Phycoerythrin from Colaconema sp. Has Immunostimulatory Effects on the Whiteleg Shrimp Litopenaeus vannamei and Increases Resistance to Vibrio parahaemolyticus and White Spot Syndrome Virus
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
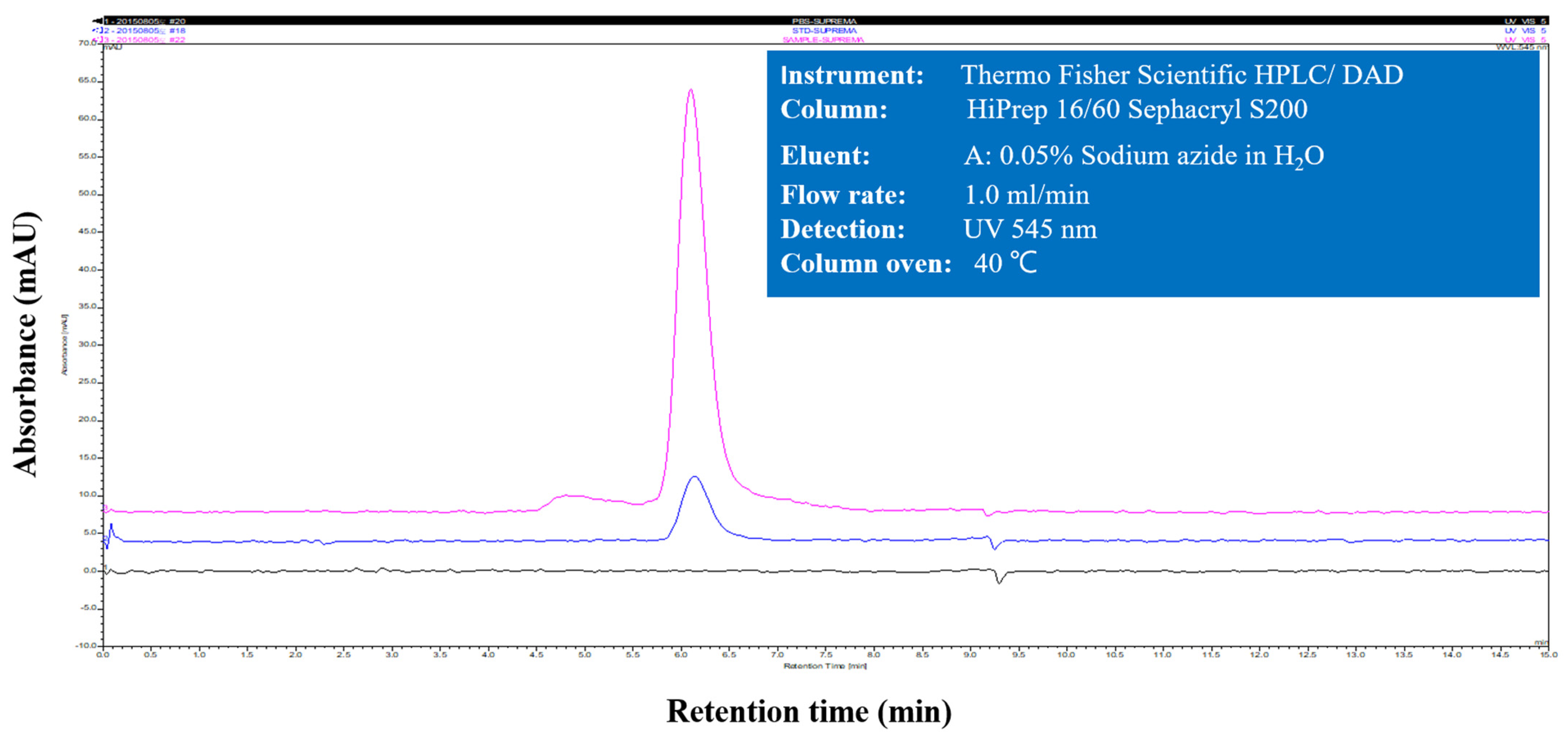
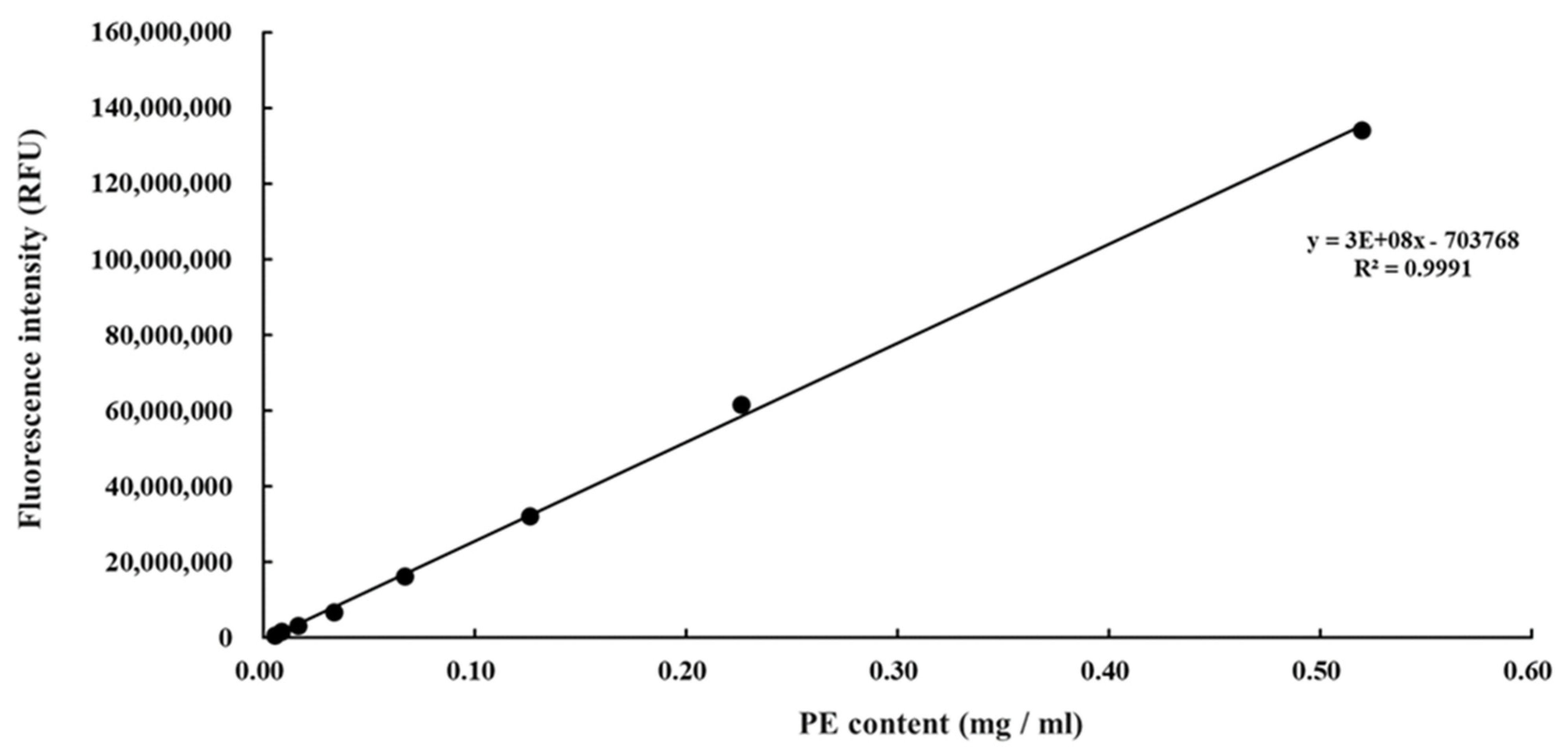
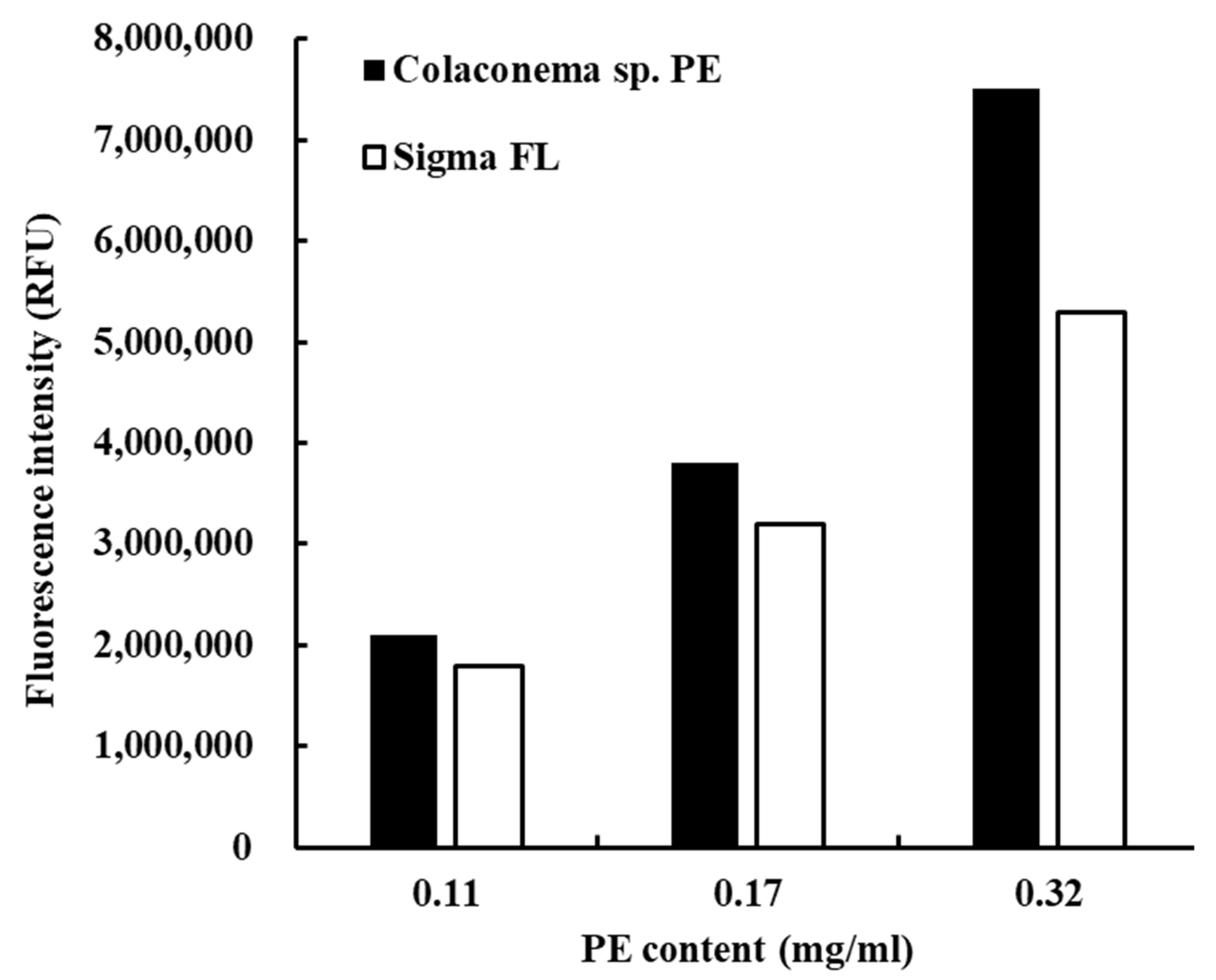
2.1. Preparation of Phycoerythrin
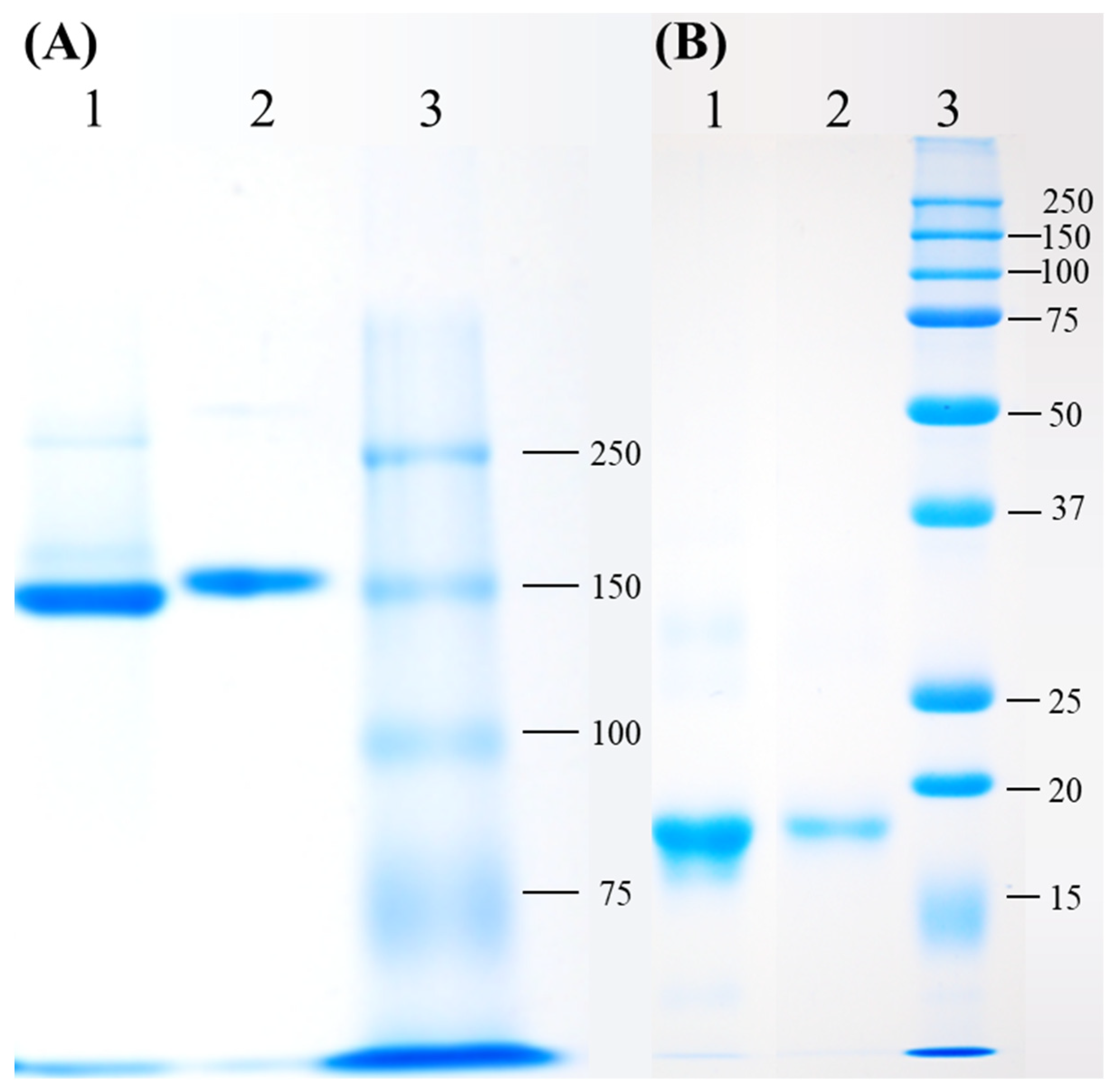
2.2. Native (PAGE) and Sodium Dodecyl Sulfate (SDS)-Polyacrylamide Gel Electrophoresis (PAGE) Analysis
2.3. Experimental Shrimp
2.4. In Vitro Effects of PE on the Immune Response of Hemocytes
2.4.1. Prophenoloxidase Activity (PO)
2.4.2. Reactive Oxygen Species (ROS) Production
2.4.3. Phagocytosis Rate
2.5. Gene Expression Analysis by Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.6. In Vivo Experimental Design
2.7. Challenge Test
2.8. Statistical Analysis
3. Results
3.1. Purification of Phycoerythrin
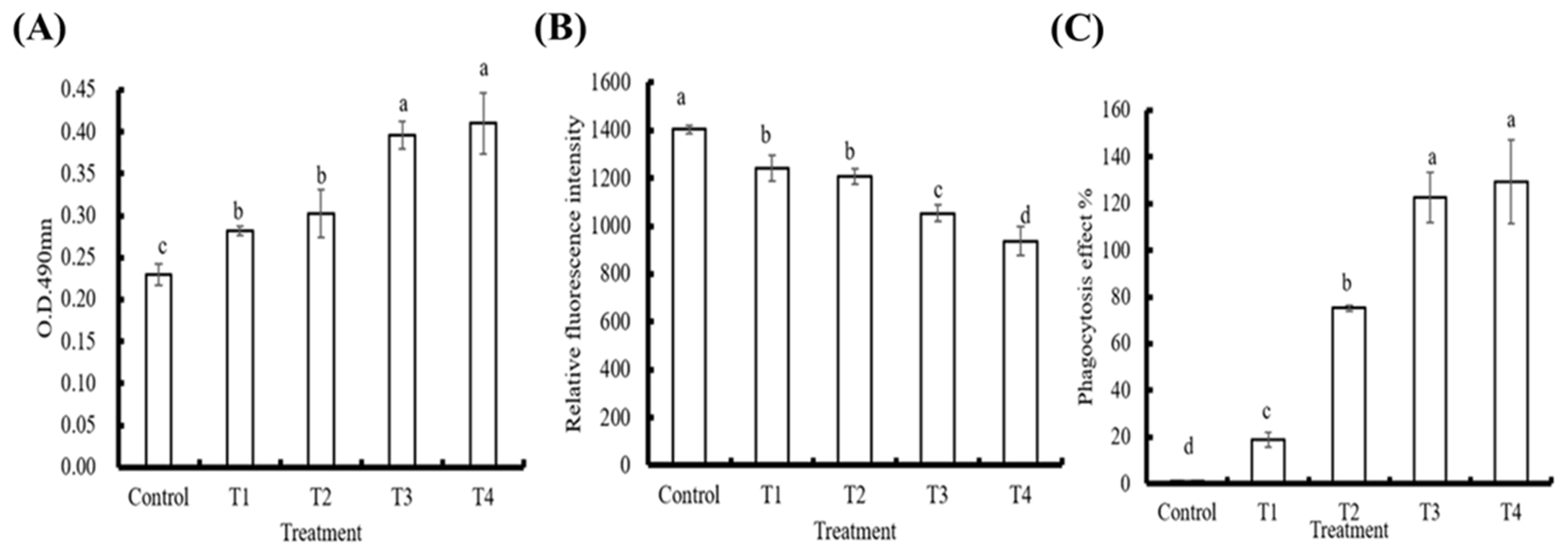
3.2. In Vitro Effects of PE on Innate Immune Parameters in Hemocytes
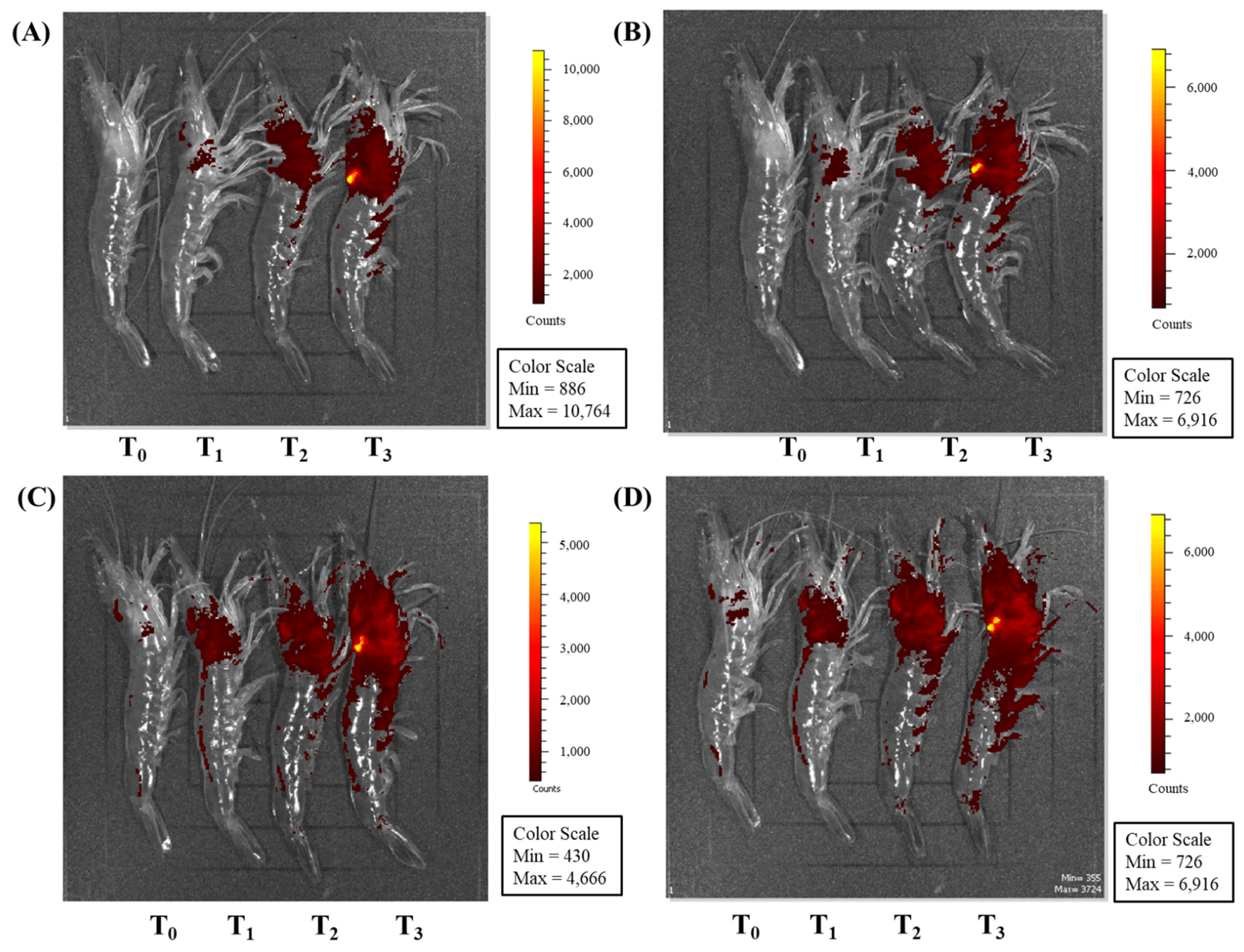
3.3. Observation Using an In Vivo Imaging System after PE Injection
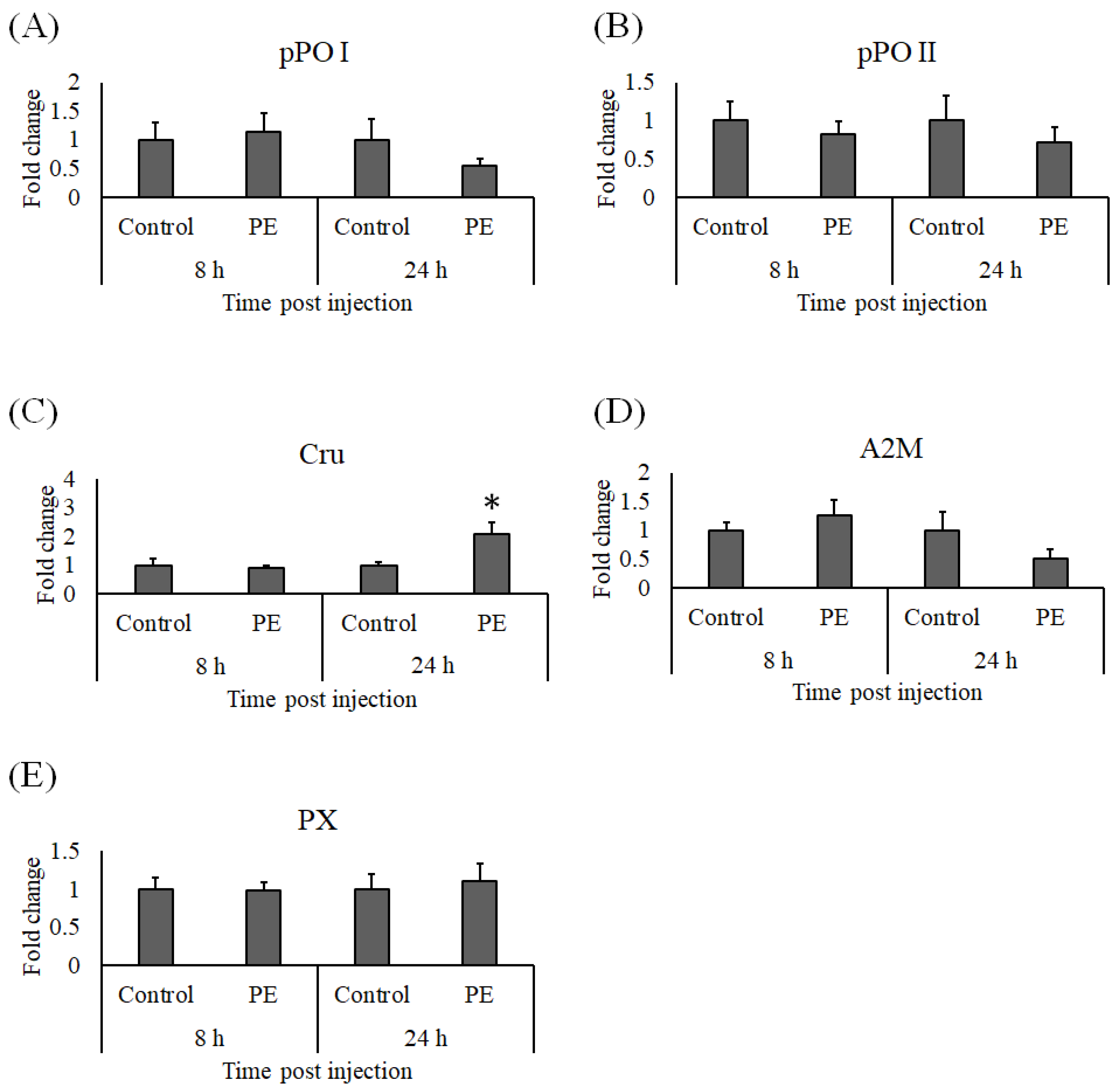
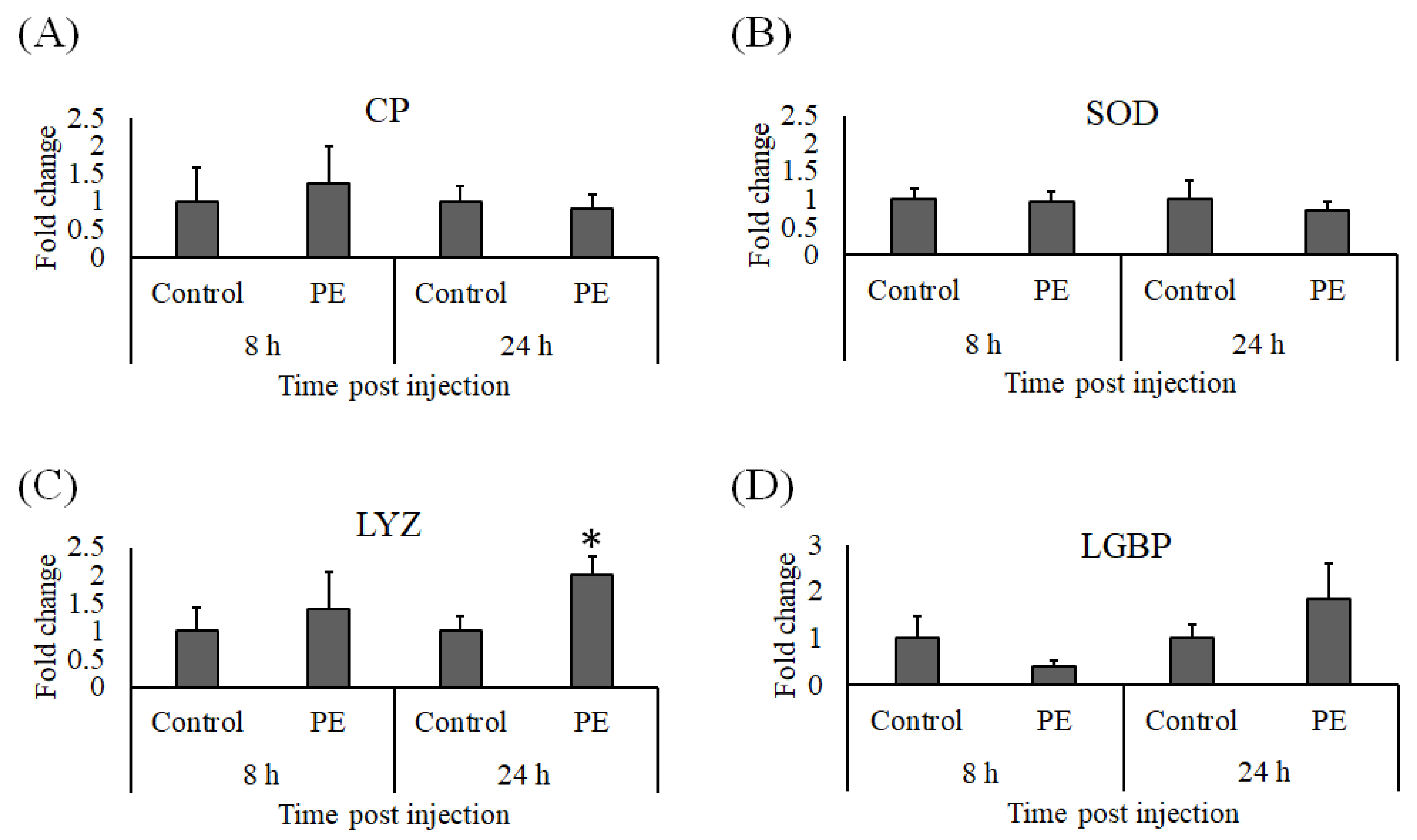
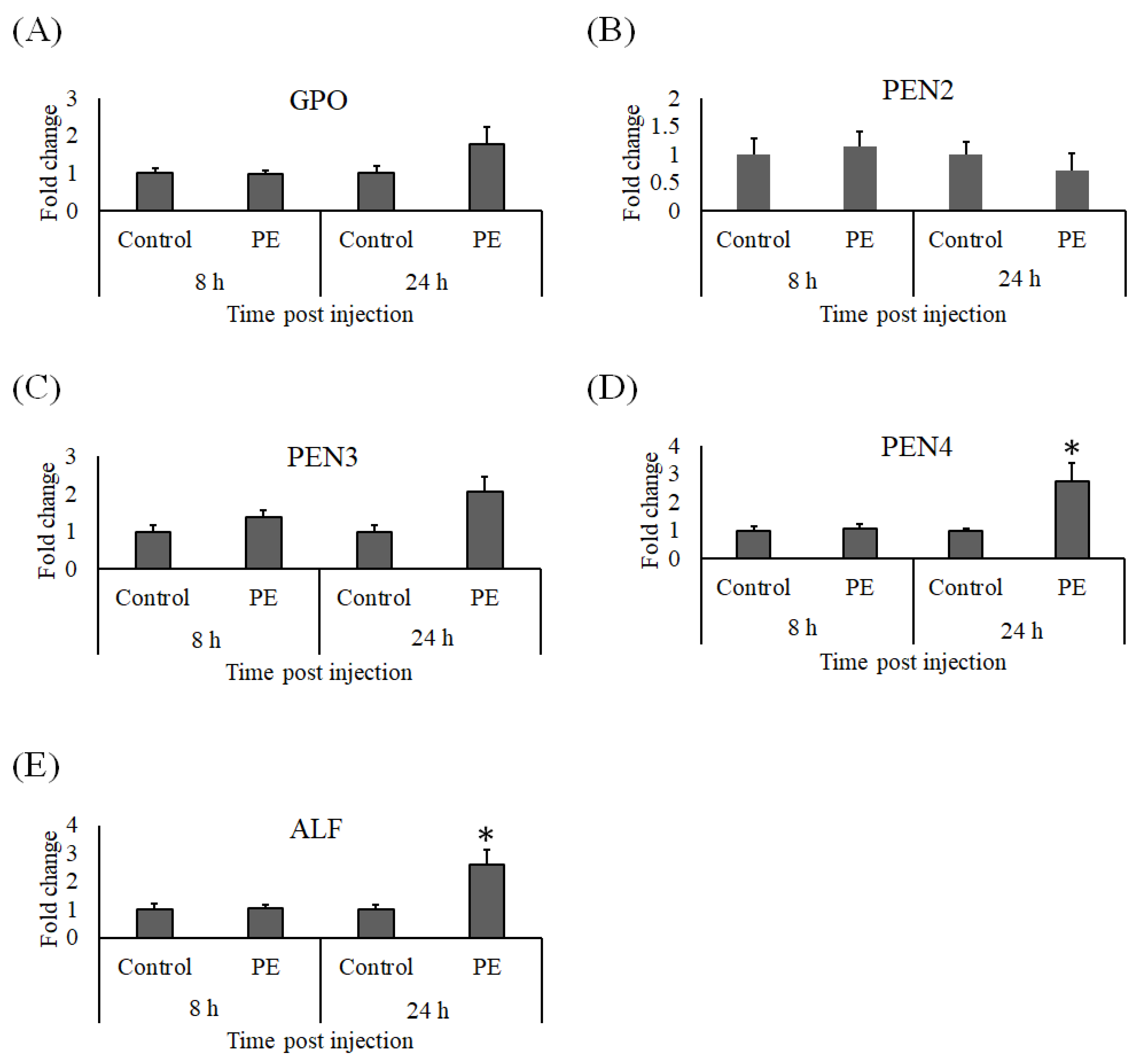
3.4. Expression Analysis of Immune Genes
3.5. Challenge Trials
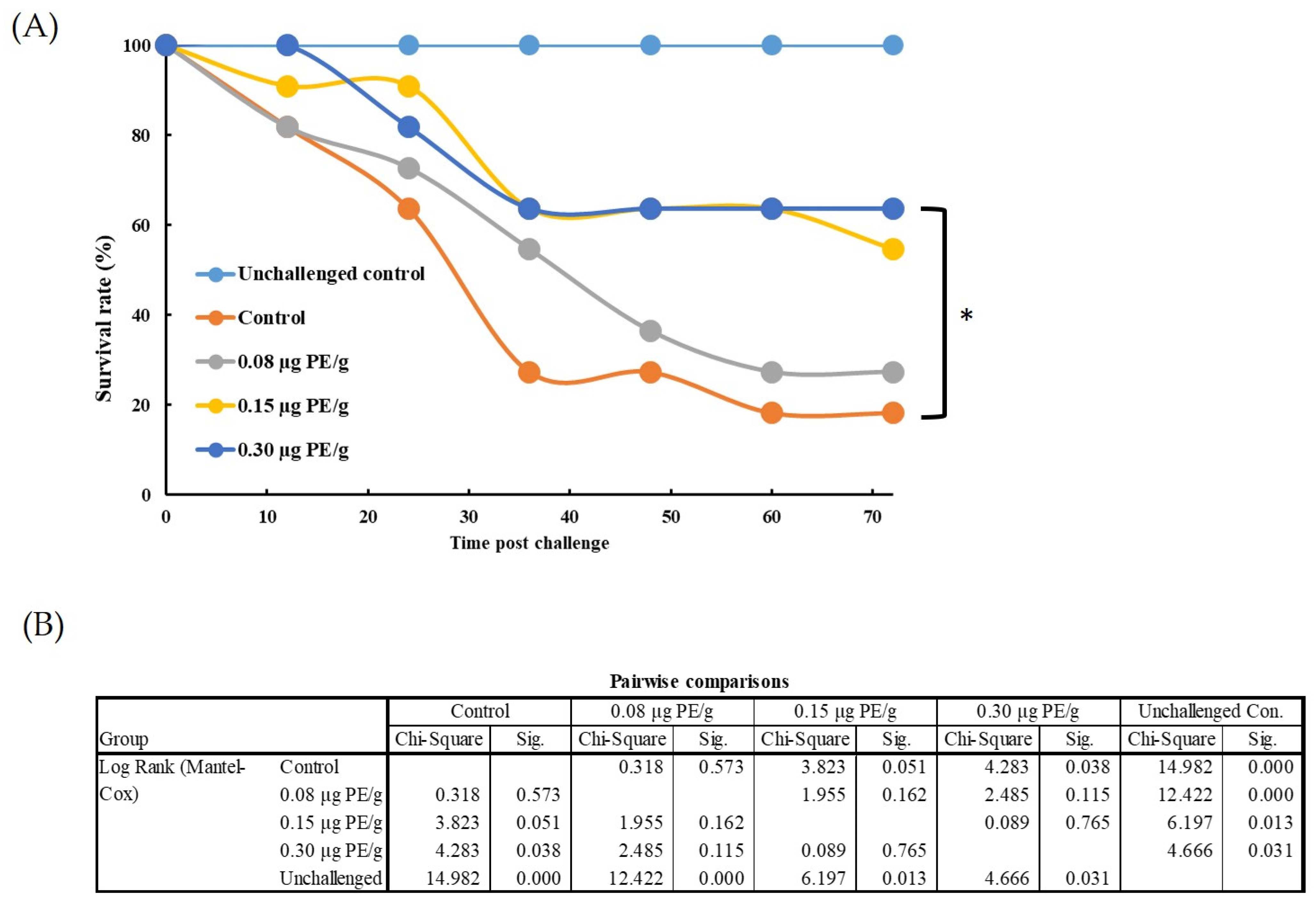
3.5.1. Effects of PE on the Survival Rate of L. vannamei Challenged with V. parahaemolyticus
3.5.2. Effects of PE on the Survival Rate of L. vannamei Challenged with White Spot Syndrome Virus
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture 2020; Sustainability in action; FAO: Rome, Italy, 2020. [Google Scholar]
- Xu, S.Y.; Huang, X.; Cheong, K.L. Recent Advances in Marine Algae Polysaccharides: Isolation, Structure, and Activities. Mar. Drugs 2017, 15, 388. [Google Scholar] [CrossRef]
- Tacon, A. Global trends in aquaculture and compound aquafeed production. World Aquac. 2018, 49, 33–46. [Google Scholar]
- Bachere, E. Anti-infectious immune effectors in marine invertebrates: Potential tools for disease control in larviculture. Aquaculture 2003, 227, 427–438. [Google Scholar] [CrossRef]
- Soto-Rodriguez, S.; Gomez-Gil, B. Shrimp Diseases and Molecular Diagnostic Methods. In Aquaculture Microbiology and Biotechnology; Taylor & Francis Group: Abingdon, UK, 2009; pp. 101–131. [Google Scholar]
