Calcinosis in Alpaca Crias (Vicugna pacos) Due to Vitamin D Intoxication—Clinical, Laboratory and Pathological Findings with a Focus on Kidney Function
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. History of the Cases and Pre-Treatments
2.1.1. Cria 1
2.1.2. Cria 2
2.1.3. Cria 3
2.2. Clinical Pathology
3. Results
3.1. Clinical and Laboratory Findings
3.1.1. Cria 1
3.1.2. Cria 2
3.1.3. Cria 3
3.2. Findings from Kidney Function Analysis
3.3. Findings from Electrophoresis
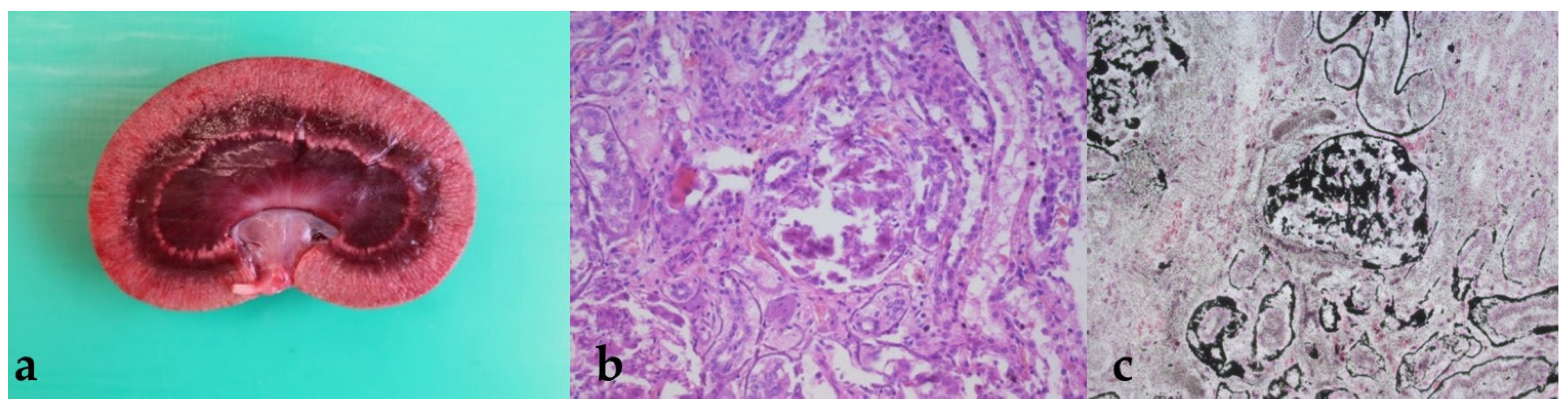
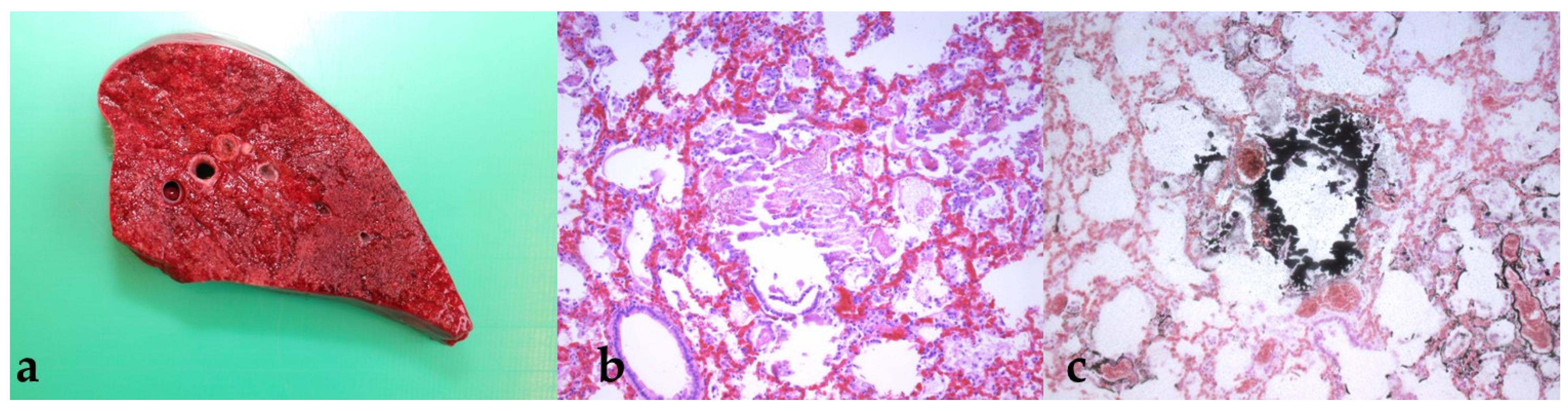
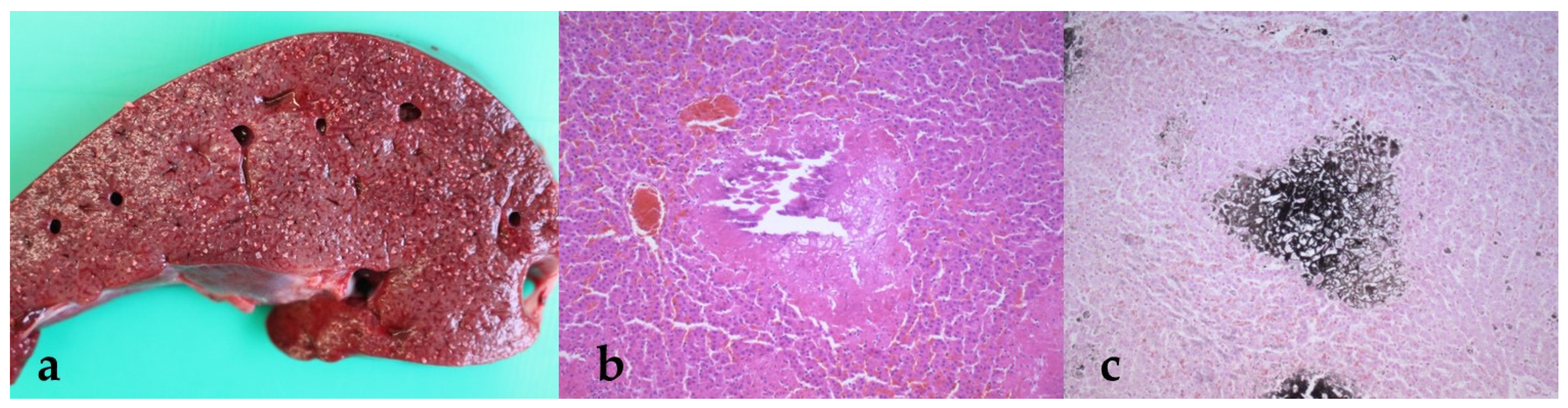
3.4. Findings from the Pathological Examination
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Neubert, S.; von Altrock, A.; Wendt, M.; Wagener, M.G. Llama and Alpaca Management in Germany—Results of an Online Survey among Owners on Farm Structure, Health Problems and Self-Reflection. Animals 2021, 11, 102. [Google Scholar] [CrossRef]
- Davis, R.; Keeble, E.; Wright, A.; Morgan, K. South American camelids in the United Kingdom: Population statistics, mortality rates and causes of death. Vet. Rec. 1998, 142, 162–166. [Google Scholar] [CrossRef]
- Hengrave Burri, I.; Martig, J.; Sager, H.; Liesegang, A.; Meylan, M. Neuweltkameliden in der Schweiz. I. Population, Haltung und Gesundheitsprobleme. Schweiz. Arch. Tierheilkd. 2005, 147, 325–334. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D metabolism, mechanism of action, and clinical applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Dittmer, K.; Thompson, K. Vitamin D metabolism and rickets in domestic animals: A review. Vet. Pathol. 2011, 48, 389–407. [Google Scholar] [CrossRef] [PubMed]
- Kofler, J.; Wittek, T. Rickets in a brown-coated Huacaya Alpaca cria—A case report. Wien. Tierärztliche Mon. 2015, 102, 80–87. [Google Scholar]
- Smith, B.B.; Van Saun, R.J. Seasonal changes in serum calcium, phosphorus, and vitamin D concentrations in llamas and alpacas. Am. J. Vet. Res. 2001, 62, 1187–1193. [Google Scholar] [CrossRef]
- Parker, J.E.; Timm, K.I.; Smith, B.B.; Van Saun, R.J.; Winters, K.M.; Sukon, P.; Snow, C.M. Seasonal interaction of serum vitamin D concentration and bone density in alpacas. Am. J. Vet. Res. 2002, 63, 948–953. [Google Scholar] [CrossRef]
- Van Saun, R.; Smith, B.; Watrous, B. Evaluation of vitamin D status of llamas and alpacas with hypophosphatemic rickets. J. Am. Vet. Med. Assoc. 1996, 209, 1128–1133. [Google Scholar]
