Predictivity of Antemortem Findings on Postmortem Inspection in Italian Heavy Pigs Slaughterhouses
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Statistical Analysis
3. Results
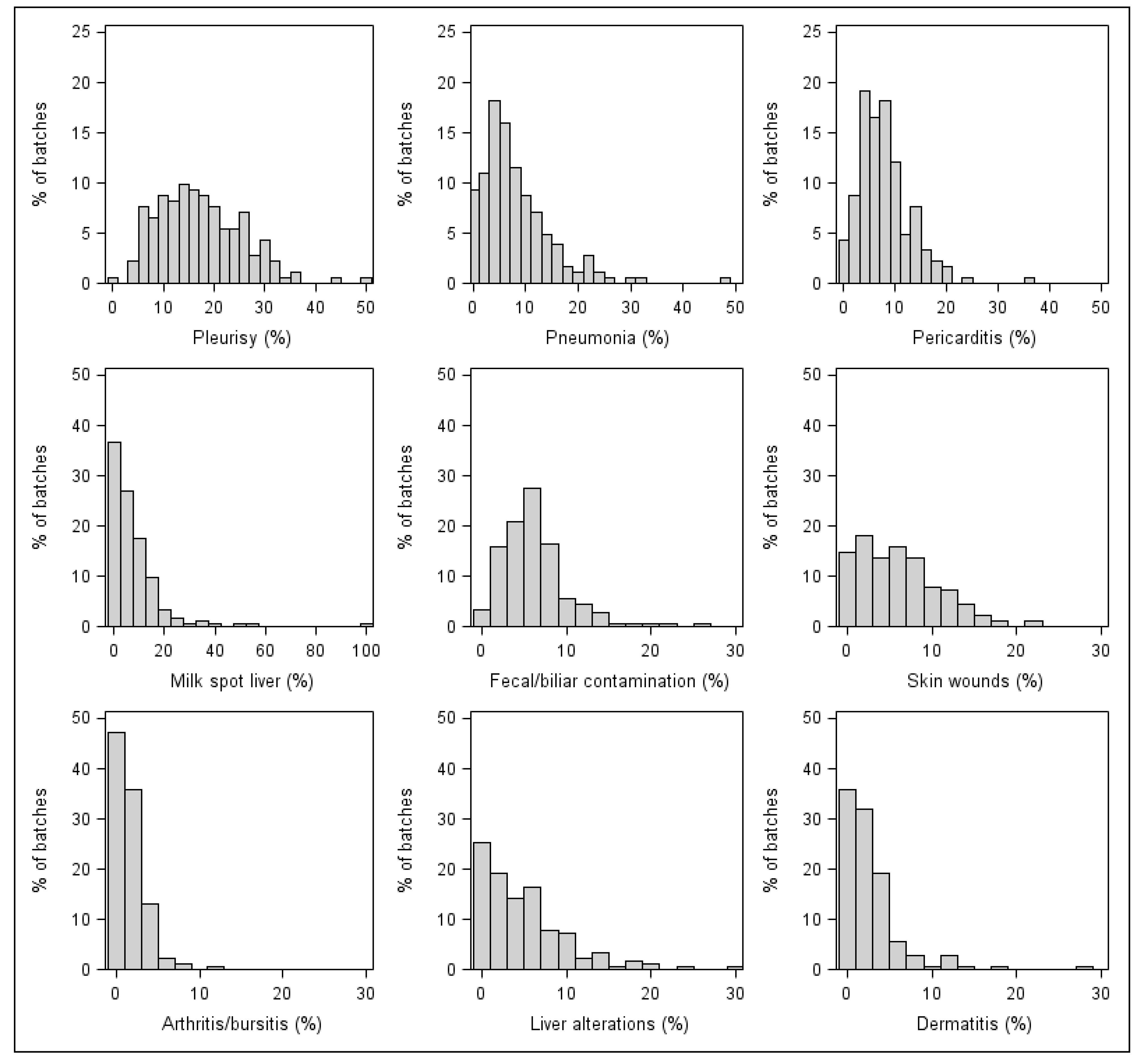
3.1. Descriptive Results
3.2. Relationship between Ante- and Postmortem Lesions
4. Discussion
4.1. Antemortem Findings
4.2. Postmortem Findings
4.3. Relationship between Ante- and Postmortem Lesions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Riess, L.E.; Hoelzer, K. Implementation of Visual-Only Swine Inspection in the European Union: Challenges, Opportunities, and Lessons Learned. J. Food Prot. 2020, 83, 1918–1928. [Google Scholar] [CrossRef]
- De Luca, S.; Zanardi, E.; Alborali, G.L.; Ianieri, A.; Ghidini, S. Abattoir-Based Measures to Assess Swine Welfare: Analysis of the Methods Adopted in European Slaughterhouses. Animals 2021, 11, 226. [Google Scholar] [CrossRef] [PubMed]
- Regulation (EU) 2017/625 of the European Parliament and of the Council of 15 March 2017 on Official Controls and other Official Activities Performed to Ensure the Application of Food and Feed Law, Rules on Animal Health and Welfare, Plant Health and Plant Protection Products, Amending Regulations (EC) No 999/2001, (EC) No 396/2005, (EC) No 1069/2009, (EC) No 1107/2009, (EU) No 1151/2012, (EU) No 652/2014, (EU) 2016/429 and (EU) 2016/2031 of the European Parliament and of the Council, Council Regulations (EC) No 1/2005 and (EC) No 1099/2009 and Council Directives 98/58/EC, 1999/74/EC, 2007/43/EC, 2008/119/EC and 2008/120/EC, and repealing Regulations (EC) No 854/2004 and (EC) No 882/2004 of the European Parliament and of the Council, Council Directives 89/608/EEC, 89/662/EEC, 90/425/EEC, 91/496/EEC, 96/23/EC, 96/93/EC and 97/78/EC and Council Decision 92/438/EEC (Official Controls Regulation) Text with EEA Relevance. 2017, Volume 095, pp. 1–142. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A02017R0625-20191214 (accessed on 30 June 2021).
- Commission Implementing Regulation (EU) 2019/627 of 15 March 2019 Laying down Uniform Practical Arrangements for the Performance of Official Controls on Products of Animal Origin Intended for Human Consumption in Accordance with Regulation (EU) 2017/625 of the European Parliament and of the Council and Amending Commission Regulation (EC) No 2074/2005 as Regards Official Controls (Text with EEA Relevance.). 2019, Volume 131. Available online: https://eur-lex.europa.eu/eli/reg_impl/2019/627/oj (accessed on 30 June 2021).
- Stärk, K.D.C.; Alonso, S.; Dadios, N.; Dupuy, C.; Ellerbroek, L.; Georgiev, M.; Hardstaff, J.; Huneau-Salaün, A.; Laugier, C.; Mateus, A.; et al. Strengths and weaknesses of meat inspection as a contribution to animal health and welfare surveillance. Food Control 2014, 39, 154–162. [Google Scholar] [CrossRef]
