Effect of Tea Tree Oil on the Expression of Genes Involved in the Innate Immune System in Goat Rumen Epithelial Cells
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Cultivation of Primary Goat Rumen Epithelial Cells
2.2. Proliferative Activity Analysis
2.3. Cell Senescence Analysis
2.4. Immunofluorescence Analysis
2.5. Identification of Goat Rumen Epithelial Cells
2.6. Experimental Design and Treatment
2.7. qRT-PCR
2.8. Statistical Analysis
3. Results
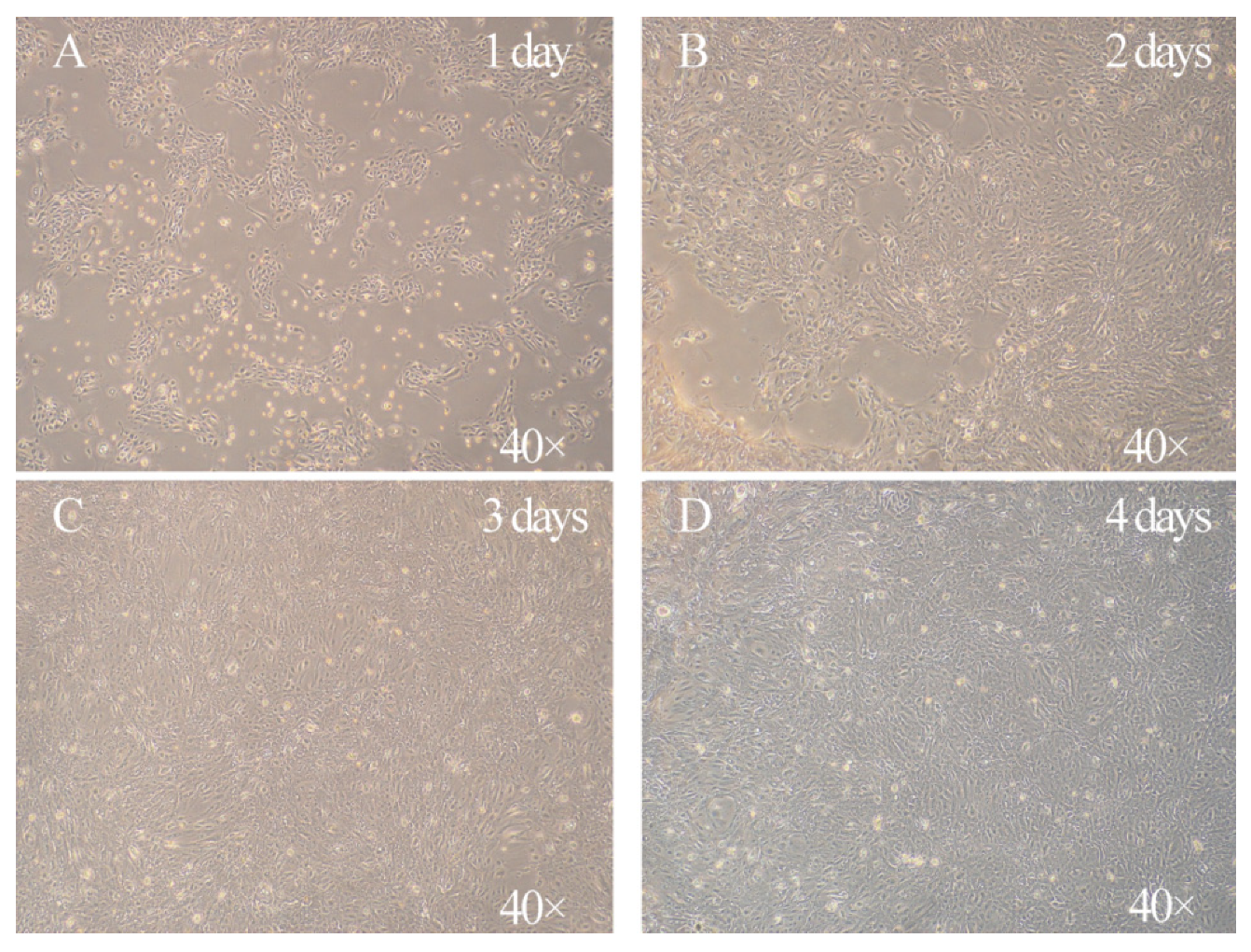
3.1. Characterization of the Morphology of Primary Goat Rumen Epithelial Cells
