Effects of Differences in Fibre Composition and Maturity of Forage-Based Diets on the Microbial Ecosystem and Its Activity in Equine Caecum and Colon Digesta and Faeces
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Design
2.2. Diets
2.3. Feed Sampling and Analyses
2.4. Caecum, Right Ventral Colon and Faecal Sampling and Measures
2.5. Microbial Analyses
2.6. Statistical Analysis
3. Results
3.1. Feed Intake
3.2. Cultured Bacterial Flora
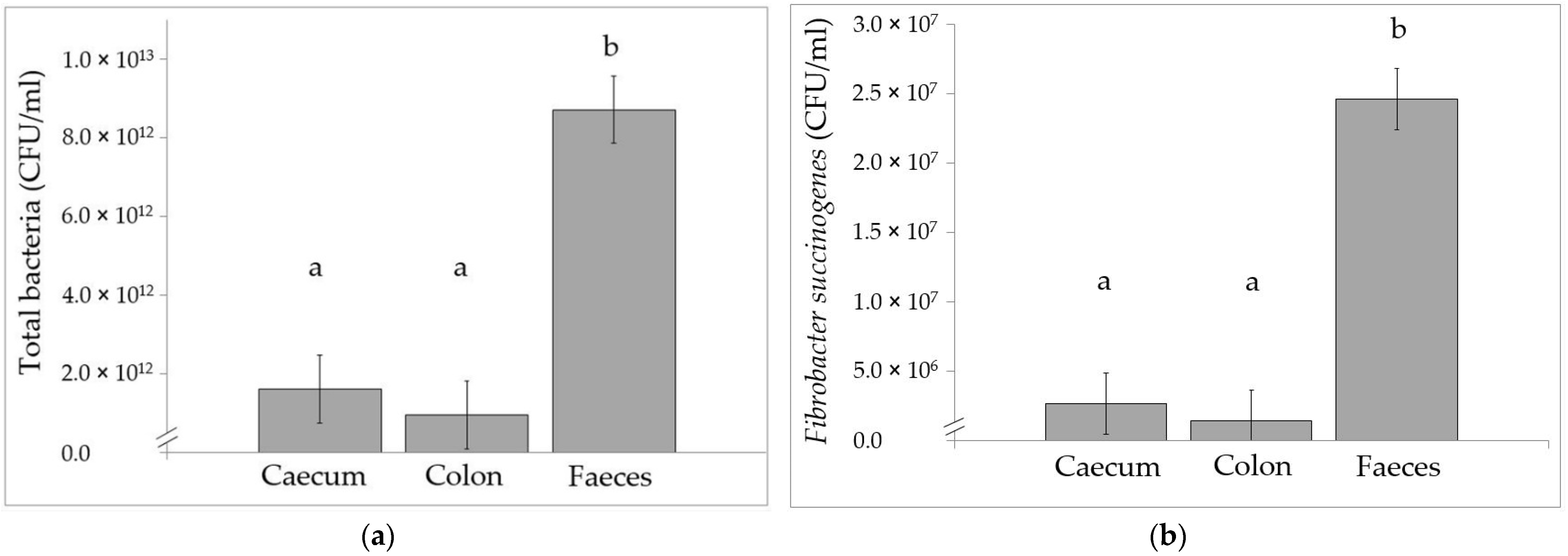
3.3. Bacterial Flora Using Real-Time qPCR Analysis
3.4. Fungi and Protozoa Using Real-Time qPCR Analysis
3.5. Short-Chain Fatty Acids, pH and Buffering Capacity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harris, P.A.; Ellis, A.D.; Fradinho, M.J.; Jansson, A.; Julliand, V.; Luthersson, N.; Santos, A.S.; Vervuert, I. Review: Feeding conserved forage to horses: Recent advances and recommendations. Animal 2017, 11, 958–967. [Google Scholar] [CrossRef] [PubMed]
- Darlington, J.M.; Hershberger, T.V. Effect of forage maturity on digestibility, intake and nutritive value of alfalfa, timothy and orchardgrass by equine. J. Anim. Sci. 1968, 27, 1572–1576. [Google Scholar] [CrossRef]
- Ragnarsson, S.; Lindberg, J.E. Nutritional value of timothy haylage in Icelandic horses. Livest. Sci. 2008, 113, 202–208. [Google Scholar] [CrossRef]
- Buxon, D.R.; Redfearn, D.D. Plant limitations to fiber digestion and utilization. J. Nutr. 1997, 127, 814S–818S. [Google Scholar] [CrossRef] [PubMed]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Hoffman, P.C.; Sievert, S.J.; Shaver, R.D.; Welch, D.A.; Combs, D.K. In situ dry matter, protein, and fiber degradation of perennial forages. J. Dairy Sci. 1993, 76, 2632–2643. [Google Scholar] [CrossRef]
- Julliand, V.; de Vaux, A.; Millet, L.; Fonty, G. Identification of Ruminococcus flavefaciens as the predominant cellulolytic bacterial species of the equine cecum. Appl. Environ. Microbiol. 1999, 65, 3738–3741. [Google Scholar] [CrossRef]
- Lin, C.; Stahl, D.A. Taxon-specific probes for the cellulolytic genus Fibrobacter reveal abundant and novel equine-associated populations. Appl. Environ. Microbiol. 1995, 61, 1348–1351. [Google Scholar] [CrossRef]
- Julliand, V.; Riondet, C.; de Vaux, A.; Alcaraz, G.; Fonty, G. Comparison of metabolic activities between Piromyces citronii, an equine fungal species, and Piromyces communis, a ruminal species. Anim. Feed Sci. Technol. 1998, 70, 161–168. [Google Scholar] [CrossRef]
- Orpin, C.G. Isolation of cellulolytic phycomycete fungi from the caecum of the horse. J. Gen. Microbiol. 1981, 123, 287–296. [Google Scholar] [CrossRef]
- Moore, B.E.; Dehority, B.A. Effects of diet and hindgut defaunation on diet digestibility and microbial concentrations in the cecum and colon of the horse. J. Anim. Sci. 1993, 71, 3350–3358. [Google Scholar] [CrossRef][Green Version]