- Liu, C.-H.; Cheng, W.; Hsu, J.-P.; Chen, J.-C. Vibrio alginolyticus infection in the white shrimp Litopenaeus vannamei confirmed by polymerase chain reaction and 16S rDNA sequencing. Dis. Aquat. Org. 2004, 61, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Karunasagar, I.; Otta, S.K.; Karunasagar, I. Disease Problems Affecting Cultured Penaeid Shrimp in India. Fish Pathol. 1998, 33, 413–419. [Google Scholar] [CrossRef]
- Hasan, M.A.; Siddique, M.; Hasan, M.; Hossain, M.; Rahman, M.S. 16S rRNA gene sequence based identification of Vibrio spp. in shrimp and tilapia hatcheries of Bangladesh. Dhaka Univ. J. Biol. Sci. 2017, 26, 45–58. [Google Scholar] [CrossRef][Green Version]
- Balasubramanian, G.; Sudhakaran, R.; Syed Musthaq, S.; Sarathi, M.; Sahul Hameed, A.S. Studies on the inactivation of white spot syndrome virus of shrimp by physical and chemical treatments, and seaweed extracts tested in marine and freshwater animal models. J. Fish Dis. 2006, 29, 569–572. [Google Scholar] [CrossRef] [PubMed]
- De Gryse, G.M.A.; Khuong, T.V.; Descamps, B.; Van Den Broeck, W.; Vanhove, C.; Cornillie, P.; Sorgeloos, P.; Bossier, P.; Nauwynck, H.J. The shrimp nephrocomplex serves as a major portal of pathogen entry and is involved in the molting process. Proc. Natl. Acad. Sci. USA 2020, 117, 28374–28383. [Google Scholar] [CrossRef]
- Osunla, C.A.; Okoh, A.I. Vibrio Pathogens: A Public Health Concern in Rural Water Resources in Sub-Saharan Africa. Int. J. Environ. Res. Public Health 2017, 14, 1188. [Google Scholar] [CrossRef]
- Immanuel, G.; Sivagnanavelmurugan, M.; Marudhupandi, T.; Radhakrishnan, S.; Palavesam, A. The effect of fucoidan from brown seaweed Sargassum wightii on WSSV resistance and immune activity in shrimp Penaeus monodon (Fab). Fish Shellfish Immunol. 2012, 32, 551–564. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.; Costa, R.; Peixoto, J.; Nascimento, F.; Carneiro, P.; Vieira, R. Tropical Atlantic marine macroalgae with bioactivity against virulent and antibiotic resistant Vibrio. Lat. Am. J. Aquat. Res. 2013, 41, 183–188. [Google Scholar] [CrossRef]
- Kanjana, K.; Radtanatip, T.; Asuvapongpatana, S.; Withyachumnarnkul, B.; Wongprasert, K. Solvent extracts of the red seaweed Gracilaria fisheri prevent Vibrio harveyi infections in the black tiger shrimp Penaeus monodon. Fish Shellfish Immunol. 2011, 30, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Selvin, J.; Manilal, A.; Sugathan, S.; Kiran, S.; Lipton, A. Efficacy of marine green alga Ulva fasciata extract on the management of shrimp bacterial diseases. Lat. Am. J. Aquat. Res. 2011, 39, 197–204. [Google Scholar] [CrossRef]