- Sánchez, P.; Pallarés, F.J.; Gómez, M.A.; Bernabé, A.; Gómez, S.; Seva, J. Importance of the knowledge of pathological processes for risk-based inspection in pig slaughterhouses (Study of 2002 to 2016). Asian Australas. J. Anim. Sci. 2018, 31, 1818–1827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vidal, E.; Tolosa, E.; Espinar, S.; de Val, B.P.; Nofrarías, M.; Alba, A.; Allepuz, A.; Grau-Roma, L.; López-Soria, S.; Martínez, J.; et al. Six-Year Follow-up of Slaughterhouse Surveillance (2008–2013): The Catalan Slaughterhouse Support Network (SESC). Vet. Pathol. 2016, 53, 532–544. [Google Scholar] [CrossRef]
- Nielsen, S.S.; Nielsen, G.B.; Denwood, M.J.; Haugegaard, J.; Houe, H. Comparison of recording of pericarditis and lung disorders at routine meat inspection with findings at systematic health monitoring in Danish finisher pigs. Acta Vet. Scand. 2015, 57, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kongsted, H.; Sørensen, J.T. Lesions found at routine meat inspection on finishing pigs are associated with production system. Vet. J. Lond. Engl. 1997 2017, 223, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Ghidini, S.; Zanardi, E.; Di Ciccio, P.A.; Borrello, S.; Belluzi, G.; Guizzardi, S.; Ianieri, A. Development and test of a visual-only meat inspection system for heavy pigs in Northern Italy. BMC Vet. Res. 2018, 14, 6. [Google Scholar] [CrossRef] [Green Version]
- Guardone, L.; Vitali, A.; Fratini, F.; Pardini, S.; Cenci Goga, B.T.; Nucera, D.; Armani, A. A Retrospective Study after 10 Years (2010–2019) of Meat Inspection Activity in a Domestic Swine Abattoir in Tuscany: The Slaughterhouse as an Epidemiological Observatory. Animals 2020, 10, 1907. [Google Scholar] [CrossRef]
- Ceccarelli, M.; Leprini, E.; Sechi, P.; Iulietto, M.F.; Grispoldi, L.; Goretti, E.; Cenci-Goga, B.T. Analysis of the causes of the seizure and destruction of carcasses and organs in a slaughterhouse in central Italy in the 2010–2016 period. Ital. J. Food Saf. 2018, 7, 6899. [Google Scholar] [CrossRef]
- Vecerek, V.; Voslarova, E.; Semerad, Z.; Passantino, A. The Health and Welfare of Pigs from the Perspective of Post Mortem Findings in Slaughterhouses. Animals 2020, 10, 825. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Agriculture (USDA). Food Safety and Inspection Service (FSIS) Directive 6100.2 Revision 1—Post-Mortem Livestock Inspection. p. 34. Available online: https://www.fsis.usda.gov/policy/fsis-directives/6100.2 (accessed on 30 June 2021).
- Laukkanen-Ninios, R.; Rahkila, R.; Oivanen, L.; Wirta, E.-R.; Fredriksson-Ahomaa, M. Views of veterinarians and meat inspectors concerning the practical application of visual meat inspection on domestic pigs in Finland. J. Consum. Prot. Food Saf. 2020, 15, 5–14. [Google Scholar] [CrossRef] [Green Version]
- Felin, E.; Jukola, E.; Raulo, S.; Heinonen, J.; Fredriksson-Ahomaa, M. Current food chain information provides insufficient information for modern meat inspection of pigs. Prev. Vet. Med. 2016, 127, 113–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cobanovic, N.; Jamnikar-Ciglenecki, U.; Kirbis, A.; Krizman, M.; Štukelj, M.; Vićić, I.; Karabasil, N. Coherence of Clinical Symptoms at Antemortem Inspection and Pathological Lesions at Postmortem Inspection in Slaughter Pigs. Kafkas Üniversitesi Vet. Fakültesi Derg. 2020, 26, 533–539. [Google Scholar]