- Schröder, C.; Seehusen, F.; Wolf, P.; Ganter, M. Rachitis bei einem Alpakafohlen-Ein Fallbericht. Tierärztliche Prax. Großtiere 2008, 36, 343–347. [Google Scholar]
- Judson, G.; Feakes, A. Vitamin D doses for alpacas (Lama pacos). Aust. Vet. J. 1999, 77, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Van Saun, R.J. Nutrient requirements of South American camelids: A factorial approach. Small Rumin. Res. 2006, 61, 165–186. [Google Scholar] [CrossRef]
- Fowler, M.E. Medicine and Surgery of Camelids; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Emmerich, I.U.; Ganter, M.; Wittek, T. Dosierungsvorschläge für Arzneimittel bei Kleinen Wiederkäuern und Neuweltkameliden; Schattauer Verlag: Stuttgart, Germany, 2013. [Google Scholar]
- Keindorf, H.-J. “Hyena disease” in cattle: A casuistic contribution. Dtsch. Tierärztliche Wochenschr. 2007, 114, 192–194. [Google Scholar]
- Kamphues, J.; Meyer, H.; Pohlenz, J.; Wirth, W. Vitamin D intoxication in Airedale puppies fed with milk replacer. Kleintierpraxis 1990, 35, 458–463. [Google Scholar]
- Lopez, I.; Pineda, C.; Muñoz, L.; Raya, A.; Lopez, G.; Aguilera-Tejero, E. Chronic vitamin D intoxication in captive iberian lynx (Lynx pardinus). PLoS ONE 2016, 11, e0156331. [Google Scholar]
- Hülsmann, H.; Stockhofe-Zurwieden, N.; Ganter, M.; Müller, E. Clinical findings in vitamin D poisoning of swine. Tierarztl. Prax. 1991, 19, 488–492. [Google Scholar] [PubMed]
- DeLuca, H.F.; Prahl, J.M.; Plum, L.A. 1,25-Dihydroxyvitamin D is not responsible for toxicity caused by vitamin D or 25-hydroxyvitamin D. Arch. Biochem. Biophys. 2011, 505, 226–230. [Google Scholar] [CrossRef]
- Kaune, R.; Schroeder, B.; Harmeyer, J. Binding properties of plasma vitamin D-binding protein and intestinal 1, 25-dihydroxyvitamin D3 receptor in piglets with pseudo-vitamin D-deficiency rickets, type I: Treatment effects with pharmacological doses of vitamin D3. Arch. Biochem. Biophys. 1990, 282, 326–332. [Google Scholar] [CrossRef]
- Lindt, S. Calcinosis of vitamin D excess in different animals. Wien. Tierärztliche Mon. 1968, 55, 148–164. [Google Scholar]
- Gerspach, C.; Bateman, S.; Sherding, R.; Chew, D.; Besier, A.; Grieves, J.; Lakritz, J. Acute renal failure and anuria associated with vitamin D intoxication in two alpaca (Vicugna pacos) cria. J. Vet. Intern. Med. 2010, 24, 443–449. [Google Scholar] [CrossRef]