- Sivagnanavelmurugan, M.; Marudhupandi, T.; Palavesam, A.; Immanuel, G. Antiviral Effect of Fucoidan Extracted from the Brown Seaweed, Sargassum wightii, on Shrimp Penaeus monodon Postlarvae against White Spot Syndrome Virus. J. World Aquac. Soc. 2012, 43, 697–706. [Google Scholar] [CrossRef]
- Chen, Y.-Y.; Sim, S.S.; Chiew, S.L.; Yeh, S.-T.; Liou, C.-H.; Chen, J.-C. Dietary administration of a Gracilaria tenuistipitata extract produces protective immunity of white shrimp Litopenaeus vannamei in response to ammonia stress. Aquaculture 2012, 370–371, 26–31. [Google Scholar] [CrossRef]
- Apt, K.E.; Collier, J.L.; Grossman, A.R. Evolution of the Phycobiliproteins. J. Mol. Biol. 1995, 248, 79–96. [Google Scholar] [CrossRef]
- Sekar, S.; Chandramohan, M. Phycobiliproteins as a commodity: Trends in applied research, patents and commercialization. J. Appl. Phycol. 2008, 20, 113–136. [Google Scholar] [CrossRef]
- Six, C.; Thomas, J.C.; Garczarek, L.; Ostrowski, M.; Dufresne, A.; Blot, N.; Scanlan, D.J.; Partensky, F. Diversity and evolution of phycobilisomes in marine Synechococcus spp.: A comparative genomics study. Genome Biol. 2007, 8, R259. [Google Scholar] [CrossRef]
- Anwer, K.; Sonani, R.; Madamwar, D.; Singh, P.; Khan, F.; Bisetty, K.; Ahmad, F.; Hassan, M.I. Role of N-terminal residues on folding and stability of C-phycoerythrin: Simulation and urea-induced denaturation studies. J. Biomol. Struct. Dyn. 2015, 33, 121–133. [Google Scholar] [CrossRef]
- Soni, B.R.; Hasan, M.I.; Parmar, A.; Ethayathulla, A.S.; Kumar, R.P.; Singh, N.K.; Sinha, M.; Kaur, P.; Yadav, S.; Sharma, S.; et al. Structure of the novel 14kDa fragment of α-subunit of phycoerythrin from the starving cyanobacterium Phormidium tenue. J. Struct. Biol. 2010, 171, 247–255. [Google Scholar] [CrossRef]
- Sonani, R.R.; Singh, N.K.; Kumar, J.; Thakar, D.; Madamwar, D. Concurrent purification and antioxidant activity of phycobiliproteins from Lyngbya sp. A09DM: An antioxidant and anti-aging potential of phycoerythrin in Caenorhabditis elegans. Process. Biochem. 2014, 49, 1757–1766. [Google Scholar] [CrossRef]
- Sonani, R.R.; Singh, N.K.; Awasthi, A.; Prasad, B.; Kumar, J.; Madamwar, D. Phycoerythrin extends life span and health span of Caenorhabditis elegans. Age 2014, 36, 9717. [Google Scholar] [CrossRef]
- Sonani, R.R.; Rastogi, R.P.; Singh, N.K.; Thadani, J.; Patel, P.J.; Kumar, J.; Tiwari, A.K.; Devkar, R.V.; Madamwar, D. Phycoerythrin averts intracellular ROS generation and physiological functional decline in eukaryotes under oxidative stress. Protoplasma 2017, 254, 849–862. [Google Scholar] [CrossRef]