- Snelling, T.J.; McEwan, N.R.; Newbold, C.J. Cellulolytic activity of ciliate protozoa in the equine caecum. In Proceedings of the Rowett-INRA 7th joint Symposium—Gut Microbiology: New Insights into Gut Microbial Ecosystems, Aberdeen, UK, 23–25 June 2010. [Google Scholar]
- Julliand, V.; Grimm, P. Horse Species Symposium: The microbiome of the horse hindgut: History and current knowledge. J. Anim. Sci. 2016, 94, 2262–2274. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Koike, S.; Miyaji, M.; Hata, H.; Tanaka, K. Hindgut microbes, fermentation and their seasonal variations in Hokkaido native horses compared to light horses. Ecol. Res. 2006, 21, 285–291. [Google Scholar] [CrossRef]
- Bonhomme-Florentin, A. Degradation of hemicellulose and pectin by horse caecum contents. Br. J. Nutr. 1988, 60, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Sadet-Bourgeteau, S.; Philippeau, C.; Dequiedt, S.; Julliand, V. Comparison of the bacterial community structure within the equine hindgut and faeces using Automated Ribosomal Intergenic Spacer Analysis (ARISA). Animal 2014, 8, 1928–1934. [Google Scholar] [CrossRef][Green Version]
- Kern, D.L.; Slyter, L.L.; Weaver, J.M.; Leffel, E.C.; Samuelsons, G. Pony cecum vs. steer rumen: The effect of oats and hay on the microbial ecosystem. J. Anim. Sci. 1973, 37, 463–469. [Google Scholar] [CrossRef] [PubMed]
- De Fombelle, A.; Varloud, M.; Goachet, A.G.; Jacotot, E.; Philippeau, C.; Drogoul, C.; Julliand, V. Characterization of the microbial and biochemical profile of the different segments of the digestive tract in horses given two distinct diets. Anim. Sci. 2003, 77, 293–304. [Google Scholar] [CrossRef]
- Nadeau, J.A.; Andrews, F.M.; Mathew, A.G.; Argenzio, R.A.; Blackford, J.T.; Sohtell, M.; Saxton, A.M. Evaluation of diet as a cause of gastric ulcers in horses. Am. J. Vet. Res. 2000, 61, 784–790. [Google Scholar] [CrossRef]
- Zeyner, A.; Geißler, C.; Dittrich, A. Effects of hay intake and feeding sequence on variables in faeces and faecal water (dry matter, pH value, organic acids, ammonia, buffering capacity) of horses. J. Anim. Physiol. Anim. Nutr. 2004, 88, 7–19. [Google Scholar] [CrossRef]
- Jansson, A.; Rundgren, M.; Lindberg, J.E.; Ronéus, M.; Hedendahl, A.; Kjellberg, L.; Lundberg, M.; Palmgren Karlsson, C.; Ekström, K. Utfodringsrekommendationer för Häst; Swedish University of Agricultural Sciences, Hippologenheten, Box 7046: Uppsala, Sweden, 2004. [Google Scholar]
- Lindgren, E. The Nutritional Value of Roughages Determined In Vivo and by Laboratory Methods; Report No. 45; Swedish University of Agricultural Sciences, Department of Animal Nutrition and Management: Uppsala, Sweden, 1979; p. 63. [Google Scholar]