- Consistenze Degli Allevamenti. Available online: http://dati.istat.it/Index.aspx?DataSetCode=DCSP_CONSISTENZE (accessed on 17 August 2021).
- ClassyFarm—Istituto Zooprofilattico Sperimentale Della Lombardia E Dell’Emilia-Romagna. Available online: http://www.classyfarm.it/ (accessed on 11 November 2020).
- Council Directive 2008/71/EC of 15 July 2008 on the Identification and Registration of Pigs (Codified Version). 2008. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32008L0071 (accessed on 30 June 2021).
- Dalmau, A.; Temple, D.; Rodriguez, P.; Llonch, P.; Velarde, A. Application of the Welfare Quality® protocol at pig slaughterhouses. Anim. Welf. 2009, 18, 497–505. [Google Scholar]
- Temple, D.; Courboulay, V.; Manteca, X.; Velarde, A.; Dalmau, A. The welfare of growing pigs in five different production systems: Assessment of feeding and housing. Animal 2012, 6, 656–667. [Google Scholar] [CrossRef]
- Scott, K.; Chennells, D.J.; Campbell, F.M.; Hunt, B.; Armstrong, D.; Taylor, L.; Gill, B.P.; Edwards, S.A. The welfare of finishing pigs in two contrasting housing systems: Fully-slatted versus straw-bedded accommodation. Livest. Sci. 2006, 103, 104–115. [Google Scholar] [CrossRef]
- Maisano, A.M.; Luini, M.; Vitale, N.; Nodari, S.R.; Scali, F.; Alborali, G.L.; Vezzoli, F. Animal-based measures on fattening heavy pigs at the slaughterhouse and the association with animal welfare at the farm level: A preliminary study. Animal 2020, 14, 108–118. [Google Scholar] [CrossRef]
- Carroll, G.A.; Boyle, L.A.; Hanlon, A.; Collins, L.; Griffin, K.; Friel, M.; Armstrong, D.; O’Connell, N.E. What can carcass-based assessments tell us about the lifetime welfare status of pigs? Livest. Sci. 2018, 214, 98–105. [Google Scholar] [CrossRef] [Green Version]
- Driessen, B.; Van Beirendonck, S.; Buyse, J. Effects of Transport and Lairage on the Skin Damage of Pig Carcasses. Animals 2020, 10, 575. [Google Scholar] [CrossRef] [Green Version]
- Driessen, B.; Van Beirendonck, S.; Buyse, J. The Impact of Grouping on Skin Lesions and Meat Quality of Pig Carcasses. Animals 2020, 10, 544. [Google Scholar] [CrossRef] [Green Version]
- Bottacini, M.; Scollo, A.; Edwards, S.A.; Contiero, B.; Veloci, M.; Pace, V.; Gottardo, F. Skin lesion monitoring at slaughter on heavy pigs (170 kg): Welfare indicators and ham defects. PLoS ONE 2018, 13, e0207115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Food Safety Authority (EFSA). Statement on the use of animal-based measures to assess the welfare of animals. EFSA J. 2012, 10, 2767. [Google Scholar]
- Teixeira, D.L.; Harley, S.; Hanlon, A.; O’Connell, N.E.; More, S.J.; Manzanilla, E.G.; Boyle, L.A. Study on the Association between Tail Lesion Score, Cold Carcass Weight, and Viscera Condemnations in Slaughter Pigs. Front. Vet. Sci. 2016, 3, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- vom Brocke, A.L.; Karnholz, C.; Madey-Rindermann, D.; Gauly, M.; Leeb, C.; Winckler, C.; Schrader, L.; Dippel, S. Tail lesions in fattening pigs: Relationships with postmortem meat inspection and influence of a tail biting management tool. Animal 2019, 13, 835–844. [Google Scholar] [CrossRef]