- Hemlata Afreen, S.; Fatma, T. Extraction, purification and characterization of phycoerythrin from Michrochaete and its biological activities. Biocatal. Agric. Biotechnol. 2018, 13, 84–89. [Google Scholar] [CrossRef]
- Hernández-López, J.; Gollas-Galván, T.; Vargas-Albores, F. Activation of the prophenoloxidase system of the brown shrimp Penaeus californiensis Holmes). Comp. Biochem. Physiol. Part C Pharmacol. Toxicol. Endocrinol. 1996, 113, 61–66. [Google Scholar] [CrossRef]
- Xian, J.-A.; Wang, A.-L.; Tian, J.-X.; Huang, J.-W.; Ye, C.-X.; Wang, W.-N.; Sun, R.-Y. Morphologic, physiological and immunological changes of haemocytes from Litopenaeus vannamei treated by lipopolysaccharide. Aquaculture 2009, 298, 139–145. [Google Scholar] [CrossRef]
- Elshopakey, G.E.; Risha, E.F.; Abdalla, O.A.; Okamura, Y.; Hanh, V.D.; Ibuki, M.; Sudhakaran, R.; Itami, T. Enhancement of immune response and resistance against Vibrio parahaemolyticus in kuruma shrimp (Marsupenaeus japonicus) by dietary supplementation of β-1,4-mannobiose. Fish Shellfish Immunol. 2018, 74, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-K.; Chew, P.-F.; Soh, B.-S.; Tham, L.Y. Enhancing phagocytic activity of hemocytes and disease resistance in the prawn Penaeus merguiensis by feeding Spirulina platensis. J. Appl. Phycol. 2003, 15, 279–287. [Google Scholar] [CrossRef]
- Xian, J.-A.; Wang, A.-L.; Ye, C.-X.; Chen, X.-D.; Wang, W.-N. Phagocytic activity, respiratory burst, cytoplasmic free-Ca2+ concentration and apoptotic cell ratio of haemocytes from the black tiger shrimp, Penaeus monodon under acute copper stress. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2010, 152, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Jia, Z.; Zhang, T.; Wang, L.; Qiu, L.; Sun, J.; Song, L. Functional characterisation of phagocytes in the Pacific oyster Crassostrea gigas. PeerJ 2016, 4, e2590. [Google Scholar] [CrossRef]
- Chen, Y.-Y.; Chen, J.-C.; Lin, Y.-C.; Yeh, S.-T.; Huang, C.-L. White Shrimp Litopenaeus vannamei That Have Received Gracilaria tenuistipitata Extract Show Early Recovery of Immune Parameters after Ammonia Stressing. Mar. Drugs 2015, 13, 3606–3624. [Google Scholar] [CrossRef]
- Cheng, W.; Liu, C.-H.; Tsai, C.-H.; Chen, J.-C. Molecular cloning and characterisation of a pattern recognition molecule, lipopolysaccharide- and β-1,3-glucan binding protein (LGBP) from the white shrimp Litopenaeus vannamei. Fish Shellfish Immunol. 2005, 18, 297–310. [Google Scholar] [CrossRef]
- Liu, C.-H.; Cheng, W.; Kuo, C.-M.; Chen, J.-C. Molecular cloning and characterisation of a cell adhesion molecule, peroxinectin from the white shrimp Litopenaeus vannamei. Fish Shellfish Immunol. 2004, 17, 13–26. [Google Scholar] [CrossRef]