3.2. Characterization of Goat Rumen Epithelial Cells
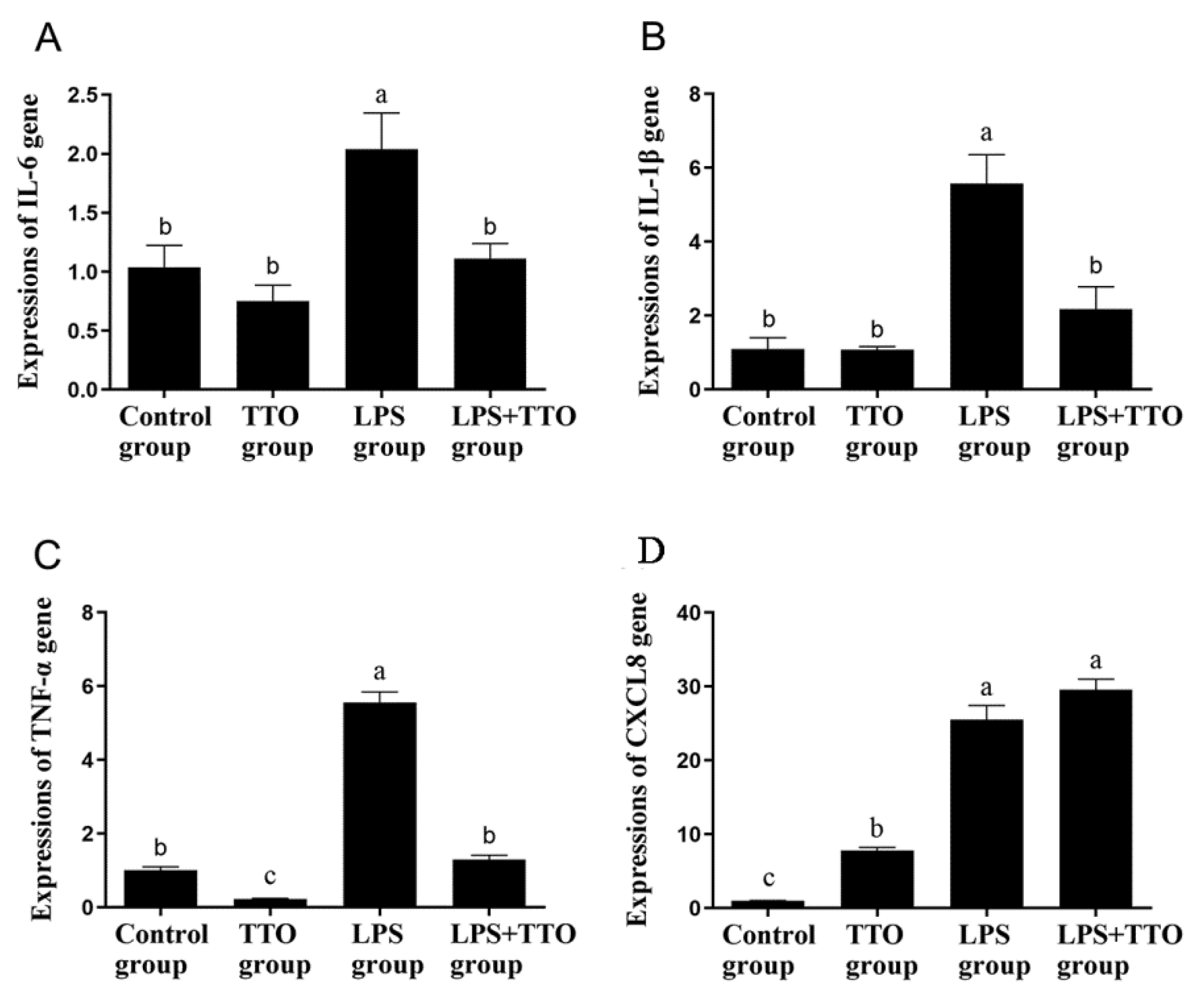
3.3. Effect of TTO on LPS-Induced Inflammatory Cytokine Expression
3.4. Effect of TTO on LPS-Induced Innate Immune Responses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Enemark, J.M. The monitoring, prevention and treatment of sub-acute ruminal acidosis (SARA): A review. Vet. J. 2008, 176, 32–43. [Google Scholar] [CrossRef]
- Mutsvangwa, T.; Wright, T. Sub-Acute Ruminal Acidosis (SARA) in Dairy Cows; Ministry of Agriculture & Food, Agriculture & Rural Division: Sejong City, Korea, 2003.