- Scollo, A.; Contiero, B.; Gottardo, F. Frequency of tail lesions and risk factors for tail biting in heavy pig production from weaning to 170 kg live weight. Vet. J. Lond. Engl. 1997 2016, 207, 92–98. [Google Scholar] [CrossRef]
- Valros, A.; Välimäki, E.; Nordgren, H.; Vugts, J.; Fàbrega, E.; Heinonen, M. Intact Tails as a Welfare Indicator in Finishing Pigs? Scoring of Tail Lesions and Defining Intact Tails in Undocked Pigs at the Abattoir. Front. Vet. Sci. 2020, 7, 405. [Google Scholar] [CrossRef]
- Tasse, M.E.; Molento, C.F.M.; Tasse, M.E.; Molento, C.F.M. Injury and condemnation data of pigs at slaughterhouses with federal inspection in the State of Paraná, Brazil, as indicators of welfare during transportation. Ciênc. Rural 2019, 49, 308–322. [Google Scholar] [CrossRef] [Green Version]
- Fraile, L.; Alegre, A.; López-Jiménez, R.; Nofrarías, M.; Segalés, J. Risk factors associated with pleuritis and cranio-ventral pulmonary consolidation in slaughter-aged pigs. Vet. J. 2010, 184, 326–333. [Google Scholar] [CrossRef]
- Steinmann, T.; Blaha, T.; Meemken, D. A simplified evaluation system of surface-related lung lesions of pigs for official meat inspection under industrial slaughter conditions in Germany. BMC Vet. Res. 2014, 10, 98. [Google Scholar] [CrossRef] [Green Version]
- Boes, J.; Kanora, A.; Havn, K.T.; Christiansen, S.; Vestergaard-Nielsen, K.; Jacobs, J.; Alban, L. Effect of Ascaris suum infection on performance of fattening pigs. Vet. Parasitol. 2010, 172, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Scollo, A.; Gottardo, F.; Contiero, B.; Mazzoni, C.; Leneveu, P.; Edwards, S.A. Benchmarking of pluck lesions at slaughter as a health monitoring tool for pigs slaughtered at 170kg (heavy pigs). Prev. Vet. Med. 2017, 144, 20–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carroll, G.A.; Boyle, L.A.; Teixeira, D.L.; Van Staaveren, N.; Hanlon, A.; O’Connell, N.E. Effects of Scalding and Dehairing of Pig Carcasses at Abattoirs on the Visibility of Welfare-Related Lesions. Animal 2016, 10, 460–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galuppi, R.; Avenoso, A.M.; Leotti, G.; Ostanello, F.; Poglayen, G.; Tampieri, M.P. Diagnosis of Sarcoptic Mange in Slaughtered Swine. Vet. Res. Commun. 2007, 31, 233–236. [Google Scholar] [CrossRef]
- Davies, P.R.; Bahnson, P.B.; Grass, J.J.; Marsh, W.E.; Garcia, R.; Melancon, J.; Dial, G.D. Evaluation of the monitoring of papular dermatitis lesions in slaughtered swine to assess sarcoptic mite infestation. Vet. Parasitol. 1996, 62, 143–153. [Google Scholar] [CrossRef]
- Wang, Q.; Chang, B.J.; Riley, T.V. Erysipelothrix rhusiopathiae. Vet. Microbiol. 2010, 140, 405–417. [Google Scholar] [CrossRef]
- Alban, L.; Petersen, J.V.; Busch, M.E. A comparison between lesions found during meat inspection of finishing pigs raised under organic/free-range conditions and conventional, indoor conditions. Porc. Health Manag. 2015, 1, 4. [Google Scholar] [CrossRef] [Green Version]