- Chen, Y.-Y.; Chen, J.-C.; Lin, Y.-C.; Putra, D.F.; Kitikiew, S.; Li, C.-C.; Hsieh, J.-F.; Liou, C.-H.; Yeh, S.-T. Shrimp that have received carrageenan via immersion and diet exhibit immunocompetence in phagocytosis despite a post-plateau in immune parameters. Fish Shellfish. Immunol. 2014, 36, 352–366. [Google Scholar] [CrossRef]
- Han-Ching Wang, K.C.; Tseng, C.-W.; Lin, H.-Y.; Chen, I.T.; Chen, Y.-H.; Chen, Y.-M.; Chen, T.-Y.; Yang, H.-L. RNAi knock-down of the Litopenaeus vannamei Toll gene (LvToll) significantly increases mortality and reduces bacterial clearance after challenge with Vibrio harveyi. Dev. Comp. Immunol. 2010, 34, 49–58. [Google Scholar] [CrossRef]
- Ezra-Elia, R.; Obolensky, A.; Ejzenberg, A.; Ross, M.; Mintz, D.; Banin, E.; Ofri, R. Can an in vivo imaging system be used to determine localization and biodistribution of AAV5-mediated gene expression following subretinal and intravitreal delivery in mice? Exp. Eye Res. 2018, 176, 227–234. [Google Scholar] [CrossRef]
- Wang, T.; Diaz-Rosales, P.; Costa, M.M.; Campbell, S.; Snow, M.; Collet, B.; Martin, S.A.M.; Secombes, C.J. Functional characterization of a nonmammalian IL-21: Rainbow trout Oncorhynchus mykiss IL-21 upregulates the expression of the Th cell signature cytokines IFN-gamma, IL-10, and IL-22. J. Immunol. 2011, 186, 708. [Google Scholar] [CrossRef] [PubMed]
- Hong, X.; Lu, L.; Xu, D. Progress in research on acute hepatopancreatic necrosis disease (AHPND). Aquac. Int. 2016, 24, 577–593. [Google Scholar] [CrossRef]
- Sánchez-Muros, M.J.; Renteria, P.; Vizcaino, A.; Barroso, F.G. Innovative protein sources in shrimp (Litopenaeus vannamei) feeding. Rev. Aquac. 2020, 12, 186–203. [Google Scholar] [CrossRef]
- Huynh, T.-G.; Yeh, S.-T.; Lin, Y.-C.; Shyu, J.-F.; Chen, L.-L.; Chen, J.-C. White shrimp Litopenaeus vannamei immersed in seawater containing Sargassum hemiphyllum var. chinense powder and its extract showed increased immunity and resistance against Vibrio alginolyticus and white spot syndrome virus. Fish Shellfish Immunol. 2011, 31, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Shen, Z.; Li, L.; Li, Y.; Zhao, H.; Jiang, X. Immunomodulatory activity of R-phycoerythrin from Porphyra haitanensis via TLR4/NF-κB-dependent immunocyte differentiation. Food Funct. 2020, 11, 2173–2185. [Google Scholar] [CrossRef]
- Bermejo, R.; Acién, F.G.; Ibáñez, M.J.; Fernández, J.M.; Molina, E.; Alvarez-Pez, J.M. Preparative purification of B-phycoerythrin from the microalga Porphyridium cruentum by expanded-bed adsorption chromatography. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2003, 790, 317–325. [Google Scholar] [CrossRef]