- Gozho, G.N.; Plaizier, J.C.; Krause, D.O.; Kennedy, A.D.; Wittenberg, K.M. Subacute Ruminal Acidosis Induces Ruminal Lipopolysaccharide Endotoxin Release and Triggers an Inflammatory Response. J. Dairy Sci. 2005, 88, 1399–1403. [Google Scholar] [CrossRef] [Green Version]
- Aschenbach, J.R.; Penner, G.B.; Stumpff, F.; Gäbel, G. Ruminant Nutrition Symposium: Role of fermentation acid absorption in the regulation of ruminal pH12. J. Anim. Sci. 2011, 89, 1092–1107. [Google Scholar] [CrossRef] [Green Version]
- Zebeli, Q.; Metzler-Zebeli, B.U. Interplay between rumen digestive disorders and diet-induced inflammation in dairy cattle. Res. Vet. Sci. 2012, 93, 1099–1108. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Wang, S.; Ni, Y.; Zhang, Y.; Zhuang, S.; Shen, X. High concentrate-induced subacute ruminal acidosis (SARA) increases plasma acute phase proteins (APPs) and cortisol in goats. Animal 2014, 8, 1433–1438. [Google Scholar] [CrossRef] [PubMed]
- Gozho, G.; Krause, D.; Plaizier, J. Ruminal Lipopolysaccharide Concentration and Inflammatory Response During Grain-Induced Subacute Ruminal Acidosis in Dairy Cows. J. Dairy Sci. 2007, 90, 856–866. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Zhu, W.; Mao, S. High-concentrate feeding upregulates the expression of inflammation-related genes in the ruminal epithelium of dairy cattle. J. Anim. Sci. Biotechnol. 2016, 7, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Ladner-Keay, C.; LeVatte, M.; Wishart, D.S. Role of polysaccharide and lipid in lipopolysaccharide induced prion protein conversion. Prion 2016, 10, 466–483. [Google Scholar] [CrossRef]
- Chow, J.C.; Young, D.W.; Golenbock, D.T.; Christ, W.J.; Gusovsky, F. Toll-like Receptor-4 Mediates Lipopolysaccharide-induced Signal Transduction. J. Biol. Chem. 1999, 274, 10689–10692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dziarski, R.; Wang, Q.; Miyake, K.; Kirschning, C.J.; Gupta, D. MD-2 Enables Toll-Like Receptor 2 (TLR2)-Mediated Responses to Lipopolysaccharide and Enhances TLR2-Mediated Responses to Gram-Positive and Gram-Negative Bacteria and Their Cell Wall Components. J. Immunol. 2001, 166, 1938–1944. [Google Scholar] [CrossRef] [Green Version]
- Shi, X.; Li, D.; Deng, Q.; Li, Y.; Sun, G.; Yuan, X.; Song, Y.; Wang, Z.; Li, X. NEFAs activate the oxidative stress-mediated NF-κB signaling pathway to induce inflammatory response in calf hepatocytes. J. Steroid Biochem. Mol. Biol. 2015, 145, 103–112. [Google Scholar] [CrossRef]
- Lv, S.; Li, J.; Qiu, X.; Li, W.; Zhang, C.; Zhang, Z.-N.; Luan, B. A negative feedback loop of ICER and NF-κB regulates TLR signaling in innate immune responses. Cell Death Differ. 2016, 24, 492–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nyati, K.K.; Masuda, K.; Zaman, M.M.-U.; Dubey, P.K.; Millrine, D.; Chalise, J.P.; Higa, M.; Li, S.; Standley, D.M.; Saito, K. TLR4-induced NF-κB and MAPK signaling regulate the IL-6 mRNA stabilizing protein Arid5a. Nucleic Acids Res. 2017, 45, 2687–2703. