On the Helminth Fauna of the Muskrat (Ondatra zibethicus (Linnaeus, 1766)) in the Barnim District of Brandenburg State/Germany
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Skyriene, G.; Paulauskas, A. Distribution of invasive muskrats (Ondatra zibethicus) and impact on ecosystems. Ekologija 2012, 58, 357–367. [Google Scholar] [CrossRef] [Green Version]
- Ulbrich, J. The Muskrat: Biology, Distribution in Europe, Economic Significance and Control; C. Heinrich: Dresden, Germany, 1930; 137p. [Google Scholar]
- Serkowa, O.P. Parasite fauna of the muskrat acclimatized in the Kalelo-Finn SSR. Parasitol. Sbornik Zool. Inst. Akad. Nauk SSSR 1948, 10, 189–192. (In Russian) [Google Scholar]
- Dobrowolski, A.W. Parasites of the muskrat. Zool. J. 1952, 31, 640–642. (In Russian) [Google Scholar]
- DAISI. European Invasive Species Gateway. 2011. Available online: http://europe-alians.org/ (accessed on 16 May 2021).
- Bern Convention on the Conservation of European Wildlife and Habitats. Recommendation No 77(1999) on the Eradication of Non-Native Vertebrates. Available online: http://rm.coe.int/09000016807466df (accessed on 16 May 2021).
- Tackmann, K.; Löschner, U.; Mix, H.; Staubach, C.; Thulke, H.-H.; Conraths, F.-J. Spatial distribution patterns of Echinococcus multilocularis (Leuckart 1863) (Cestoda: Cyclophyllidea: Taeniidae) among red foxes in an endemic focus in Brandenburg, Germany. Epidemiol. Infect. 1998, 120, 101–109. [Google Scholar] [CrossRef]
- Abuladze, K.I. Taeniata—Cestods of animals and man and diseases caused by them. In Essentials of Cestodology; Skrabin, K.I., Ed.; Nauka: Moskva, Russia, 1964; p. 530. (In Russian) [Google Scholar]
- Ganoe, L.S.; Brown, J.D.; Yabsley, M.J.; Lovallo, M.J.; Walter, W.D. A review of pathogens, diseases, and contaminants of muskrats (Ondatra zibethicus) in North America. Front. Vet. Sci. 2020, 7, 233. [Google Scholar] [CrossRef]
- Müller, G. The muskrat in Saxony-Anhalt as intermediate host for cestodes. Zool. Garten Leipzig. N.F. 1952, 19, 42–44. (In German) [Google Scholar]
- Schuster, R. Contributions to the parasite fauna of the GDR. 8th communication: On the helminth fauna of Ondatra zibethica. Angew. Parasitol. 1987, 28, 21–25. (In German) [Google Scholar] [PubMed]