- Sakai, S.; Komura, Y.; Nishimura, Y.; Sugawara, T.; Hirata, T. Inhibition of Mast Cell Degranulation by Phycoerythrin and Its Pigment Moiety Phycoerythrobilin, Prepared from Porphyra yezoensis. Food Sci. Technol. Res. 2011, 17, 171–177. [Google Scholar] [CrossRef]
- Senthilkumar, N.; Suresh, V.; Thangam, R.; Kurinjimalar, C.; Kavitha, G.; Murugan, P.; Kannan, S.; Rengasamy, R. Isolation and characterization of macromolecular protein R-Phycoerythrin from Portieria hornemannii. Int. J. Biol. Macromol. 2013, 55, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Smith, V.J.; Brown, J.H.; Hauton, C. Immunostimulation in crustaceans: Does it really protect against infection? Fish Shellfish Immunol. 2003, 15, 71–90. [Google Scholar] [CrossRef]
- Song, Y.-L.; Hsieh, Y.-T. Immunostimulation of tiger shrimp (Penaeus monodon) hemocytes for generation of microbicidal substances: Analysis of reactive oxygen species. Dev. Comp. Immunol. 1994, 18, 201–209. [Google Scholar] [CrossRef]
- Yang, H.-T.; Yang, M.-C.; Sun, J.-J.; Guo, F.; Lan, J.-F.; Wang, X.-W.; Zhao, X.-F.; Wang, J.-X. Catalase eliminates reactive oxygen species and influences the intestinal microbiota of shrimp. Fish Shellfish Immunol. 2015, 47, 63–73. [Google Scholar] [CrossRef]
- Nordberg, J.; Arner, E.S. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free. Radic. Biol. Med. 2001, 31, 1287–1312. [Google Scholar] [CrossRef]
- Holmblad, T.; Söderhäll, K. Cell adhesion molecules and antioxidative enzymes in a crustacean, possible role in immunity. Aquaculture 1999, 172, 111–123. [Google Scholar] [CrossRef]
- Amparyup, P.; Charoensapsri, W.; Tassanakajon, A. Prophenoloxidase system and its role in shrimp immune responses against major pathogens. Fish Shellfish Immunol. 2013, 34, 990–1001. [Google Scholar] [CrossRef]
- Wang, X.-W.; Wang, J.-X. Pattern recognition receptors acting in innate immune system of shrimp against pathogen infections. Fish Shellfish Immunol. 2012, 34, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Jiravanichpaisal, P.; Lee, B.L.; Söderhäll, K. Cell-mediated immunity in arthropods: Hematopoiesis, coagulation, melanization and opsonization. Immunobiology 2006, 211, 213–236. [Google Scholar] [CrossRef] [PubMed]
- Cerenius, L.; Lee, B.L.; Söderhäll, K. The proPO-system: Pros and cons for its role in invertebrate immunity. Trends Immunol. 2008, 29, 263–271. [Google Scholar] [CrossRef]
