejo, M.E. The Study of Growth and Performance in Local Chicken Breeds and Varieties: A Review of Methods and Scientific Transference. Animals 2021, 11, 2492. https://doi.org/10.3390/ani11092492
González Ariza A, Arando Arbulu A, Navas González FJ, Nogales Baena S, Delgado Bermejo JV, Camacho Vallejo ME. The Study of Growth and Performance in Local Chicken Breeds and Varieties: A Review of Methods and Scientific Transference. Animals. 2021; 11(9):2492. https://doi.org/10.3390/ani11092492
Chicago/Turabian StyleGonzález Ariza, Antonio, Ander Arando Arbulu, Francisco Javier Navas González, Sergio Nogales Baena, Juan Vicente Delgado Bermejo, and María Esperanza Camacho Vallejo. 2021. "The Study of Growth and Performance in Local Chicken Breeds and Varieties: A Review of Methods and Scientific Transference" Animals 11, no. 9: 2492. https://doi.org/10.3390/ani11092492
APA StyleGonzález Ariza, A., Arando Arbulu, A., Navas González, F. J., Nogales Baena, S., Delgado Bermejo, J. V., & Camacho Vallejo, M. E. (2021). The Study of Growth and Performance in Local Chicken Breeds and Varieties: A Review of Methods and Scientific Transference. Animals, 11(9), 2492. https://doi.org/10.3390/ani11092492