- Hansson, I.; Hamilton, C.; Ekman, T.; Forslund, K. Carcass quality in certified organic production compared with conventional livestock production. J. Vet. Med. B Infect. Dis. Vet. Public Health 2000, 47, 111–120. [Google Scholar] [CrossRef]
- Damriyasa, I.M.; Failing, K.; Volmer, R.; Zahner, H.; Bauer, C. Prevalence, risk factors and economic importance of infestations with Sarcoptes scabiei and Haematopinus suis in sows of pig breeding farms in Hesse, Germany. Med. Vet. Entomol. 2004, 18, 361–367. [Google Scholar] [CrossRef]
- Martínez, J.; Segalés, J.; Aduriz, G.; Atxaerandio, R.; Jaro, P.; Ortega, J.; Peris, B.; Corpa, J.M. Pathological and aetiological studies of multifocal interstitial nephritis in wasted pigs at slaughter. Res. Vet. Sci. 2006, 81, 92–98. [Google Scholar] [CrossRef]
- Baker, T.F.; McEwen, S.A.; Prescott, J.F.; Meek, A.H. The prevalence of leptospirosis and its association with multifocal interstitial nephritis in swine at slaughter. Can. J. Vet. Res. 1989, 53, 290–294. [Google Scholar] [PubMed]
- Ngugi, J.N.; Fèvre, E.M.; Mgode, G.F.; Obonyo, M.; Mhamphi, G.G.; Otieno, C.A.; Cook, E.A.J. Seroprevalence and associated risk factors of leptospirosis in slaughter pigs; a neglected public health risk, western Kenya. BMC Vet. Res. 2019, 15, 403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez-Vazquez, M.J.; Smith, R.P.; Kang, S.; Lewis, F.; Nielen, M.; Gunn, G.J.; Edwards, S.A. Identification of factors influencing the occurrence of milk spot livers in slaughtered pigs: A novel approach to understanding Ascaris suum epidemiology in British farmed pigs. Vet. Parasitol. 2010, 173, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Vial, F.; Reist, M. Evaluation of Swiss slaughterhouse data for integration in a syndromic surveillance system. BMC Vet. Res. 2014, 10, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ala-Kurikka, E.; Heinonen, M.; Mustonen, K.; Peltoniemi, O.; Raekallio, M.; Vainio, O.; Valros, A. Behavior changes associated with lameness in sows. Appl. Anim. Behav. Sci. 2017, 193, 15–20. [Google Scholar] [CrossRef] [Green Version]
- Heinonen, M.; Peltoniemi, O.; Valros, A. Impact of lameness and claw lesions in sows on welfare, health and production. Livest. Sci. 2013, 156, 2–9. [Google Scholar] [CrossRef]
- Prunier, A.; Averos, X.; Dimitrov, I.; Edwards, S.A.; Hillmann, E.; Holinger, M.; Ilieski, V.; Leming, R.; Tallet, C.; Turner, S.P.; et al. Review: Early life predisposing factors for biting in pigs. Animal 2020, 14, 570–587. [Google Scholar] [CrossRef] [Green Version]

| Condition/Lesions | Guideline | AM/PM |
|---|---|---|
| Dirt >30% | Detection of the presence of manure on the body covering 30% or more of the surface of a side. | AM |
| Skin lesions | Detection of at least one deep lesion or multiple bruises or injuries due to mismanagement during loading/unloading of a side of the body. | AM |
| Ear lesions | Detection of the presence of bruises and injuries or outcomes of wounds such missing ear parts or ear necrosis. | AM |
| Lameness | Detection of the pig being severely lame with minimum weight-bearing on the affected limb. | AM |