- Thiess, A.; Schuster, R.; Nöckler, K.; Mix, H. Helminth findings inindigenous raccoon dogs, Nycteroides procyonoides (Gray, 1834). Berl. Münch. Tierärztl. Wschr. 2001, 114, 273–276. (In German) [Google Scholar]
- Schwarz, S.; Sutor, A.; Staubach, C.; Mattis, R.; Tackmann, K.; Conraths, F.J. Estimated prevalence of Echinococcus multilocularis in raccoon dogs Nyctereutes procyonoides in northern Brandenburg, Germany. Curr. Zool. 2011, 57, 655–661. [Google Scholar] [CrossRef]
- Schuster, R.K.; Shimalov, V.V. A comparative study of helminths of raccoon dogs (Nyctereutes procynoides) and red foxes (Vulpes vulpes) sharing the same territory. Asian Pac. J. Trop. Dis. 2017, 7, 705–711. [Google Scholar] [CrossRef]
- Frank, B.; Zeyhle, E. Echinococcus and other tapeworm larvae in muskrat (Ondatra zibethicus). Nachrichtenbl. Dtsch. Pflanzenschutzd. 1981, 33, 166–170. (In German) [Google Scholar]
- Zeyhle, E.; Abel, M.; Frank, W. Epidemiological invstigations on the occurrence of Echinococcus multilocularis in final and intermediate hosts in the Federal Republic of Germany. Mitt. Österr. Ges. Tropenmed. Parasitol. 1990, 12, 221–232. (In German) [Google Scholar]
- Ewald, D. Distribution of the tapeworm Echinococcus multilocularis in the fox Vulpes vulpes and muskrat Ondatra zibethicus in the Freiburg administrative district. Mitt. Bad. Landesver. Naturkd. Naturschutz. 1990, 15, 81–100. (In German) [Google Scholar]
- Baumeister, S.; Pohlmeyer, K.; Kuschfeldt, S.; Stoye, M. On the prevalence of Echinococcus multilocularis and other metacestodes and cestodes of the muskrat (Ondrata zibethicus LINK 1795) in Niedersachsen. Dtsch. tierärztl. Wschr. 1997, 104, 448–452. (In German) [Google Scholar]
- Hartel, K.S.; Spittler, H.; Doering, H.; Winkelmann, J.; Hoerauf, A.; Reiter-Owona, I. The function of wild nutria (Myocastor coypus) as intermediate hosts for Echinococcus multilocularis in comparison to wild muskrats (Ondatra zibethicus). Int. J. Med. Microbiol. 2004, 293, 62–63. [Google Scholar] [CrossRef] [Green Version]
- Schichowski, H.D. Investigations on the occurrence of finned stadia of Echinococcus multilocularis in muskrats (Ondatra zibethicus) in the district of Arnsberg North-Rhine). Z. Jagdwissenschaft 2002, 48, 119–124. (In German) [Google Scholar] [CrossRef]