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Zhang, Q.; Chen, K.; Xu, W.; Xiang, X.; Xia, S. The regulatory effect of oxymatrine on the TLR4/MyD88/NF-κB signaling pathway in lipopolysaccharide-induced MS1 cells. Phytomedicine 2017, 36, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Lu, C.; Liu, Z.; Zhang, P.; Guo, H.; Wang, T. Schizandrin B protects LPS-induced sepsis via TLR4/NF-κB/MyD88 signaling pathway. Am. J. Transl. Res. 2018, 10, 1155. [Google Scholar] [PubMed]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Ghosh, S. Negative Regulation of Toll-like Receptor-mediated Signaling by Tollip. J. Biol. Chem. 2002, 277, 7059–7065. [Google Scholar] [CrossRef] [Green Version]
- Brissoni, B.; Agostini, L.; Kropf, M.; Martinon, F.; Swoboda, V.; Lippens, S.; Everett, H.; Aebi, N.; Janssens, S.; Meylan, E.; et al. Intracellular Trafficking of Interleukin-1 Receptor I Requires Tollip. Curr. Biol. 2006, 16, 2265–2270. [Google Scholar] [CrossRef]
- Burns, K.; Clatworthy, J.P.; Martin, L.; Martinon, F.; Plumpton, C.J.; Maschera, B.; Lewis, A.T.; Ray, K.; Tschopp, J.; Volpe, F. Tollip, a new component of the IL-1RI pathway, links IRAK to the IL-1 receptor. Nat. Cell Biol. 2000, 2, 346–351. [Google Scholar] [CrossRef]
- Subhashini, R.; Geetha, R. Action of tea tree oil and cinnamon leaf oil against oral pathogens. Asian J. Pharm. Clin. Res. 2015, 8, 80–81. [Google Scholar]
- Shemesh, A.; Mayo, W. Australian tea tree oil: A natural antiseptic and fungicidal agent. Aust. J. Pharm. 1991, 72, 802–803. [Google Scholar]
- Brophy, J.J.; Davies, N.; Southwell, I.A.; Stiff, I.A.; Williams, L.R. Gas chromatographic quality control for oil of Melaleuca terpinen-4-ol type (Australian tea tree). J. Agric. Food Chem. 1989, 37, 1330–1335. [Google Scholar] [CrossRef]
- Keszei, A.; Hassan, Y.; Foley, W.J. A Biochemical Interpretation of Terpene Chemotypes in Melaleuca alternifolia. J. Chem. Ecol. 2010, 36, 652–661. [Google Scholar] [CrossRef] [PubMed]
- Hart, P.H.; Brand, C.; Carson, C.; Riley, T.V.; Prager, R.H.; Finlay-Jones, J.J. Terpinen-4-ol, the main component of the essential oil of Melaleuca alternifolia (tea tree oil), suppresses inflammatory mediator production by activated human monocytes. Inflamm. Res. 2000, 49, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Brand, C.; Townley, S.; Finlay-Jones, J.; Hart, P. Tea tree oil reduces histamine-induced oedema in murine ears. Inflamm. Res. 2002, 51, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Brand, C.; Ferrante, A.; Prager, R.H.; Riley, T.V.; Carson, C.; Finlay-Jones, J.J.; Hart, P.H. The water-soluble components of the essential oil of Melaleuca alternifolia (tea tree oil) suppress the production of superoxide by human monocytes, but not neutrophils, activated in vitro. Inflamm. Res. 2001, 50, 213–219. [Google Scholar] [CrossRef]
- Abe, S.; Maruyama, N.; Hayama, K.; Ishibashi, H.; Inoue, S.; Oshima, H.; Yamaguchi, H. Suppression of tumor necrosis factor-alpha-induced neutrophil adherence responses by essential oils. Mediat. Inflamm. 2003, 12, 323–328. [Google Scholar] [CrossRef]