| Umbilical/inguinal hernia | Detection of the presence of umbilical or inguinal hernia. | AM |
| Suppressed | Detection of the pig requiring suppression in the truck or in the pen due to severe lesions or injuries. | AM |
| Dead on arrival | Detection of dead pigs during transport. | AM |
| Non-walking | Detection of the pigs being unable to walk with no weightbearing on the affected limb. | AM |
| Tail lesion | Detection of a presence of fresh blood, inflammation, infection, or missing part of tail tissue. | AM |
| Dyspnea | Detection of pigs with difficulty breathing. | AM |
| Anemia | Detection of carcasses with pale skin and mucosae. | AM |
| Cachexia | Detection of pigs with poor body condition. | AM |
| Erysipelas | Detection of typical skin lesions. | AM |
| Pleurisy | Detection of adhesions present on the carcass and/or fibrin present on the visceral layer of the pleura. | PM |
| Pneumonia | Detection of pneumonia and the outcomes of pneumonia. Detection of pneumonia when an entire lobe is involved or when the lesion involves two contralateral lobes. Lung abscesses (even one) are considered as pneumonia. | PM |
| Pericarditis | Detection of fibrin on the heart surface. | PM |
| Myocarditis | Detection of acute/chronic myocarditis. | PM |
| Milk spot liver | Detection of typical milk spot lesions. | PM |
| Liver alterations | Detection of hepatitis and outcomes of hepatitis. The presence of fibrin on the capsule should be classified as peritonitis. Detection of steatosis and liver necrosis. | PM |
| Liver cirrhosis | Record of liver affected by extended scar tissue and cirrhosis. | PM |
| Dermatitis | Detection of a thickening of the skin. Detection of lesions exceeding 50% of the body surface or less when confined to the abdominal region and chest. Detection of carcasses massively affected by bites of ectoparasites and dermatitis. | PM |
| Cryptorchidism | Detection of testicles in abdomen. | PM |
| Skin wounds | Wounds from intraspecific fights and numerous injuries that deepen into the derma, with possible infection. | PM |
| Arthritis/bursitis | Record of at least one bursa and/or inflamed joint. | PM |
| Stomach repletion | Record of not-emptied stomach. | PM |
| Nephrosis/hydronephrosis | Detection of degenerative process of the kidneys. | PM |
| Interstitial nephritis | Record of interstitial nephritis. | PM |
| Abscesses | Detection of abscesses that are not located in the lung or in the liver. Also Detection of phlegmon as abscesses. | PM |
| Enteritis/colitis | Record of thickening of the small intestine, with or without hemorrhages or necrosis. | PM |
| Splenomegaly | Detection of more than 50% of the organ affected. | PM |
| Peritonitis | Report of inflammation and/or infection of the peritoneum. | PM |
| Generalized lymphadenitis | Detection of an increased volume of lymph nodes in the carcass. | PM |
| Umbilical/inguinal hernia | Detection of umbilical or inguinal hernia. | PM |
| Intestinal ascariasis | Record of the presence of ascariasis at intestinal level. | PM |