- Tavella, A.; Stefani, A.; Zanardello, C.; Bettini, A.; Gauly, M.; Zanolari, P. Dystrophic mineralization of the arterial fibrovascular tissue associated with a vitamin D hypervitaminosis in an 8-year-old female Alpaca (Vicugna pacos). Ir. Vet. J. 2016, 69, 19. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Helmer, C.; Wagener, M.G.; Hannemann, R.; Schwennen, C.; Altrock, A.V.; Kleinschmidt, S.; Kammeyer, P.; Puff, C.; Becker, K.; Ganter, M. Hypervitaminosis D in new world camelids—Two case reports. Prakt. Tierarzt 2017, 98, 952–962. [Google Scholar]
- Jankovsky, J.M.; Newman, S.J. Pathology in Practice. J. Am. Vet. Med. Assoc. 2017, 251, 531–534. [Google Scholar] [CrossRef] [PubMed]
- Wagener, M.G.; Grimm, L.M.; Ganter, M. Anaemia in a llama (Lama glama): Treatment, regeneration and differential diagnoses. Vet. Rec. Case Rep. 2018, 6, e000638. [Google Scholar] [CrossRef]
- Wagener, M.G.; Grossmann, T.; Stöter, M.; Ganter, M. Hematological diagnostics in llamas and alpacas. Prakt. Tierarzt 2018, 99, 481–493. [Google Scholar]
- Wendt, M.; Waldmann, K.H.; Bickhardt, K. Comparison between the Clearance of Inulin and Endogenous Creatinine in Pigs. J. Vet. Med. Ser. A 1990, 37, 752–759. [Google Scholar] [CrossRef]
- Waldmann, K.; Wendt, M.; Bickhardt, K. Kreatinin-Clearance als Grundlage klinischer Nierenfunktionsbestimmung beim Schwein. Tierärztliche Prax. 1991, 19, 373–380. [Google Scholar]
- Bickhardt, K.; Deegen, E.; Espelage, W. Nierenfunktionsuntersuchungen bei Pferden–Methodik und Referenzwerte bei gesunden Tieren. Dtsch. Tierärztliche Wochenschr. 1996, 103, 113–122. [Google Scholar]
- Brandt, K.; Deegen, E.; Glitz, F.; Bickhardt, K. Nierenfunktionsanalysen bei Pferden mit Nephropathien. Pferdeheilkunde 1997, 13, 335–344. [Google Scholar] [CrossRef]
- Henniger, T.; Schwert, B.; Henniger, P.; Distl, O.; Ganter, M. Renal function tests in milk fed calves—Reference values and influence of bovine neonatal pancytopenia (BNP). Tierärztliche Prax. Großtiere 2013, 41, 345–352. [Google Scholar]
- Dawson, D.R.; DeFrancisco, R.J.; Mix, S.D.; Stokol, T. Reference intervals for biochemical analytes in serum and heparinized plasma and serum protein fractions in adult alpacas (Vicugna pacos). Vet. Clin. Pathol. 2011, 40, 538–548. [Google Scholar] [CrossRef] [PubMed]
- Hengrave Burri, I.; Tschudi, P.; Martig, J.; Liesegang, A.; Meylan, M. South American camelids in Switzerland. II. Reference values for blood parameters. Schweiz. Arch. Tierheilkd. 2005, 147, 335–343. [Google Scholar] [CrossRef]