- Boussinesq, M.; Bresson, S.; Liance, M.; Houin, R. A new natural intermediate host of Echinococcus multilocularis in France: The muskrat (Ondatra zibethicus L.). Ann. Parasitol. Hum. Comp. 1986, 61, 431–434. (In French) [Google Scholar] [CrossRef] [Green Version]
- Umhang, G.; Richomme, C.; Boucher, J.M.; Guedon, G.; Boué, F. Nutrias and muskrats as bioindicators for the presence of Echinococcus multilocularis in new endemic areas. Vet. Parasitol. 2013, 197, 283–287. [Google Scholar] [CrossRef]
- Hanosset, R.; Saegerman, C.; Adant, S.; Massart, L.; Losson, B. Echinococcus multilocularis in Belgium: Prevalence in red foxes (Vulpes vulpes) and in different species of potential intermediate hosts. Vet. Parasitol. 2008, 151, 212–217. [Google Scholar] [CrossRef]
- Mathy, A.; Hanosset, R.; Adant, S.; Losson, B. The carriage of larval Echinococcus multilocularis and other cestodes by the musk rat (Ondatra zibethicus) along the Ourthe river and its tributaries (Belgium). J. Wildl. Dis. 2009, 45, 279–287. [Google Scholar] [CrossRef] [Green Version]
- Borgsteede, F.H.M.; Tibben, J.H.; van der Giessen, J.W.B. The musk rat (Ondatra zibethicus) as intermediate host of cestodes in the Netherlands. Vet. Parasitol. 2003, 117, 29–36. [Google Scholar] [CrossRef]
- Sprehn, C. On diseases of German fur animals based on own research in 1929. Mitt. Reichszentr. Pelzt. Rauchw. Leipzig. 1930, 2, 61–69. (In German) [Google Scholar]
- Gässlein, H. Cestodes of vertebrates in the surroundings of Erlangen. Z. Parasitenk. 1954, 16, 443–468. (In German) [Google Scholar]
- Eble, H. Infection of muskrats with Cysticercus fasciolaris in the territory of the GDR. Wiss. Z. Univ. Halle 1957, 4, 159–166. (In German) [Google Scholar]
- Schuster, R. Cysticercus fasciolaris in the liver of a muskrat (Ondatra zibethica). Angew. Parasitol. 1982, 23, 223–227. (In German) [Google Scholar]
- Friedland, T.; Steiner, B.; Boeckeler, W. Prevalence of cysticercosis in muskrats Ondatra zibethica in Schleswig-Holstein west Germany. Z. Jagdwissenschaft 1985, 31, 134–139. (In German) [Google Scholar]
- Schuster, R.; Kaufmann, A.; Hering, S. Investigations on the endoparasite fauna of domestic cats in eastern Brandenburg. Berl. Münch. Tierärztl. Wschr. 1997, 110, 48–50. (In German) [Google Scholar]
- Schmidt, S. Examination of Wild Mice in Germany for the Occurrence of Capillaria hepatica and Cyclophyllidea. Ph.D. Thesis, University of Leipzig, Leipzig, Germany, 2001. [Google Scholar]