| Jaundice | Record of the presence of generalized jaundice. | PM |
| Muscular lesion/color alteration | Detection of any change in the color or degenerative process on the carcasses. | PM |
| Tail lesion | Detection of inflammation, infection, or missing part of tail tissue. | PM |
| Gastroesophageal ulcer | Detection of hemorrhages or ulcer at gastric level. | PM |
| Boar taint | Report of carcasses with pungent and abnormal odor. | PM |
| Purulent spondylitis | Report of abscesses in the vertebral bodies. | PM |
| Neoplastic process | Record of any tumor present in the carcass regardless of size and distribution. | PM |
| Edema (pancreatic) | Detection of edema at pancreatic level. | PM |
| Erysipelas | Detection of typical skin lesions. | PM |
| Anemia | Detection of carcasses with pale skin and mucosae. | PM |
| Cachexia | Detection of carcasses with poor body condition. | PM |
| Insufficient bleeding | Record of intense congestion of the head and neck area. | PM |
| Goiter | Report of any swelling in the neck due to an enlarged thyroid gland. | PM |
| Variable | No. of Positive Batches | Within-Batch Prevalence (%) | ||
|---|---|---|---|---|
| Mean | 95% CI | Range (Min–Max) | ||
| Dirt >30% | 177 | 37.13 | 33.85–40.41 | 0–92.31 |
| Skin lesions | 167 | 9.07 | 7.83–10.32 | 0–57.78 |
| Ear lesions | 106 | 3.30 | 2.47–4.14 | 0–55.38 |
| Lameness | 52 | 0.30 | 0.22–0.39 | 0–4.23 |
| Umbilical hernia | 37 | 0.25 | 0.16–0.34 | 0–3.20 |
| Suppressed | 23 | 0.11 | 0.05–0.17 | 0–3.01 |
| Tail-biting | 19 | 0.11 | 0.07–0.16 | 0–1.54 |
| Non-walking | 19 | 0.09 | 0.05–0.14 | 0–1.54 |
| Dead on arrival | 7 | 0.04 | 0.01–0.07 | 0–2.22 |
| Dyspnea | 3 | 0.01 | 0–0.03 | 0–0.77 |
| Anemia | 0 | 0 | 0 | 0 |
| Cachexia | 0 | 0 | 0 | 0 |
| Erysipelas | 0 | 0 | 0 | 0 |
| Variable | No. of Positive Batches | Within-Batch Prevalence (%) | ||
|---|---|---|---|---|
| Mean | 95% CI | Range (Min–Max) | ||
| Pleurisy | 181 | 17.21 | 16.00–18.43 | 0–49.29 |
| Pericarditis | 177 | 7.82 | 7.03–9.30 | 0–35.23 |
| Pneumonia | 171 | 8.16 | 6.40–7.15 | 0–57.78 |
| Skin wounds | 167 | 6.03 | 5.34–6.71 | 0–21.60 |
| Milk spot liver | 151 | 7.60 | 5.97–9.23 | 0–100 |
| Liver alterations | 153 | 4.89 | 4.15–5.62 | 0–29.23 |
| Dermatitis | 133 | 2.70 | 2.18–3.21 | 0–28.52 |
| Arthritis/bursitis | 125 | 1.59 | 1.33–1.85 | 0–11.11 |
| Stomach repletion | 112 | 5.35 | 4.05–6.64 | 0–76.92 |
| Nephrosis/Hydronephrosis | 66 | 1.33 | 0.98–1.68 | 0–11.11 |
| Abscesses | 60 | 0.91 | 0.76–1.07 | 0–6.00 |
| Enteritis/colitis | 51 | 0.52 | 0.32–0.71 | 0–13.64 |
| Interstitial nephritis | 50 | 1.26 | 0.87–1.66 | 0–12.77 |
| Splenomegaly | 40 | 0.29 | 0.18–0.40 | 0–5.26 |
| Peritonitis | 16 | 0.11 | 0.03–0.18 | 0–4.44 |
| Cryptorchidism | 12 | 0.07 | 0.01–0.13 | 0–4.41 |
| Umbilical/inguinal hernia | 10 | 0.04 | 0.01–0.06 | 0–1.41 |
| Intestinal ascariasis | 9 | 0.28 | 0.04–0.52 | 0–12.41 |
| Jaundice | 5 | 0.02 | 0–0.03 | 0–0.78 |
| Muscular lesions/color alterations | 2 | 0.01 | 0–0.02 | 0–0.77 |
| Tail-biting | 2 | 0.01 | 0–0.01 | 0–0.70 |