- Larsson, K.; Bengtsson, S. Determination of readily available carbohydrates in plant material. In National Laboratory of Agriculture Chemistry Methods; Report No. 22; National Laboratory of Agriculture Chemistry: Uppsala, Sweden, 1983; p. 10. (In Swedish) [Google Scholar]
- Weissbach, F. Bestimmung der Pufferkapazität (Determination of Buffering Capacity); Method Description Federal Research Center of Agriculture, Institute of Grassland and Forage Research: Braunschweig, Germany, 1992; 3p. [Google Scholar]
- Jouany, J.P. Volatile fatty acid and alcohol determination in digestive contents, silage juices, bacterial cultures and anaerobic fermentor contents. Sci. Alim. 1982, 2, 131–144. [Google Scholar]
- Leedle, J.A.; Hespell, R.B. Differential carbohydrate media and anaerobic replica plating techniques in delineating carbohydrate-utilizing subgroups in rumen bacterial populations. Appl. Environ. Microbiol. 1980, 39, 709–719. [Google Scholar] [CrossRef]
- Halliwell, G.; Bryant, M.P. The cellulolytic activity of pure culture strains of bacteria from the rumen of cattle. J. Gen. Microbiol. 1963, 32, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Hughes, B.; Plantat, J.L. Calculation of the most probable number acceptance limit in case of important number of inoculums by dilution. Chemosphere 1983, 12, 1679–1684. [Google Scholar] [CrossRef]
- Grimm, P.; Julliand, V.; Philippeau, C.; Sadet-Bourgeteau, S. Effect of yeast supplementation on hindgut microbiota and digestibility of horses subjected to an abrupt change of hays. Livest. Sci. 2016, 186, 34–40. [Google Scholar] [CrossRef]
- Mackie, R.I.; Heath, S. Enumeration and isolation of lactate-utilizing bacteria from the rumen of sheep. Appl. Environ. Microbiol. 1979, 38, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Varloud, M. Implication des Micro-Organismes de L’estomac dans la Digestion de L’amidon par le Cheval. Ph.D. Thesis, INRA Paris-Grignon, Paris, France, 2006. [Google Scholar]
- Yu, Z.; Morrison, M. Improved extraction of PCR-quality community DNA from digesta and fecal samples. Biotechniques 2004, 36, 808–812. [Google Scholar] [CrossRef]
- Lyons, S.R.; Griffen, A.L.; Leys, E.J. Quantitative real-time PCR for Porphyromonas gingivalis and total bacteria. J. Clin. Microbiol. 2000, 38, 2362–2365. [Google Scholar] [CrossRef]
- Denman, S.E.; McSweeney, C.S. Development of a real-time PCR assay for monitoring anaerobic fungal and cellulolytic bacterial populations within the rumen. FEMS Microbiol. Ecol. 2006, 58, 572–582. [Google Scholar] [CrossRef]
- Denman, S.E.; Tomkins, N.W.; McSweeney, C.S. Quantitation and diversity analysis of ruminal methanogenic populations in response to the antimethanogenic compound bromochloromethane. FEMS Microbiol. Ecol. 2007, 62, 313–322. [Google Scholar] [CrossRef]