- Cerenius, L.; Söderhäll, K. The prophenoloxidase-activating system in invertebrates. Immunol. Rev. 2004, 198, 116–126. [Google Scholar] [CrossRef]
- Nappi, A.J.; Christensen, B.M. Melanogenesis and associated cytotoxic reactions: Applications to insect innate immunity. Insect Biochem. Mol. Biol. 2005, 35, 443–459. [Google Scholar] [CrossRef]
- Cerenius, L.; Babu, R.; Söderhäll, K.; Jiravanichpaisal, P. In vitro effects on bacterial growth of phenoloxidase reaction products. J. Invertebr. Pathol. 2010, 103, 21–23. [Google Scholar] [CrossRef]
- Cerenius, L.; Söderhäll, K. 2—Crustacean immune responses and their implications for disease control. In Infectious Disease in Aquaculture; Austin, B., Ed.; Woodhead Publishing: Sawston, UK, 2012; pp. 69–87. [Google Scholar]
- Novoa, B.; Figueras, A. 3—Immune responses in molluscs and their implications for disease control. In Infectious Disease in Aquaculture; Austin, B., Ed.; Woodhead Publishing: Sawston, UK, 2012; pp. 88–110. [Google Scholar]
- Maningas, M.B.; Kondo, H.; Hirono, I. Molecular mechanisms of the shrimp clotting system. Fish Shellfish Immunol. 2012, 34, 968–972. [Google Scholar] [CrossRef]
- Chaikeeratisak, V.; Somboonwiwat, K.; Tassanakajon, A. Shrimp Alpha-2-Macroglobulin Prevents the Bacterial Escape by Inhibiting Fibrinolysis of Blood Clots. PLoS ONE 2012, 7, e47384. [Google Scholar] [CrossRef]
- Tanaka, S.; Nakamura, T.; Morita, T.; Iwanaga, S. Limulus anti-LPS factor: An anticoagulant which inhibits the endotoxin mediated activation of Limulus coagulation system. Biochem. Biophys. Res. Commun. 1982, 105, 717–723. [Google Scholar] [CrossRef]
- Zhan, W.; He, L.; Wei, X.; Wang, X.; Tang, X. An Anti-Lipopolysaccharide Factor in Litopenaeus Vannamei Participates in the Immune Defense Against WSSV and Vibrio Anguillarum. J. Crustacean Biol. 2015, 35, 670–675. [Google Scholar] [CrossRef][Green Version]
- Loker, E.S.; Adema, C.M.; Zhang, S.M.; Kepler, T.B. Invertebrate immune systems--not homogeneous, not simple, not well understood. Immunol. Rev. 2004, 198, 10–24. [Google Scholar] [CrossRef] [PubMed]

| Gene | Sequence 5′–3′ | Reference |
|---|---|---|
| Lipopolysaccharide and β-1,3-glucan binding protein (LGBP) | CGGCAACCAGTACGGAGGAAC | (Cheng et al., 2005) [34] |
| GTGGAAATCATCGGCGAAGGAG | ||
| Peroxinectin (PX) | ATCCAGCAGCCAGGTATG | (Liu et al., 2004) [35] |
| CAGACTCATCAGATCCATTCC | ||
| Prophenoloxidase I (proPO I) | ACGTCACTTCCGGCAAGCGA | (Chen et al., 2014) [36] |
| CCTCCTTGTGAGCGTTGTCAGG | ||
| Prophenoloxidase II (proPO II) | ACCACTGGCACTGGCACCTCGTCTA | |
| TCGCCAGTTCTCGAGCTTCTGCAC | ||