- Caldefie-Chézet, F.; Guerry, M.; Chalchat, J.-C.; Fusillier, C.; Vasson, M.-P.; Guillot, J. Anti-inflammatory Effects of Melaleuca alternifolia Essential Oil on Human Polymorphonuclear Neutrophils and Monocytes. Free. Radic. Res. 2004, 38, 805–811. [Google Scholar] [CrossRef]
- Eskan, M.A.; Rose, B.G.; Benakanakere, M.R.; Zeng, Q.; Fujioka, D.; Martin, M.H.; Lee, M.-J.; Kinane, D.F. TLR4 and S1P receptors cooperate to enhance inflammatory cytokine production in human gingival epithelial cells. Eur. J. Immunol. 2008, 38, 1138–1147. [Google Scholar] [CrossRef] [Green Version]
- de Souza, J.A.C.; Junior, C.R.; Garlet, G.; Nogueira, A.V.B.; Cirelli, J.A. Modulation of host cell signaling pathways as a therapeutic approach in periodontal disease. J. Appl. Oral Sci. 2012, 20, 128–138. [Google Scholar] [CrossRef] [Green Version]
- Nogueira, M.N.M.; de Aquino, S.; Junior, C.R.; Spolidorio, D.M.P. Terpinen-4-ol and alpha-terpineol (tea tree oil components) inhibit the production of IL-1β, IL-6 and IL-10 on human macrophages. Inflamm. Res. 2014, 63, 769–778. [Google Scholar] [CrossRef]
- Zhan, K.; Gong, X.; Chen, Y.; Jiang, M.; Yang, T.; Zhao, G. Short-Chain Fatty Acids Regulate the Immune Responses via G Protein-Coupled Receptor 41 in Bovine Rumen Epithelial Cells. Front. Immunol. 2019, 10, 2042. [Google Scholar] [CrossRef] [PubMed]
- Bulgari, O.; Dong, X.; Roca, A.L.; Caroli, A.M.; Loor, J.J. Innate immune responses induced by lipopolysaccharide and lipoteichoic acid in primary goat mammary epithelial cells. J. Anim. Sci. Biotechnol. 2017, 8, 29. [Google Scholar] [CrossRef] [PubMed]
- Bionaz, M.; Loor, J.J. Identification of reference genes for quantitative real-time PCR in the bovine mammary gland during the lactation cycle. Physiol. Genom. 2007, 29, 312–319. [Google Scholar] [CrossRef]
- Graham, C.; Simmons, N.L. Functional organization of the bovine rumen epithelium. Am. J. Physiol. Integr. Comp. Physiol. 2005, 288, R173–R181. [Google Scholar] [CrossRef] [Green Version]
- Ehmann, K.U.; DeVries, J.; Chen, M.; Adamos, A.; Guzman, R.; Omary, M. An in vitro model of epithelial cell growth stimulation in the rodent mammary gland. Cell Prolif. 2003, 36, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Vogler, S.; Martens, H. Short-chain fatty acids and CO2 as regulators of Na+ and Cl? absorption in isolated sheep rumen mucosa. J. Comp. Physiol. B 1991, 161, 419–426. [Google Scholar] [CrossRef]
- Penner, G.B.; Aschenbach, J.R.; Gäbel, G.; Rackwitz, R.; Oba, M. Epithelial Capacity for Apical Uptake of Short Chain Fatty Acids Is a Key Determinant for Intraruminal pH and the Susceptibility to Subacute Ruminal Acidosis in Sheep. J. Nutr. 2009, 139, 1714–1720. [Google Scholar] [CrossRef] [Green Version]
- Bilk, S.; Huhn, K.; Honscha, K.U.; Pfannkuche, H.; Gäbel, G. Bicarbonate exporting transporters in the ovine ruminal epithelium. J. Comp. Physiol. B 2005, 175, 365–374. [Google Scholar] [CrossRef]
- Halestrap, A.P.; Meredith, D. The SLC16 gene family? From monocarboxylate transporters (MCTs) to aromatic amino acid transporters and beyond. Pflügers Archiv 2004, 447, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Koho, N.; Taponen, J.; Tiihonen, H.; Manninen, M.; Pösö, A. Effects of age and concentrate feeding on the expression of MCT 1 and CD147 in the gastrointestinal tract of goats and Hereford finishing beef bulls. Res. Vet. Sci. 2011, 90, 301–305. [Google Scholar] [CrossRef]