- Kozlov, D.P. Key to the Helminths of Carnivorous Mammals of the USSR; Nauka: Moskva, Russia, 1977; 274p. [Google Scholar]
- Schuster, R.; Heidecke, D. Helminth findings in Castor fiber albicus MATSCHIE, 1907. Semiaquatische Säugetiere Wiss. Beitr. Univ. Halle 1992, 207–213. (In German) [Google Scholar]
- Schierhorn, K.; Stubbe, M.; Schuster, R.; Heidecke, D. Helminth fauna of the otter (Lutra lutra L., 1758). In Habitat 6; Reuter, C., Röchert, R., Eds.; GN—Gruppe Naturschutz.: Hamkensbüttel, Germany, 1991; pp. 133–142. [Google Scholar]
- Schuster, R.; Benitz, R. On the development of Taenia martis (Zeder, 1803) in the intermediate host. Helminthologia 1992, 29, 13–18. [Google Scholar]
- Kinsella, J.M. Growth, development, and intraspecific variation of Quinqueserialis quinqueseruialis (Trematoda: Notocotylidae) in rodent hosts. J. Parasitol. 1971, 57, 62–70. [Google Scholar] [CrossRef]
- Seegers, G.; Baumeister, S.; Kuschfeldt, S.; Pohlmeyer, K.; Stoye, M. Nematode and trematode fauna of the muskrat (Ondatra zibethicus LINK, 1795) in Lower Saxony. Dtsch. Tierärztl. Wochenschr. 1997, 104, 503–504. (In German) [Google Scholar]
- Schuster, R. Echinostoma echinatum, Notocotylus noyeri and Quinqueserialis quinqueserialis as rare parasites of Rattus norvegicus. Angew. Parasitol. 1986, 27, 221–225. (In German) [Google Scholar]
- Mühling, P. The helminth fauna of vertebrates of Eastern Prussia. Arch. Naturgesch. 1888, 21, 1–118. (In German) [Google Scholar]
- Luv, B. The life cycle of Psilotrema simillimum (Mühling, 1898) (Trematoda: Psillostomidae). Parazitologija 1978, 12, 62–67. (In Russian) [Google Scholar]
- Sonin, M.D. Trematods of Fish-Eating Birds of the Palearctic (Opisthorchiidae, Renicolidae, Strigeidae); Nauka: Moskva, Russia, 1985; 214p. (In Russian) [Google Scholar]
- Ryzikov, K.I.; Gvozdev, E.V.; Tokobaev, M.M.; Shaldyrin, L.C.; Macaberidze, G.V.; Merkusheva, I.V.; Nadtocij, E.V.; Chochlova, I.G.; Sharpilo, L.D. Key to the Helminths of the Fauna of the USSR. Cestods and Trematods; Nauka: Moskva, Russia, 1978; 229p. (In Russian) [Google Scholar]
- Hering-Hagenbeck, S.; Schuster, R. A focus of opisthorchiidosis in Germany. Appl. Parasitol. 1996, 37, 260–265. [Google Scholar]