- Dawson, D.R.; DeFrancisco, R.J.; Stokol, T. Reference intervals for hematologic and coagulation tests in adult alpacas (Vicugna pacos). Vet. Clin. Pathol. 2011, 40, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Garry, F.; Weiser, M.G.; Belknap, E. Clinical pathology of llamas. Vet. Clin. N. Am. Food Anim. Pract. 1994, 10, 201–209. [Google Scholar] [CrossRef]
- Wehner, A.; Katzenberger, J.; Groth, A.; Dorsch, R.; Koelle, P.; Hartmann, K.; Weber, K. Vitamin D intoxication caused by ingestion of commercial cat food in three kittens. J. Feline Med. Surg. 2013, 15, 730–736. [Google Scholar] [CrossRef] [PubMed]
- Araki, T.; Holick, M.F.; Alfonso, B.D.; Charlap, E.; Romero, C.M.; Rizk, D.; Newman, L.G. Vitamin D intoxication with severe hypercalcemia due to manufacturing and labeling errors of two dietary supplements made in the United States. J. Clin. Endocrinol. Metab. 2011, 96, 3603–3608. [Google Scholar] [CrossRef]
- Klontz, K.C.; Acheson, D.W. Dietary supplement-induced vitamin D intoxication. N. Engl. J. Med. 2007, 357, 308–309. [Google Scholar] [CrossRef]
- Renner, J.E. “Hyena disease” in cattle—A review. Dtsch. Tierärztliche Wochenschr. 1985, 92, 433–434. [Google Scholar]
- Van Saun, R.J.; Cebra, C. Nutritional diseases. In Llama and Alpaca Care: Medicine, Surgery, Reproduction, Nutrition, and Herd Health: First Edition; Elsevier Inc.: Amsterdam, The Netherlands, 2013; pp. 124–139. [Google Scholar]
- Fairweather, A.; Eason, C.; Elder, P.; Eason, C.; Arthur, D. Reference concentrations of cholecalciferol in animals: A basis for establishing non-target exposure. N. Z. J. Zool. 2013, 40, 280–289. [Google Scholar] [CrossRef]
- Jones, G. Pharmacokinetics of vitamin D toxicity. Am. J. Clin. Nutr. 2008, 88, 582S–586S. [Google Scholar] [CrossRef]
- Mello, J. Calcinosis—Calcinogenic plants. Toxicon 2003, 41, 1–12. [Google Scholar] [CrossRef]
- Bockisch, F.; Aboling, S.; Coenen, M.; Vervuert, I. Yellow oat grass intoxication in horses: Pitfalls by producing hay from extensive landscapes? A case report. Tierärztliche Prax. Großtiere 2015, 43, 296–304. [Google Scholar] [CrossRef]
- Kozikowski, T.A.; Magdesian, K.G.; Puschner, B. Oleander intoxication in New World camelids: 12 cases (1995–2006). J. Am. Vet. Med. Assoc. 2009, 235, 305–310. [Google Scholar] [CrossRef]
- Chamorro, M.F.; Passler, T.; Joiner, K.; Poppenga, R.H.; Bayne, J.; Walz, P.H. Acute renal failure in 2 adult llamas after exposure to Oak trees (Quercus spp.). Can. Vet. J. 2013, 54, 61. [Google Scholar] [PubMed]