- Koho, N.; Maijala, V.; Norberg, H.; Nieminen, M.; Pösö, A.R. Expression of MCT1, MCT2 and MCT4 in the rumen, small intestine and liver of reindeer (Rangifer tarandus tarandus L.). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2005, 141, 29–34. [Google Scholar] [CrossRef]
- Kirat, D.; Matsuda, Y.; Yamashiki, N.; Hayashi, H.; Kato, S. Expression, cellular localization, and functional role of monocarboxylate transporter 4 (MCT4) in the gastrointestinal tract of ruminants. Gene 2007, 391, 140–149. [Google Scholar] [CrossRef]
- Dibrov, P.; Fliegel, L. Comparative molecular analysis of Na+/H+ exchangers: A unified model for Na+/H+ antiport? FEBS Lett. 1998, 424, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Thwaites, D.T.; Anderson, C. H+-coupled nutrient, micronutrient and drug transporters in the mammalian small intestine. Exp. Physiol. 2007, 92, 603–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Etschmann, B.; Heipertz, K.S.; von der Schulenburg, A.; Schweigel, M. A vH+-ATPase is present in cultured sheep ruminal epithelial cells. Am. J. Physiol.-Gastrointest. Liver Physiol. 2006, 291, G1171–G1179. [Google Scholar] [CrossRef]
- Albrecht, E.; Kolisek, M.; Viergutz, T.; Zitnan, R.; Schweigel, M. Molecular identification, immunolocalization, and functional activity of a vacuolar-type H+-ATPase in bovine rumen epithelium. J. Comp. Physiol. B 2007, 178, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Gäbel, G.; Aschenbach, J. Ruminal SCFA Absorption: Channelling Acids without Harm. In Ruminant Physiology: Digestion, Metabolism and Impact of Nutrition on Gene Expression, Immunology and Stress; Sejrsen, K., Hvelplund, T., Nielsen, M.O., Eds.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2006; pp. 173–195. [Google Scholar]
- Khafipour, E.; Plaizier, J.; Aikman, P.; Krause, D. Population structure of rumen Escherichia coli associated with subacute ruminal acidosis (SARA) in dairy cattle. J. Dairy Sci. 2011, 94, 351–360. [Google Scholar] [CrossRef] [Green Version]
- Busquet, M.; Calsamiglia, S.; Ferret, A.; Kamel, C. Plant Extracts Affect In Vitro Rumen Microbial Fermentation. J. Dairy Sci. 2006, 89, 761–771. [Google Scholar] [CrossRef]
- Castillejos, L.; Calsamiglia, S.; Ferret, A. Effect of Essential Oil Active Compounds on Rumen Microbial Fermentation and Nutrient Flow in In Vitro Systems. J. Dairy Sci. 2006, 89, 2649–2658. [Google Scholar] [CrossRef]
- Busquet, M.; Calsamiglia, S.; Ferret, A.; Kamel, C. Screening for the effects of natural plant extracts and secondary plant metabolites on rumen microbial fermentation in continuous culture. Anim. Feed Sci. Technol. 2005, 123, 597–613. [Google Scholar] [CrossRef]
- Koh, K.; Pearce, A.; Marshman, G.; Finlay-Jones, J.; Hart, P. Tea tree oil reduces histamine-induced skin inflammation. Br. J. Dermatol. 2002, 147, 1212–1217. [Google Scholar] [CrossRef]