- Grabda, J. Les parasites internes du rat musque—Ondatra zibethica (L.) des environments de Bydgoszcz (Pologne). Acta Parasit. Pol. 1954, 5, 17–36. [Google Scholar]
- Sey, O. Modification of the American muskrat’s (Ondatra zibethica L.) parasite fauna due to acclimatization. Acta Zool. Acad. Sci. Hung. 1967, 8, 409–416. [Google Scholar]
- Matskasi, I. The trematode fauna of rodents and insectivore (Mammalia) in Hungary. 3. The occurrence of Psilotrema simillimum and P. spiculigerum (Mühling, 1898) (=P. marki Skvorcov, 1934 syn. n.) in rodents. Parasit. Hung. 1974, 7, 99–109. [Google Scholar]
- Sharpilo, V.P. Parasitic Worms of Reptiles of the USSR Fauna; Nauka: Kiev, Russia, 1976; 287p. (In Russian) [Google Scholar]
- Styczynska-Jurewicz, E. The life cycle of Plagiorchis elegans (Rud., 1802) and the revision of the genus Plagiorchis Lühe, 1889. Acta Parasitol. Polonica 1962, 10, 419–445. [Google Scholar]
- Krasnolobova, T.A. Biological peculiarities of trematodes of the genus Plagiorchis. Development of P. laricola in the final host (part 2). Tr. Gel’mintol. Lab. 1971, 22, 92–118. (In Russian) [Google Scholar]
- Krasnolobova, T.A. Concept of the systematics of the trematode genus Plagiorchis Lühe, 1899. Tr. Gel’mintol. Lab. 1977, 27, 65–110. (In Russian) [Google Scholar]
- Krasnolobova, T.A. On the development of Plagiorchis elegans Lühe, 1899 under conditions of the Volga delta. Tr. Gel’mintol. Lab. 1979, 29, 75–80. (In Russian) [Google Scholar]
- Gormann, A.M. Studies on the Biology of. Plagiorchis Elegans (Rudolphi, 1802), (Trematoda: Digenea) in Its Mammalian and Molluscan-Hosts. Ph.D. Thesis, University of Leeds, Leeds, UK, 1980. [Google Scholar]
- Bock, D. The life cycle of Plagiorchis spec. 1, a species of the Plagiorchis elegans group (Trematoda, Plagiorchiidae). Parasitol. Res. 1984, 70, 359–371. [Google Scholar] [CrossRef]
- Krasnolobova, T.A. Trematodes of the USSR. Genus Plagiorchis; Nauka: Moskva, Russia, 1987; 164p. (In Russian) [Google Scholar]
- Simon-Vicente, F.; Mascoma, S.; Lopez-Roman, R.; Tenora, F.; Gallego, J. Review of Notocotylus species (Trematoda, Notocotylidae) parasitizing rodents in Europe. Folia Parasitol. 1985, 32, 21–33. [Google Scholar]
- Filimonova, L.V. Trematodes of the USSR. Notocotylids; Nauka: Moskva, Russia, 1985; 127p. (In Russian) [Google Scholar]