- Mosoni, P.; Chaucheyras-Durand, F.; Béra-Maillet, C.; Forano, E. Quantification by real-time PCR of cellulolytic bacteria in the rumen of sheep after supplementation of a forage diet with readily fermentable carbohydrates: Effect of a yeast additive. J. Appl. Microbiol. 2007, 103, 2676–2685. [Google Scholar] [CrossRef]
- Muhonen, S.; Connysson, M.; Lindberg, J.E.; Julliand, V.; Bertilsson, J.; Jansson, A. Effects of crude protein intake from grass silage-only diets on the equine colon ecosystem after an abrupt feed change. J. Anim. Sci. 2008, 86, 3465–3472. [Google Scholar] [CrossRef]
- Muhonen, S.; Julliand, V.; Lindberg, J.E.; Bertilsson, J.; Jansson, A. Effects on the equine colon ecosystem of grass silage and haylage diets after an abrupt change from hay. J. Anim. Sci. 2009, 87, 2291–2298. [Google Scholar] [CrossRef]
- Jouany, J.P.; Medina, B.; Bertin, G.; Julliand, V. Effect of live yeast culture supplementation on hindgut microbial communities and their polysaccharide and glycoside hydrolase activities in horses fed a high-fiber or high-starch diet. J. Anim. Sci. 2009, 87, 2844–2852. [Google Scholar] [CrossRef]
- Philippeau, C.; Sadet-Bourgeteau, S.; Varloud, M.; Julliand, V. Impact of barley form on equine total tract fibre digestibility and colonic microbiota. Animal 2015, 9, 1943–1948. [Google Scholar] [CrossRef] [PubMed]
- Hastie, P.M.; Mitchell, K.; Murray, J.M.D. Semi-quantitative analysis of Ruminococcus flavefaciens, Fibrobacter succinogenes and Streptococcus bovis in the equine large intestine using real-time polymerase chain reaction. Br. J. Nutr. 2008, 100, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Kern, D.L.; Slyter, L.L.; Leffel, E.C.; Weaver, J.M.; Oltjen, R.R. Ponies vs. steers: Microbial and chemical characteristics of intestinal digesta. J. Anim. Sci. 1974, 38, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Varel, V.H.; Robinson, I.M.; Jung, H.J.G. Influence of dietary fiber on xylanolytic and cellulolytic bacteria of adult pigs. Appl. Environ. Microbiol. 1987, 53, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Teunissen, M.J.; Huub, J.M.; Op den Camp, H.J.M. Anaerobic fungi and their cellulolytic and xylanolytic enzymes. Antonie Van Leeuwenhoek 1993, 63, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-∆∆CT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Mura, E.; Edwards, J.; Kittelmann, S.; Kaerger, K.; Voigt, K.; Mrázek, J.; Moniello, G.; Fliegerova, K. Anaerobic fungal communities differ along the horse digestive tract. Fungal Biol. 2019, 123, 240–246. [Google Scholar] [CrossRef]
- Salyers, A.A.; Vercellotti, J.R.; West, S.E.; Wilkins, T.D. Fermentation of mucin and plant polysaccharides by strains of Bacteroides from the human colon. Appl. Environ. Microbiol. 1977, 33, 319–322. [Google Scholar] [CrossRef] [PubMed]