- Warnke, P.H.; Becker, S.T.; Podschun, R.; Sivananthan, S.; Springer, I.N.; Russo, P.A.; Wiltfang, J.; Fickenscher, H.; Sherry, E. The battle against multi-resistant strains: Renaissance of antimicrobial essential oils as a promising force to fight hospital-acquired infections. J. Cranio-Maxillofac. Surg. 2009, 37, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Zhan, K.; Yang, T.; Feng, B.; Zhu, X.; Chen, Y.; Huo, Y.; Zhao, G. The protective roles of tea tree oil extracts in bovine mammary epithelial cells and polymorphonuclear leukocytes. J. Anim. Sci. Biotechnol. 2020, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Tobias, P.S.; Mathison, J.C.; Ulevitch, R.J. A family of lipopolysaccharide binding proteins involved in responses to gram-negative sepsis. J. Biol. Chem. 1988, 263, 13479–13481. [Google Scholar] [CrossRef]
- Hailman, E.; Lichenstein, H.S.; Wurfel, M.M.; Miller, D.S.; Johnson, D.A.; Kelley, M.; Busse, L.A.; Zukowski, M.M.; Wright, S.D. Lipopolysaccharide (LPS)-binding protein accelerates the binding of LPS to CD14. J. Exp. Med. 1994, 179, 269–277. [Google Scholar] [CrossRef]
- Guha, M.; Mackman, N. LPS induction of gene expression in human monocytes. Cell. Signal. 2001, 13, 85–94. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. Toll-like receptor downstream signaling. Arthritis Res. 2005, 7, 12–19. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.; Tanaka, T.; Kishimoto, T. Therapeutic uses of anti-interleukin-6 receptor antibody. Int. Immunol. 2015, 27, 21–29. [Google Scholar] [CrossRef] [Green Version]
- Skotnicka, B.; Hassmann, E. Proinflammatory and immunoregulatory cytokines in the middle ear effusions. Int. J. Pediatr. Otorhinolaryngol. 2008, 72, 13–17. [Google Scholar] [CrossRef]
- Le, J.; Vilček, J. Interleukin 6: A Multifunctional Cytokine Regulating Immune Reactions and the Acute Phase Protein Response. Pathol. Rev. 1990 1990, 97–111. [Google Scholar] [CrossRef]
- Harada, A.; Sekido, N.; Akahoshi, T.; Wada, T.; Mukaida, N.; Matsushima, K. Essential involvement of interleukin-8 (IL-8) in acute inflammation. J. Leukoc. Biol. 1994, 56, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Cao, X. Regulation of Toll-like receptor signaling pathways in innate immune responses. Ann. N. Y. Acad. Ences 2013, 1283, 67–74. [Google Scholar]
- Rossi, D.; Zlotnik, A. The Biology of Chemokines and their Receptors. Annu. Rev. Immunol. 2000, 18, 217–242. [Google Scholar] [CrossRef]
- Fu, Q.; Chen, X.; Cui, H.; Guo, Y.; Chen, J.; Shen, N.; Bao, C. Association of elevated transcript levels of interferon-inducible chemokines with disease activity and organ damage in systemic lupus erythematosus patients. Arthritis Res. Ther. 2008, 10, R112. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Gene | Primer Sequence, 5′ to 3′ | Accession Number | Product Size (bp) |
|---|---|---|---|
| MCT4 | F: GTTTGGGATAGGCTACAGTGACACA | NM_001109980.1 | 106 |
| R: GCAGCCAAAGCGATTCACA | |||
| NHE1 | F: CCTCTACAGCTACATGGCCTAC | Etschmann et al. (2006) | 113 |
| R: GGGAGATGTTGGCTTCCA | |||
| PAT1 | F: CCTTGAGGCACGGCTAC | BC123616.1 | 123 |