- Odening, K. Physids and planorbids as hosts in the life cycles of indigenous Notocotylidae (Trematoda: Paramphistomida). Z. Parasitenkd. 1966, 27, 210–239. (In German) [Google Scholar] [CrossRef]
- Chai, J.-Y.; Cho, J.; Chang, T.; Jung, B.-K.; Sohn, W.-M. Taxonomy of Echinostoma revolutum and 37-collar spined Echinostoma spp.: A Historical Review. Korean J. Parasitol. 2020, 58, 343–371. [Google Scholar] [CrossRef]
- Kanev, I.; Fried, B.; Dimitrov, V.; Radev, V. Redescription of Echinostoma trivolvis (Cort, 1914) (Trematoda: Echinostomatidae) with a discussion on its identity. Syst. Parasitol. 1995, 32, 61–70. [Google Scholar] [CrossRef]
- Detwiler, J.T.; Zajac, A.M.; Minchella, D.J.; Belden, L.K. Revealing cryptic parasite diversity in a definitive host: Echinostoma in muskrats. J. Parasitol. 2012, 98, 1148–1155. [Google Scholar] [CrossRef] [PubMed]
- Kanev, I. On mprphology, BIOLOGY, Ecology and Taxonomy of Species of the “Revolutum” Group (Trematoda: Echinostomatidae: Echinostoma. Ph.D. Thesis, B’lgarska Akademia na Naukite, Plovdiv, Bulgaria, 1985; 467p. (In Bulgarian). [Google Scholar]
- Konstadinova, A. Echinostoma echinatum (Zeder, 1803) sensu Kanev (Digenea: Echinostomatidae): A note of caution. Syst. Parasitol. 1995, 32, 23–26. [Google Scholar] [CrossRef]
- Shusteev, M.M. Parasite fauna of the upper Bora. Parazitologiya 1977, 11, 538–540. (In Russian) [Google Scholar]
- Schuster, R.; Bonin, J.; Staubach, C.; Heidrich, R. Liver fluke (Opisthorchiidae) findings in red foxes (Vulpes vulpes) in the eastern part of the Federal State Brandenburg, Germany--a contribution to the epidemiology of opisthorchiidosis. Parasitol, Res. 1999, 85, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Schuster, R.; Bonin, J.; Staubach, C.; Nitschke, B. On the distribution of opisthorchiid liverflukes in red foxes (Vulpes vulpes) in western Brandenburg. Berl. Münch. Tierärztl. Wschr. 2000, 113, 407–411. [Google Scholar]
- Schuster, R.; Wanjek, C.; Bartnik, C.; Wittstatt, U.; Baumann, M.; Schein, E. Liverfluke infection and sarcoptic mange in red foxes in Berlin. Berl. Münch. Tierärztl. Wschr. 2001, 114, 193–196. [Google Scholar]
- Schuster, R.K.; Heidrich, J.; Pauly, A.; Nöckler, K. Liver flukes in dogs and treatment with praziquantel. Vet. Parasitol. 2007, 150, 326–365. [Google Scholar] [CrossRef] [PubMed]