- Poulsen, K.P.; Gerard, M.P.; Spaulding, K.A.; Geissler, K.A.; Anderson, K.L. Bilateral renal agenesis in an alpaca cria. Can. Vet. J. 2006, 47, 159. [Google Scholar] [PubMed]
- Hardefeldt, L.Y.; Textor, J.; Dart, A. Renal agenesis in an alpaca cria. Aust. Vet. J. 2007, 85, 185–187. [Google Scholar] [CrossRef] [PubMed]
- Angus, K.; Hodgson, J.; Hosie, B.; Low, J.; Mitchell, G.; Dyson, D.; Holliman, A. Acute nephropathy in young lambs. Vet. Rec. 1989, 124, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Wagener, M.G.; Lehmbecker, A.; Bühler, M.; Wilkens, M.; Punsmann, T.; Ganter, M. Calcinosis in a roe deer fawn (Capreolus capreolus) in northern Germany. BMC Vet. Res. 2020, 16, 406. [Google Scholar] [CrossRef] [PubMed]
- Giori, L.; Tricomi, F.M.; Zatelli, A.; Roura, X.; Paltrinieri, S. High-resolution gel electrophoresis and sodium dodecyl sulphate–agarose gel electrophoresis on urine samples for qualitative analysis of proteinuria in dogs. J. Vet. Diagn. Investig. 2011, 23, 682–690. [Google Scholar] [CrossRef]
- Chaudhary, Z.; Iqbal, J.; Rashid, J. Serum protein electrophoretic pattern in young and adult camels. Aust. Vet. J. 2003, 81, 625–626. [Google Scholar] [CrossRef] [PubMed]
| Animal | Cria 1 | Cria 2 | Cria 3 |
|---|---|---|---|
| Sex | male | male | female |
| Age | 4.5 months | 4 months | 3 months |
| Body weight (BW) | 25.5 kg | 19 kg | 14.5 kg |
| Recommended amount of vitamin D (2.000 IU per kg BW) | 51,000 | 38,000 | 29,000 |
| Injected amount of vitamin D (IU total) | 2,000,000 | 1,500,000 | 1,000,000 |
| Injected vitamin D (IU/kg BW) | 78,000 | 79,000 | 69,000 |
| Overdosing vitamin D x | 39.2 x | 39.5 x | 34.5 x |
| Time span of vitamin D injection until death | 18 days | 23 days | 15 days |
| Time span of vitamin D injection until symptoms appear | 7 days | 12 days | 7 days |
| Time span from onset of symptoms to death | 11 days | 11 days | 8 days |
| Cria 1 | Cria 2 | Cria 3 | ||||||
|---|---|---|---|---|---|---|---|---|
| Reference | Day 15 | Day 16 | Day 15 | Day 16 | Day 17 | Day 21 | Day 15 | |
| Hemoglobin (g/L) | 134–166 | 122 | 145 | 121 | 116 | |||
| PCV (L/L) | 0.29–0.37 | 0.25 | 0.30 | 0.25 | 0.23 | |||
| Leucocytes (G/L) | 7.3–16.0 | 3.9 | 14.1 | 26 | 8.7 | |||
| Lymphocytes (G/L) | 1.4–5.9 | 3.22 | 1.13 | 6.5 | 3.22 | |||
| Neutrophils (G/L) | 2.9–8.0 | 1.9 | 8.95 | 11.7 | 3.7 | |||
| Band neutrophils (G/L) | 0–0.2 | 0.47 | 0.35 | 5.59 | 1.18 | |||
| Eosinophils (G/L) | 0.1–3.1 | 0.06 | 3.38 | 0 | 0.17 | |||