| R: GCACCAGACTCCGAGACATA | |||
| AE2 | F: AGCAGCAACAACCTGGAGT | NM_001205664.1 | 123 |
| R: GGTGAAACGGGAGACGAA | |||
| vH+ ATPas | F: TTTTATTGAACAAGAAGCCAATGA | Albrecht et al. (2008) | 182 |
| R: GATTCATCAAATTGGACATCTGAA | |||
| IL-1β | F: GAAGAGCTGCACCCAACA | XM_013967700.2 | 172 |
| R: CAGGTCATCATCACGGAAG | |||
| IL-6 | F: AGATATACCTGGACTTCCT | NM_001285640.1 | 80 |
| R: TGTTCTGATACTGCTCTG | |||
| TNF-α | F: TGGTTCAGACACTCAGGT | NM_001286442.1 | 75 |
| R: CGCTGATGTTGGCTACAA | |||
| CXCL8 | F: TGTGTGAAGCTGCAGTTCTGT | XM_005681749 | 186 |
| R: TGGGGTCTAAGCACACCTCT | |||
| CCL5 | F: GTCTGCCTCCCCATATGCCTC | XM_005693201 | 187 |
| R: CTCTCGCACCCACTTCTTCTC | |||
| CXCL6 | F: CCAAGGTGGAAGTGGTAGCC | XM_005681937 | 149 |
| R: CTGGGCAATTCTTCCAACGC | |||
| TLR-2 | F: TTGACAAGAAGGCCATCCCC | NM_001285603 | 105 |
| R: AGAACGCTTCCTGCTGAGTC | |||
| TLR-4 | F: TTCAACCGTATCACGGCCTC | NM_001285574 | 127 |
| R: TGACCCACTGCAGGAAACTC | |||
| MyD88 | F: TTGAGAAGAGGTGCCGTCG | XM_013973392 | 187 |
| R: CAGACAGTGATGAAGCGCAG | |||
| Tollip | F: CGACGTAGGCTTAGCGTGAA | XM_013976999 | 142 |
| R: CTGGTCTCACGCATCTACCG | |||
| IRF3 | F: TTGTGAACTCAGGGGTCAGG | XM_013971473 | 125 |
| R: TGGGCTCAAGTCCATGTCAC | |||
| IFIT3 | F: AAATTCTGAGGCAGGCCGTT | XM_005698196 | 127 |
| R: TTTCCCAGAGCCTCGACAAC | |||
| NF-κB | F: CTGGAAGCACGAATGACAGA | XM_005681365 | 197 |
| R: GCTGTAAACATGAGCCGTACC | |||
| GAPDH | F: CAAAGTGGACATCGTTGCCA | XM_005681365 | 197 |
| R: TGGAAGATGGTGATGGCCTT |
| Symbol | Treatment 1 | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| Control | TTO | LPS | TTO + LPS | |||
| TLR-2 | 1.00 b | 0.70 c | 3.15 a | 1.84 b | 0.30 | <0.001 |
| TLR-4 | 1.00 b | 1.09 a,b | 0.92 b | 1.44 a | 0.07 | 0.018 |
| CCL5 | 1.02 | 0.94 | 1.78 | 1.55 | 0.15 | 0.105 |
| MYD88 | 1.00 | 0.78 | 0.83 | 1.24 | 0.80 | 0.178 |
| TOLLIP | 1.01 | 0.64 | 0.84 | 0.98 | 0.06 | 0.049 |
| IRF3 | 1.00 b | 1.19 b | 1.15 b | 1.45 a | 0.05 | 0.001 |
| NF-κB | 1.01 b | 0.73 b | 1.82 a | 1.67 a | 0.14 | <0.001 |
| IFIT3 | 1.01 a | 0.62 b | 1.20 a | 0.43 b | 0.10 | 0.002 |
| CXCL6 | 1.00 c | 0.76 c | 12.31 a | 4.28 b | 1.44 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Z.; Lin, M.; Ma, X.; Zhao, G.; Zhan, K. Effect of Tea Tree Oil on the Expression of Genes Involved in the Innate Immune System in Goat Rumen Epithelial Cells. Animals 2021, 11, 2460. https://doi.org/10.3390/ani11082460
Hu Z, Lin M, Ma X, Zhao G, Zhan K. Effect of Tea Tree Oil on the Expression of Genes Involved in the Innate Immune System in Goat Rumen Epithelial Cells. Animals. 2021; 11(8):2460. https://doi.org/10.3390/ani11082460
Chicago/Turabian StyleHu, Zixuan, Miao Lin, Xiaoyu Ma, Guoqi Zhao, and Kang Zhan. 2021. "Effect of Tea Tree Oil on the Expression of Genes Involved in the Innate Immune System in Goat Rumen Epithelial Cells" Animals 11, no. 8: 2460. https://doi.org/10.3390/ani11082460
APA StyleHu, Z., Lin, M., Ma, X., Zhao, G., & Zhan, K. (2021). Effect of Tea Tree Oil on the Expression of Genes Involved in the Innate Immune System in Goat Rumen Epithelial Cells. Animals, 11(8), 2460. https://doi.org/10.3390/ani11082460