- Schuster, R.; Dell, K.; Wanjek, C.; Nöckler, K. Morphological features of opisthorchiid metacercariae infective for man. In Proceedings of the 4th World Congress Foodborne Infections and Intoxications, Berlin, Germany, 7–12 June 1998; Nöckler, K., Teufel, P., Schmidt, K., Weise, E., Eds.; Federal Institute for Health Protection of Consumers and Veterinary Medicine: Berlin, Germany, 1998; pp. 802–806. [Google Scholar]
- Ryzikov, K.i.; Gvozdev, E.V.; Tokobaev, M.M.; Shaldybin, L.C.; Macaberidze, G.V.; Merkusheva, I.V.; Nadtocij, E.V.; Chochlova, I.G.; Sharpilo, L.D. Key to the Helminths of the Fauna of the USSR. Nematods and Acanthocephalans; Nauka: Moskva, Russia, 1979; 272p. (In Russian) [Google Scholar]
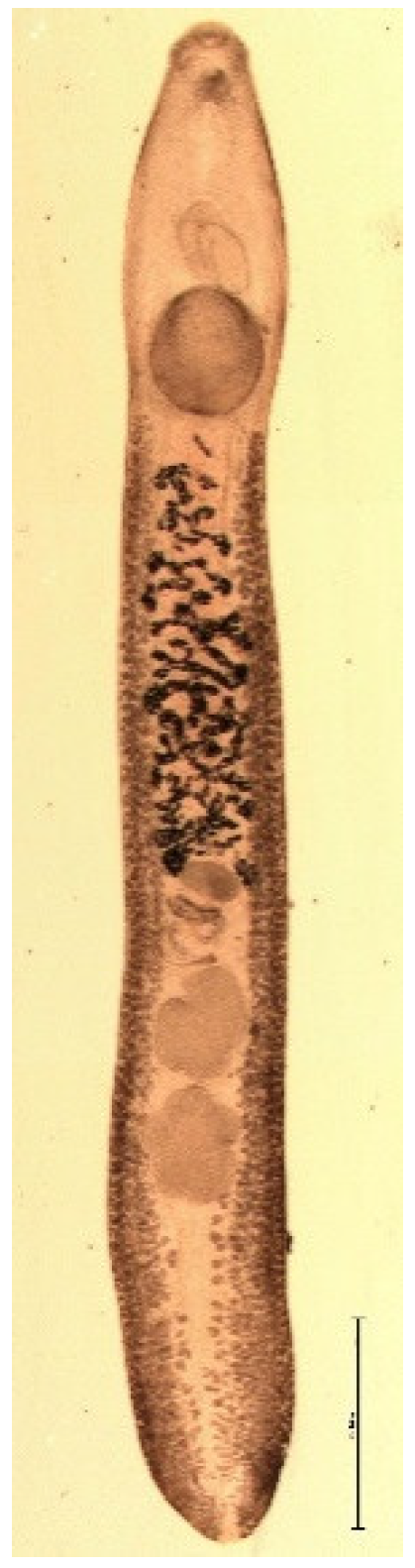
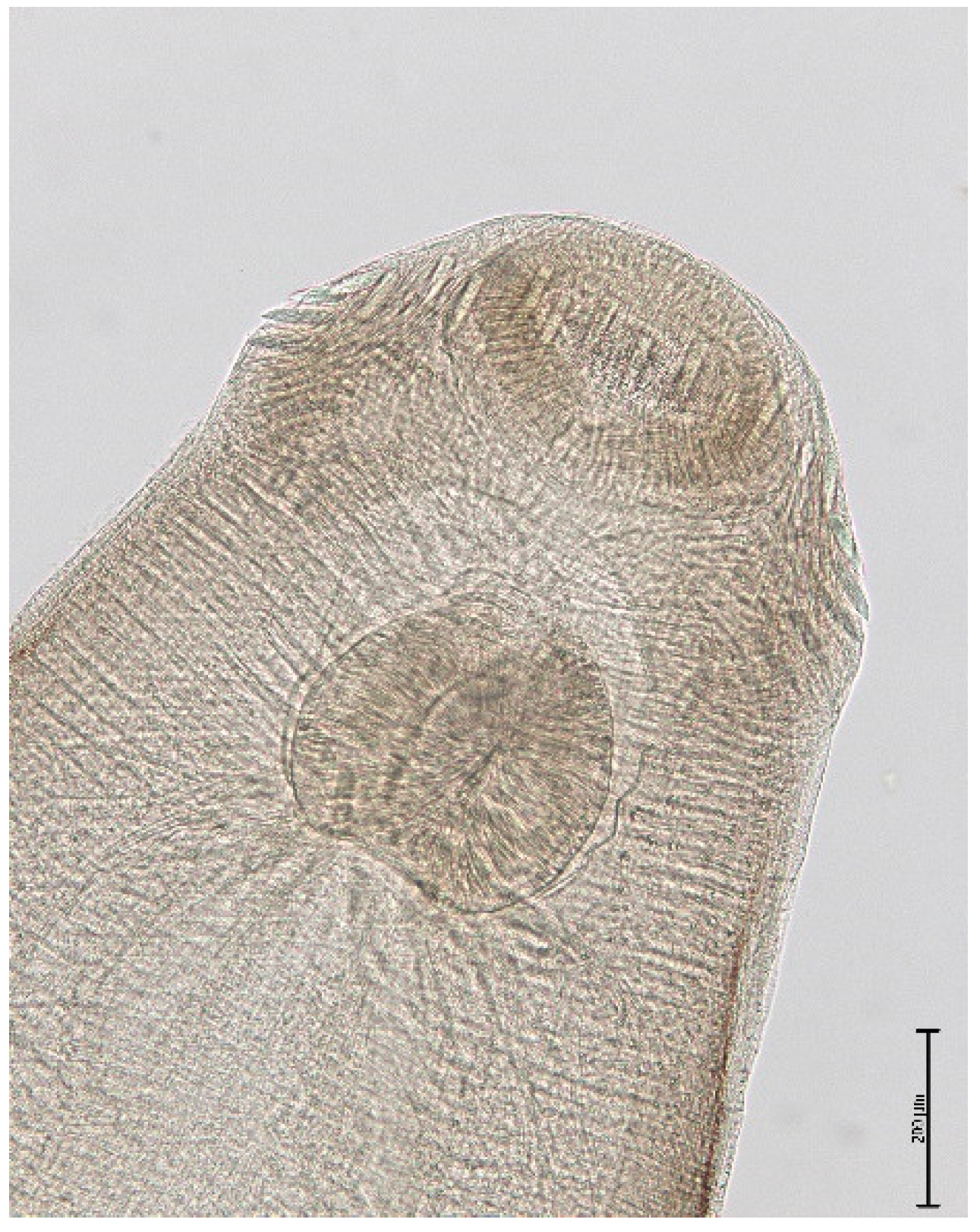
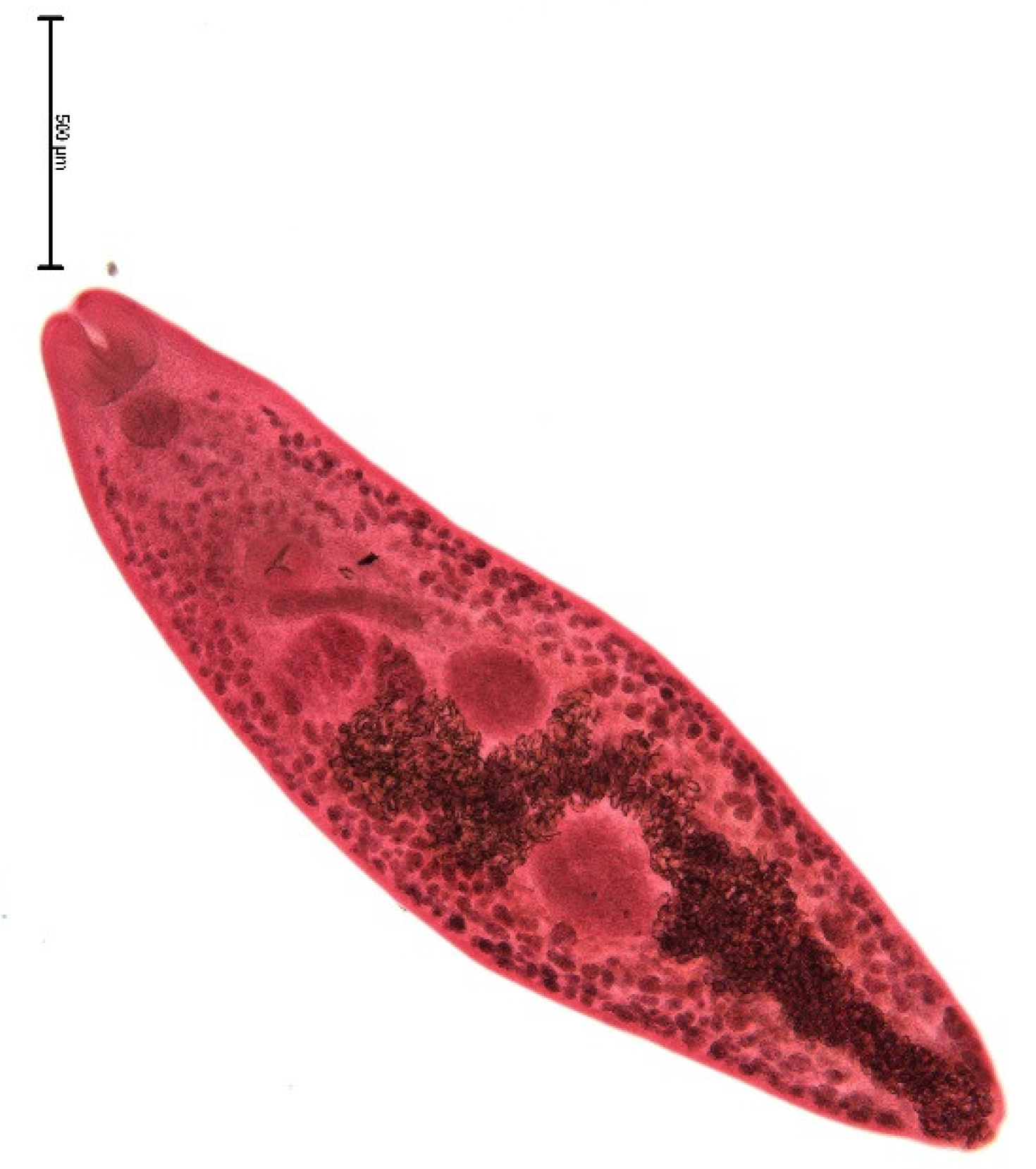

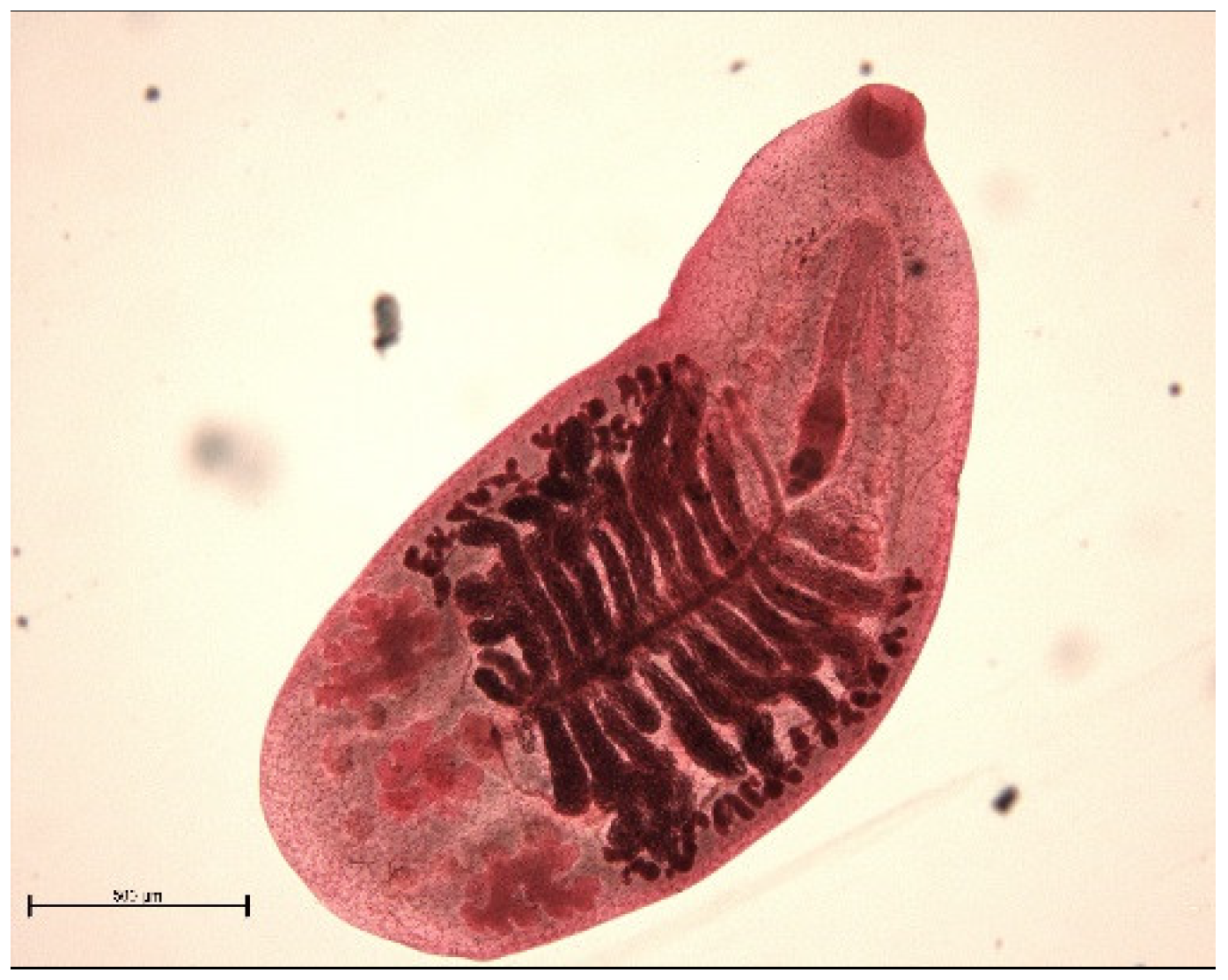
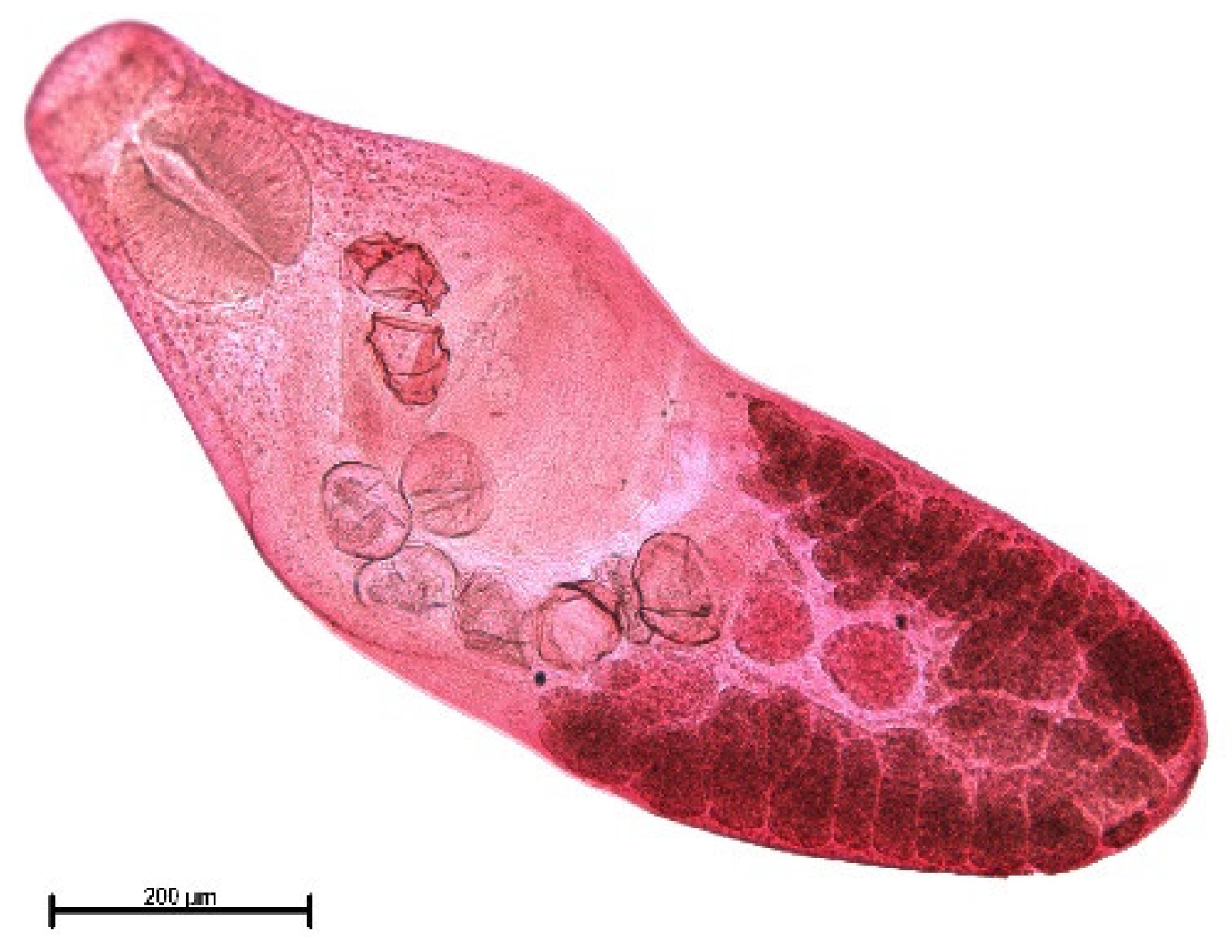
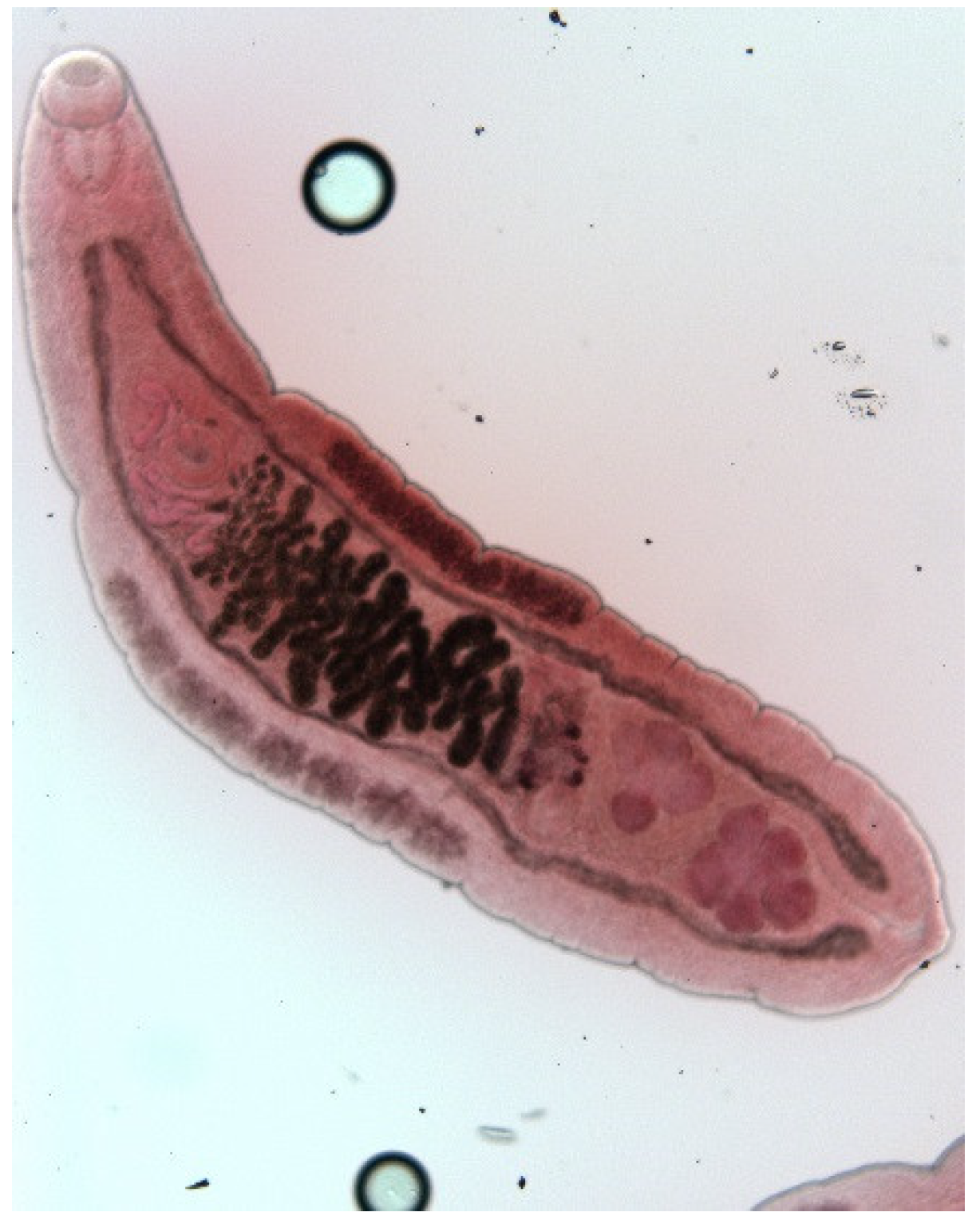

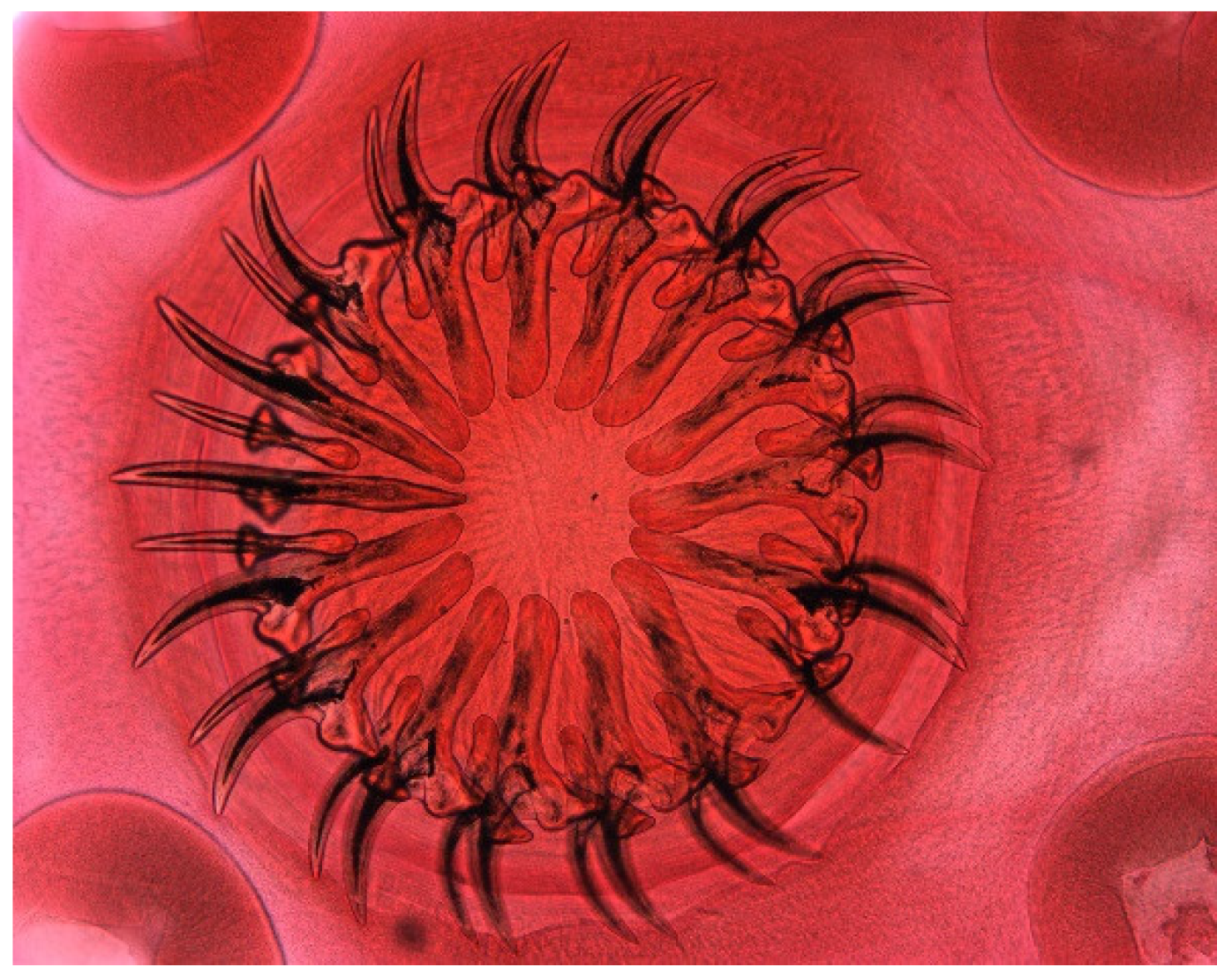

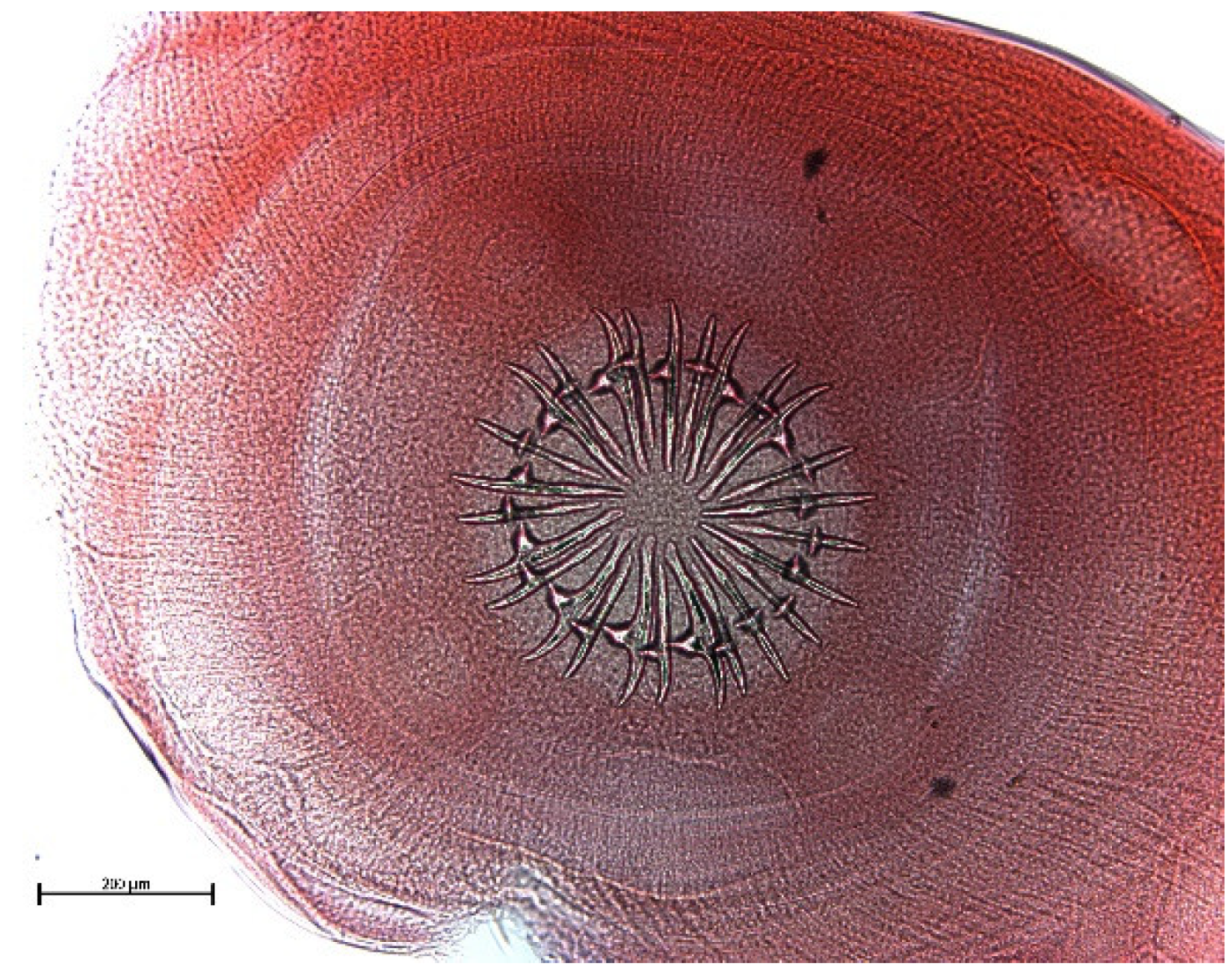

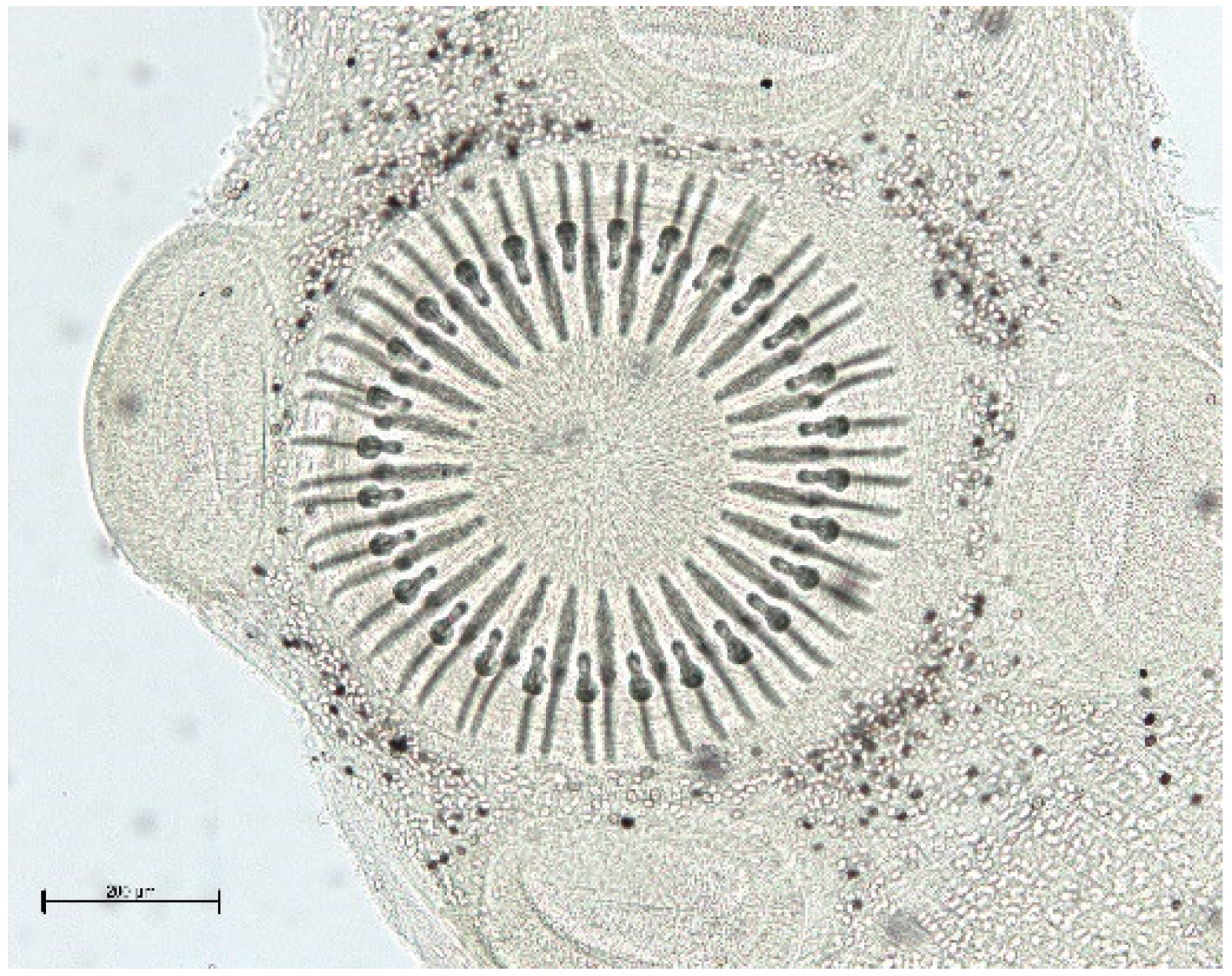
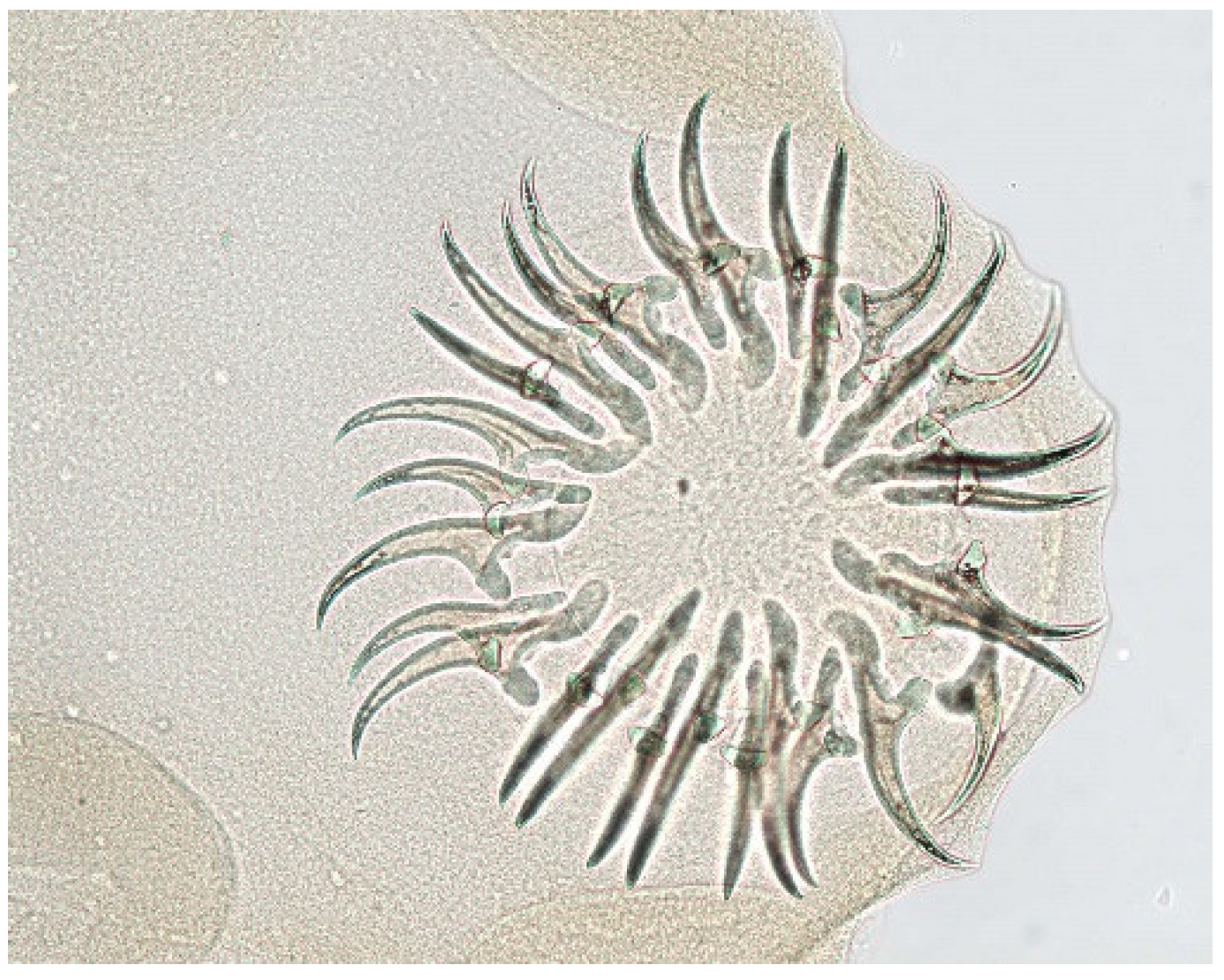
| Age Group | Habitats | Amount | ||
|---|---|---|---|---|
| Cut off Meanders | Lakes | Running Waters | ||
| Adults | 8 | 24 | 15 | 47 |
| Subadults | 12 | 16 | 3 | 31 |
| Juveniles | 19 | 17 | 16 | 52 |
| total | 39 | 57 | 34 | 130 |
| Habitat | Number of Muskrats Infected with Parasites | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Echinostoma sp. | Plagiorchis elegans | Plagiorchis arvicola | Notocotylus noyeri | Psilotrema spp. | Opisthorchis felineus | Hydatigera taeniaeformis | Taenia martis | Taenia polyacantha | Taenia crassiceps | |
| Lakes (n = 57) | 2 | 13 | 0 | 1 | 14 | 0 | 19 | 3 | 3 | 1 |
| Cut off meanders (n = 39) | 3 | 7 | 0 | 0 | 18 | 1 | 5 | 5 | 1 | 0 |
| Running waters (n = 34) | 7 | 1 | 1 | 0 | 1 | 0 | 6 | 0 | 0 | 0 |
| Total (n = 130) | 12 | 21 | 1 | 1 | 33 | 1 | 30 | 8 | 4 | 1 |
| Age Group | Parasites | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Echinostoma sp. | Plagiorchis elegans | Plagiorchis arvicola | Notocotylus noyeri | Psilotrema spp. | Opisthorchis felineus | Hydatigera taeniaeformis | Taenia martis | Taenia polyacantha | Taenia crassiceps | |
| Adults (n = 47) | 8.5 (5–62) | 17.0 (1–10) | 2.1 (5) | 0.0 (0) | 17.0 (1–15) | 2.1 (12) | 48.9 (1–15) | 10.6 (1–17) | 6.4 (1–69) | 2.1 (37) |