- Ziołecki, A.; Wojciechowicz, M. Small pectinolytic spirochetes from the rumen. Appl. Environ. Microbiol. 1980, 39, 919–922. [Google Scholar] [CrossRef] [PubMed]
- Bayliss, C.E.; Houston, A.P. Characterization of plant polysaccharide- and mucin-fermenting anaerobic bacteria from human feces. Appl. Environ. Microbiol. 1984, 48, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Cornick, N.A.; Jensen, N.S.; Stahl, D.A.; Hartman, P.A.; Allison, M.J. Lachnospira pectinoschiza sp. nov., an anaerobic pectinophile from the pig intestine. Int. J. Syst. Bacteriol. 1994, 44, 87–93. [Google Scholar] [CrossRef]
- Jensen, N.S.; Canale-Parola, E. Nutritionally limited pectinolytic bacteria from the human intestine. Appl. Environ. Microbiol. 1985, 50, 172–173. [Google Scholar] [CrossRef]
- Hatfield, R.D.; Weimer, P.J. Degradation characteristics of isolated and in situ cell wall lucerne pectic polysaccharides by mixed ruminal microbes. J. Sci. Food Agric. 1995, 69, 185–196. [Google Scholar] [CrossRef]
- Ozeki, K.; Imai, S.; Katsuno, M. On the distribution of the ciliated protozoa in the large intestine of horse. Tohoku J. Agric. Res. 1973, 24, 86–101. [Google Scholar]
- Al Jassim, R.A.M.; Rowe, J.B. Better understanding of acidosis and its control. Proc. Recent Adv. Anim. Nutr. Aust. 1999, 12, 91–97. [Google Scholar]
- Al Jassim, R.A.M.; Scott, P.T.; Trebbin, A.L.; Trott, D.; Pollitt, C.C. The genetic diversity of lactic acid producing bacteria in the equine gastrointestinal tract. FEMS Microbiol. Lett. 2005, 248, 75–81. [Google Scholar] [CrossRef]
- Medina, B.; Girard, I.D.; Jacotot, E.; Julliand, V. Effect of a preparation of Saccharomyces cerevisiae on microbial profiles and fermentation patterns in the large intestine of horses fed a high fiber or a high starch diet. J. Anim. Sci. 2002, 80, 2600–2609. [Google Scholar] [CrossRef]
- Strobel, H.J.; Russell, J.B. Effect of pH and energy spilling on bacterial protein synthesis by carbohydrate-limited cultures of mixed rumen bacteria. J. Dairy Sci. 1986, 69, 2941–2947. [Google Scholar] [CrossRef]
- Ding, Z.; Rowe, J.B.; Godwin, I.R.; Xu, Y. The buffering capacity of caecal digesta exceeds that of rumen digesta from sheep fed pasture or roughage diets. Aust. J. Agr. Res. 1997, 48, 723–728. [Google Scholar] [CrossRef]
- Argenzio, R.A.; Stevens, C.E. Cyclic changes in ionic composition of digesta in the equine intestinal tract. Am. J. Physiol. 1975, 228, 1224–1230. [Google Scholar] [CrossRef] [PubMed]
- Gamble, C.T.; Chamberlain, C.C.; Merriman, G.M.; Lidvall, E.R. Effects of pelleting, pasture and selected diet ingredients on the incidence of esophagogastric ulcers in swine. J. Anim. Sci. 1967, 26, 1054–1058. [Google Scholar] [CrossRef] [PubMed]