| Gastroesophageal ulcer | 1 | <0.01 | 0–0.01 | 0–0.82 |
| Boar taint | 1 | <0.01 | 0–0.01 | 0–0.77 |
| Liver cirrhosis | 1 | <0.01 | 0–0.01 | 0–0.71 |
| Purulent spondylitis | 1 | <0.01 | 0–0.01 | 0–0.70 |
| Neoplastic processes | 1 | <0.01 | 0–0.01 | 0–0.27 |
| Edema (pancreatic) | 0 | 0 | 0 | 0 |
| Erysipelas | 0 | 0 | 0 | 0 |
| Death before stunning | 0 | 0 | 0 | 0 |
| Anemia | 0 | 0 | 0 | 0 |
| Cachexia | 0 | 0 | 0 | 0 |
| Insufficient bleeding | 0 | 0 | 0 | 0 |
| Goiter | 0 | 0 | 0 | 0 |
| Generalized lymphadenitis | 0 | 0 | 0 | 0 |
| Myocarditis | 0 | 0 | 0 | 0 |
| Response Variable | Explanatory Variables | Parameter Estimate (± SE) | χ21 | p-Value |
|---|---|---|---|---|
| Respiratory diseases | Dirt >30% | 0.65 ± 0.21 | 9.58 | 0.0020 |
| Ear lesions | 1.37 ± 0.78 | 3.04 | 0.081 | |
| Lameness | 12.5 ± 8.15 | 2.34 | 0.13 | |
| Pericarditis | Dirt >30% | 0.36 ± 0.21 | 2.87 | 0.090 |
| Skin wounds | Skin lesions | 1.31 ± 0.60 | 4.67 | 0.0308 |
| Dirt >30% | 0.51 ± 0.25 | 4.01 | 0.0453 | |
| Suppressed | −30.8 ± 20.0 | 2.38 | 0.12 | |
| Milk spot liver | Standardized herd size | −0.54 ± 0.15 | 13.54 | 0.0002 |
| Skin lesions | 2.16 ± 0.93 | 5.41 | 0.020 | |
| Dirt >30% | 0.65 ± 0.44 | 2.16 | 0.15 | |
| Liver alterations | Skin lesions | 1.40 ± 0.78 | 3.21 | 0.073 |
| Ear lesions | 2.04 ± 1.16 | 3.12 | 0.077 | |
| Dermatitis | Dirt >30% | 1.15 ± 0.39 | 8.73 | 0.0031 |
| Skin lesions | 2.96 ± 0.69 | 18.52 | 0.0002 | |
| Standardized herd size | 0.008 ± 0.11 | 0.01 | 0.94 | |
| Arthritis/bursitis | Standardized herd size | 0.13 ± 0.05 | 7.42 | 0.0064 |
| Lameness | 17.91 ± 10.98 | 2.66 | 0.10 | |
| Dirt >30% | 0.53 ± 0.35 | 2.27 | 0.13 | |
| Kidney lesions | Dirt >30% | 1.69 ± 0.54 | 9.76 | 0.0018 |
| Umbilical hernia | 26.6 ± 16.7 | 2.55 | 0.11 | |
| Abscesses | Ear lesions | 2.81 ± 0.98 | 8.25 | 0.0041 |
| Lameness | 35.18 ± 10.32 | 11.60 | 0.0007 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghidini, S.; Alborali, G.L.; De Luca, S.; Maisano, A.M.; Guadagno, F.; Conter, M.; Ianieri, A.; Zanardi, E. Predictivity of Antemortem Findings on Postmortem Inspection in Italian Heavy Pigs Slaughterhouses. Animals 2021, 11, 2470. https://doi.org/10.3390/ani11082470
Ghidini S, Alborali GL, De Luca S, Maisano AM, Guadagno F, Conter M, Ianieri A, Zanardi E. Predictivity of Antemortem Findings on Postmortem Inspection in Italian Heavy Pigs Slaughterhouses. Animals. 2021; 11(8):2470. https://doi.org/10.3390/ani11082470
Chicago/Turabian StyleGhidini, Sergio, Giovanni Loris Alborali, Silvio De Luca, Antonio Marco Maisano, Federica Guadagno, Mauro Conter, Adriana Ianieri, and Emanuela Zanardi. 2021. "Predictivity of Antemortem Findings on Postmortem Inspection in Italian Heavy Pigs Slaughterhouses" Animals 11, no. 8: 2470. https://doi.org/10.3390/ani11082470
APA StyleGhidini, S., Alborali, G. L., De Luca, S., Maisano, A. M., Guadagno, F., Conter, M., Ianieri, A., & Zanardi, E. (2021). Predictivity of Antemortem Findings on Postmortem Inspection in Italian Heavy Pigs Slaughterhouses. Animals, 11(8), 2470. https://doi.org/10.3390/ani11082470