| Subadults (n = 31) | 6.5 (1–5) | 25.8 (1–8) | 0.0 (0) | 0.0 (0) | 35.5 (1–35) | 0.0 (0) | 12.9 (1–3) | 6.5 (1) | 3.2 (5) | 0.0 (0) |
| Juveniles (n = 52) | 11.5 (1–20) | 9.6 (1–2) | 0.0 (0) | 1.9 (3) | 25.0 (1–18) | 0.0 (0) | 3.8 (1–2) | 1.9 (1) | 0.0 (0) | 0.0 (0) |
| total (n = 130) | 9.2 (1–62) | 16.2 (1–10) | 0.8 (5) | 0.8 (3) | 24.6 (1–35) | 0.8 (12) | 23.1 (1–15) | 6.2 (1–17) | 3.1 (1–10) | 0.8 (37) |
| Cestode Larval Stage | [10] | [15] | [30] | [11] | [18] | [25] | [24] | This Paper |
|---|---|---|---|---|---|---|---|---|
| n = 630 | n = 437 | n = 670 | n = 80 | n = 991 | n = 1726 | n = 657 | n = 130 | |
| Germany | Germany | Germany | Germany | Germany | Netherlands | Belgium | Germany | |
| Echinococcus multilocularis | 0 | 1.8 | 0 | 0 | 4.1 | 0.1 | 22.1 | 0 |
| Hydatigera taeniaeformis | 33.17 | 48.1 | 99.6 | 63.75 | 42.3 | 44.8 | 65.8 | 23.1 |
| Taenia martis | 3.02 | 48.3 | 0 | 18.75 | 3.4 | 6.1 | 22.2 | 10.6 |
| Taenia crassiceps | 0.48 | 0.9 | 1.1 | 6.25 | 2.2 | 0.3 | 0.9 | 0 |
| Taenia polyacantha | 0.32 | 7.3 | 0 | 1.25 | 0.4 | 0.2 | 2.6 | 6.4 |
| Taenia pisiformis | 2.38 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Taenia mustellae | 4.8 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Mesocestoides sp. | 3.79 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schuster, R.K.; Specht, P.; Rieger, S. On the Helminth Fauna of the Muskrat (Ondatra zibethicus (Linnaeus, 1766)) in the Barnim District of Brandenburg State/Germany. Animals 2021, 11, 2444. https://doi.org/10.3390/ani11082444
Schuster RK, Specht P, Rieger S. On the Helminth Fauna of the Muskrat (Ondatra zibethicus (Linnaeus, 1766)) in the Barnim District of Brandenburg State/Germany. Animals. 2021; 11(8):2444. https://doi.org/10.3390/ani11082444
Chicago/Turabian StyleSchuster, Rolf K., Peter Specht, and Siegfried Rieger. 2021. "On the Helminth Fauna of the Muskrat (Ondatra zibethicus (Linnaeus, 1766)) in the Barnim District of Brandenburg State/Germany" Animals 11, no. 8: 2444. https://doi.org/10.3390/ani11082444
APA StyleSchuster, R. K., Specht, P., & Rieger, S. (2021). On the Helminth Fauna of the Muskrat (Ondatra zibethicus (Linnaeus, 1766)) in the Barnim District of Brandenburg State/Germany. Animals, 11(8), 2444. https://doi.org/10.3390/ani11082444