- Argenzio, R.A.; Lowe, J.E.; Pickard, D.W.; Stevens, C.E. Digesta passage and water exchange in the equine large intestine. Am. J. Physiol. 1974, 226, 1035–1042. [Google Scholar] [CrossRef]

| Diet C | Diet G | Diet L | SEM | p-Values | |
|---|---|---|---|---|---|
| DM (kg/100 kg BW and day) | 1.4 a | 1.2 b | 1.4 a | 0.04 | 0.001 |
| Energy (MJ/100 kg BW and day) 2 | 12.1 ab | 12.5 a | 11.7 b | 0.39 | 0.032 |
| Crude protein | 181 a | 199 b | 191 a,b | 5.8 | 0.016 |
| Crude fibre | 420 a | 337 b | 499 c | 15.4 | <0.001 |
| Neutral detergent fibre | 775 a | 617 b | 742 a | 23.3 | 0.001 |
| Acid detergent fibre | 465 a | 362 b | 544 c | 15.8 | <0.001 |
| Acid detergent lignin | 54 a | 38 b | 97 c | 3.1 | <0.001 |
| Hemicellulose 3 | 309 a | 256 b | 197 c | 7.7 | <0.001 |
| Cellulose 3 | 407 a | 329 b | 446 c | 12.9 | <0.001 |
| Non-structural carbohydrates 4 | 316 a | 228 b | 301 a | 9.2 | <0.001 |
| Non-starch polysaccharides 4 | 161 a | 174 a | 204 b | 9.8 | 0.016 |
| Starch | 91 a | 4 b | 16 c | 1.8 | <0.001 |
| Water soluble carbohydrates 5 | 61 a | 53 b | 21 c | 2.1 | <0.001 |
| Glucose | 11 a | 10 a | 6 b | 0.4 | <0.001 |
| Fructose | 21 a | 24 b | 9 c | 0.6 | <0.001 |
| Sucrose | 13 a | 5 b | 0.1 c | 0.4 | <0.001 |
| Fructans | 9 a | 6 b | 5 c | 0.6 | 0.010 |
| Maltodextrins | 7 | 6 | 3 | 1.9 | 0.131 |
| Calcium | 17 a | 18 b | 21 c | 0.6 | <0.001 |
| Phosphorus | 3 | 3 | 3 | 0.1 | 0.235 |
| Magnesium | 3 a | 3 a | 5 b | 0.2 | <0.001 |
| Sodium | 4 a | 4 b | 4 a | 0.1 | 0.010 |
| Potassium | 25 a | 33 b | 34 b | 1.3 | 0.001 |
| Target | Forward Primer | Reverse Primer | Reference |
|---|---|---|---|
| Total bacteria | 5′-CGGCAACGAGCGCAACCC-3′ | 5′-CCATTGTAGCACGTGTGTAGC-3′ | [34] |
| Fibrobacter succinogenes | 5′ GTTCGGAATTACTGGGCGTAAA 3′ | 5′ CGCCTGCCCCTGAACTATC 3′ | [35] |
| Ruminococcus flavefaciens | 5′ CGAACGGAGATAATTTGAGTTTACTTAGG 3′ | 5′ CGGTCTCTGTATGTTATGAGGTATTACC 3′ | [34] |
| Ruminococcus albus | 5′ CCCTAAAAGCAGTCTTAGTTCG 3′ | 5′ CCTCCTTGCGGTTAGAACA 3′ | [36] |
| Fungi | 5′-GAGGAAGTAAAAGTCGTAACAAGGTTTC-3′ | 5′-CAAATTCACAAAGGGTAGGATGATT-3′ | [34] |
| Protozoa | 5′-GCTTTCGWTGGTAGTGTATT-3′ | 5′-CTTGCCCTCYAATCGTWCT-3′ | [35] |
| Diets | SEM | p-Values Diet | |||
|---|---|---|---|---|---|
| Diet C | Diet G | Diet L | |||
| Total anaerobic bacteria | 8.1 | 7.5 | 7.7 | 0.21 | 0.182 |
| Pectinolytic bacteria | 7.7 a | 7.0 b | 7.2 b | 0.15 | 0.022 |
| Xylanolytic bacteria | 7.6 a | 6.7 b | 7.1 b | 0.15 | 0.011 |
| Cellulolytic bacteria | 5.7 | 5.5 | 5.7 | 0.22 | 0.846 |
| Amylolytic bacteria | 6.0 | 5.7 | 5.3 | 0.27 | 0.202 |
| Lactate-utilising bacteria | 7.4 a | 6.4 b | 6.6 b | 0.18 | 0.007 |
| Segment | SEM | p-Value Segment | |||
|---|---|---|---|---|---|
| Caecum | Colon | Faeces | |||
| Fungi | |||||
| Diet C vs. G | 1.33 a | 2.06 a | 14.25 b | 3.337 | 0.014 |