| α2-macroglobulin (A2M) | GCACGTAATCAAGATCCG | |
| CCCATCTCATTAGCACAAAC | ||
| Anti-lipopolysaccharide factor (ALF) | CTGTGGAGGAACGAGGAGAC | (Wang et al., 2010) [37] |
| CCACCGCTTAGCATCTTGTT | ||
| Crustin (Cru) | GAGGGTCAAGCCTACTGCTG | |
| ACTTATCGAGGCCAGCACAC | ||
| Penaeidin 2 (Pen2) | TCGTGGTCTGCCTGGTCTT | |
| CAGGTCTGAACGGTGGTCTTC | ||
| Penaeidin 3 (Pen3) | CACCCTTCGTGAGACCTTTG | |
| AATATCCCTTTCCCACGTGAC | ||
| Penaeidin 4 (Pen4) | GCCCGTTACCCAAACCATC | |
| CCGTATCTGAAGCAGCAAAGTC | ||
| Lysozyme (Lyz) | GAAGCGACTACGGCAAGAAC | |
| AACCGTGAGACCAGCACTCT | ||
| Superoxidase dismutase (SOD) | ATCCACCACACAAAGCATCA | |
| AGCTCTCGTCAATGGCTTGT | ||
| Glutathione peroxidase (GPO) | TTTTTCCGTGCAAAAAGGAC | |
| TAATACGCGATGCCCCTAAC | ||
| Clotting protein (CP) | TCTTTGCGCAGTTGGTGATC | |
| TGAGGTGACCGAGTGCAAAA | ||
| Elongation factor 1α (EF1α) | ATGGTTGTCAACTTTGCCCC | (Chen et al., 2014) [36] |
| TTGACCTCCTTGATCACACC |
| Pigments Source | Phycoerythrin | Allophycocyanin | Phycocyanin |
|---|---|---|---|
| Colaconema sp. | 93.6% | 4.54% | 1.8% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, P.-T.; Huang, J.; Huang, C.-Y.; Liu, Z.-X.; Yeh, H.-Y.; Huang, H.-T.; Chen, L.-L.; Nan, F.-H.; Lee, M.-C. Phycoerythrin from Colaconema sp. Has Immunostimulatory Effects on the Whiteleg Shrimp Litopenaeus vannamei and Increases Resistance to Vibrio parahaemolyticus and White Spot Syndrome Virus. Animals 2021, 11, 2371. https://doi.org/10.3390/ani11082371
Lee P-T, Huang J, Huang C-Y, Liu Z-X, Yeh H-Y, Huang H-T, Chen L-L, Nan F-H, Lee M-C. Phycoerythrin from Colaconema sp. Has Immunostimulatory Effects on the Whiteleg Shrimp Litopenaeus vannamei and Increases Resistance to Vibrio parahaemolyticus and White Spot Syndrome Virus. Animals. 2021; 11(8):2371. https://doi.org/10.3390/ani11082371
Chicago/Turabian StyleLee, Po-Tsang, Jing Huang, Chin-Yi Huang, Zi-Xuan Liu, Han-Yang Yeh, Huai-Ting Huang, Li-Li Chen, Fan-Hua Nan, and Meng-Chou Lee. 2021. "Phycoerythrin from Colaconema sp. Has Immunostimulatory Effects on the Whiteleg Shrimp Litopenaeus vannamei and Increases Resistance to Vibrio parahaemolyticus and White Spot Syndrome Virus" Animals 11, no. 8: 2371. https://doi.org/10.3390/ani11082371
APA StyleLee, P.-T., Huang, J., Huang, C.-Y., Liu, Z.-X., Yeh, H.-Y., Huang, H.-T., Chen, L.-L., Nan, F.-H., & Lee, M.-C. (2021). Phycoerythrin from Colaconema sp. Has Immunostimulatory Effects on the Whiteleg Shrimp Litopenaeus vannamei and Increases Resistance to Vibrio parahaemolyticus and White Spot Syndrome Virus. Animals, 11(8), 2371. https://doi.org/10.3390/ani11082371