| Diet C vs. L | 1.12 | 0.70 | 1.97 | 0.639 | 0.369 |
| Diet G vs. L | 9.17 | 1.40 | 0.16 | 3.900 | 0.208 |
| Protozoa | |||||
| Diet C vs. G | 1.15 | 1.17 | 2.41 | 1.140 | 0.660 |
| Diet C vs. L | 1.02 | 3.17 | 2.37 | 0.943 | 0.346 |
| Diet G vs. L | 1.89 | 1.00 | 2.24 | 0.775 | 0.566 |
| Segment | SEM | p-Value Segment | |||
|---|---|---|---|---|---|
| Caecum | Colon | Faeces | |||
| Total SCFA | 60.4 a | 64.7 a | 38.3 b | 3.91 | <0.001 |
| Acetate | 41.9 a | 45.2 a | 26.7 b | 2.44 | <0.001 |
| Propionate | 11.9 a | 12.4 a | 6.9 b | 0.95 | 0.001 |
| Butyrate | 5.0 a | 5.1 a | 2.8 b | 0.43 | 0.003 |
| Valerate | 0.8 | 0.6 | 0.4 | 0.15 | 0.134 |
| Iso-butyrate | 0.3 a | 0.6 a,b | 0.8 b | 0.11 | 0.038 |
| Iso-valerate | 0.6 | 0.8 | 0.7 | 0.13 | 0.245 |
| Diets | SEM | p-Values | |||||
|---|---|---|---|---|---|---|---|
| Diet C | Diet G | Diet L | Diet | Segment | Diet × Segment | ||
| D-lactate (mmol/L) | |||||||
| Caecum | 0.8 | 0.6 | 0.3 | 0.37 | 0.174 | 0.025 | 0.111 |
| Colon | 1.6 | 0.4 | 0.8 | ||||
| Faeces | 1.4 | 1.9 | 1.0 | ||||
| L-lactate (mmol/L) | |||||||
| Caecum | 1.1 | 0.5 | 0.3 | 0.23 | 0.022 | 0.640 | 0.127 |
| Colon | 1.3 | 0.3 | 0.7 | ||||
| Faeces | 0.7 | 0.7 | 0.6 | ||||
| pH | |||||||
| Caecum | 7.0 A | 7.0 A | 7.0 A | 0.08 | 0.012 | 0.027 | 0.002 |
| Colon | 6.9 A,B | 7.0 A | 7.1 A,B | ||||
| Faeces | 6.7 a,B | 6.5 a,B | 7.2 b,B | ||||
| BC (g lactic acid/kg dry digesta) | |||||||
| Caecum | 353 | 590 | 387 | 92.4 | 0.071 | <0.001 | 0.499 |
| Colon | 513 | 709 | 471 | ||||
| Faeces | 78 | 77 | 48 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muhonen, S.; Sadet-Bourgeteau, S.; Julliand, V. Effects of Differences in Fibre Composition and Maturity of Forage-Based Diets on the Microbial Ecosystem and Its Activity in Equine Caecum and Colon Digesta and Faeces. Animals 2021, 11, 2337. https://doi.org/10.3390/ani11082337
Muhonen S, Sadet-Bourgeteau S, Julliand V. Effects of Differences in Fibre Composition and Maturity of Forage-Based Diets on the Microbial Ecosystem and Its Activity in Equine Caecum and Colon Digesta and Faeces. Animals. 2021; 11(8):2337. https://doi.org/10.3390/ani11082337
Chicago/Turabian StyleMuhonen, Sara, Sophie Sadet-Bourgeteau, and Véronique Julliand. 2021. "Effects of Differences in Fibre Composition and Maturity of Forage-Based Diets on the Microbial Ecosystem and Its Activity in Equine Caecum and Colon Digesta and Faeces" Animals 11, no. 8: 2337. https://doi.org/10.3390/ani11082337
APA StyleMuhonen, S., Sadet-Bourgeteau, S., & Julliand, V. (2021). Effects of Differences in Fibre Composition and Maturity of Forage-Based Diets on the Microbial Ecosystem and Its Activity in Equine Caecum and Colon Digesta and Faeces. Animals, 11(8), 2337. https://doi.org/10.3390/ani11082337