| Basophils (G/L) | 0–0.1 | 0.02 | 0.14 | 0 | 0.04 | |||
| Monocytes (G/L) | 0.3–3.9 | 0.14 | 0.14 | 2.21 | 0.35 | |||
| Normoblasts % | 0–3 | 2 | 0 | 0 | 1 | |||
| 25-OH vitamin D (nmol/L) | 50–200 | >3750 | >3750 | >3750 | ||||
| Creatinine (µmol/L) | 88–212 | 2340 | 2601 | 1116 | 1068 | 1054 | 2905 | |
| Urea (mmol/L) | 1.67–5 | 93.35 | 83.71 | 68.94 | 67.17 | 128.7 | 140.3 | |
| Calcium (total) (mmol/L) | 2.09–2.54 | 3.39 | 3.84 | 2.76 | 2.49 | 3.31 | 3.55 | |
| Phosphate (mmol/L) | 1.1–2.62 | 4.81 | 5.18 | 5.32 | 5.46 | 7.18 | 7.16 | |
| Sodium (mmol/L) | 144–156 | 138.3 | 142.4 | 159.4 | ||||
| Potassium (mmol/L) | 3.9–5.5 | 5.41 | 6.07 | 9.04 | ||||
| Protein (g/L) | 58–73 | 38.8 | 37.7 | 35.8 | 36.0 | 33.9 | ||
| L-lactate (mmol/L) | 4.06 | 3.99 | 4.96 | 1.22 | 1.09 | |||
| D-lactate (mmol/L) | 0.16 | 0.18 | 0.09 | 0.16 | 0.29 | |||
| pH | 7.45 | 7.33 | 7.19 | 7.27 | 7.30 | 7.54 | ||
| Cria 1 | Cria 2 | |
|---|---|---|
| Physical parameters | ||
| pH | 6.5 | 5.5 |
| Specific gravity | 1.037 | 1.017 |
| Color | yellow | light yellow |
| Metabolites | ||
| Protein (g/L) | 40.5 | 6.8 |
| Glucose (g/L) | 0 | 0 |
| Ketone | - | - |
| Blood | +++ | ++++ |
| Nitrite | - | - |
| Cells | ||
| Leucozytes | ++ | +++ |
| Bacteria | ++ | + |
| Erythrocytes | ++++ | +++ |
| Squamous epithelial cells | - | ++ |
| Round epithelial cells | ++ | + |
| Crystals | ||
| Ca-carbonate | ++ | - |
| Ca-oxalate | (+) | - |
| Tripelphosphate | - | - |
| Amorphous phosphate | - | - |
| Nonspecific crystals | + | (+) |
| Reference | Cria 1 | Cria 2 | |
|---|---|---|---|
| Protein (g/L) | 58–73 | 41.5 | 31.5 |
| Albumin (g/L) | 28–43 | 23.1 | 13.7 |
| Globulin/albumin | 0.9–1.7 | 0.8 | 1.3 |
| CK (U/L) | 28–144 | 98 | 53 |
| ASAT (U/L) | 125–317 | 280 | 100 |
| CK/ASAT | 0.4 | 0.5 | |
| GLDH (U/L) | 3–19 | 12 | 29 |
| Creatinine (µmol/L) | 88–212 | 2191 | 977 |
| Urea (mmol/L) | 1.67–5 | 99.38 | 58.06 |
| Calcium (mmol/L) | 2.09–2.54 | 3.36 | 2.45 |
| Phosphate (mmol/L) | 1.1–2.62 | 5.56 | 4.54 |
| Sodium (mmol/L) | 144–156 | 137.3 | 147.7 |
| Potassium (mmol/L) | 3.9–5.5 | 4.15 | 4.65 |
| Reference A | Reference B | Cria 1 | Cria 2 | |
|---|---|---|---|---|
| Creatinine (µmol/L) | 9.162 | 9.183 | ||
| Creatinin-clearence (mL/min/kg) | 1.24–2.59 | 0.08 | 0.17 | |
| Calcium (mmol/L) | 2.58 | 0.82 | ||
| FE Calcium (%) | 1.3–33.2 | 18.36 | 3.56 | |
| Phosphate (mmol/L) | 3.54 | 20.92 | ||
| FE phosphate (%) | 0.02–4.86 | 15.23 | 49.02 | |
| Sodium (mmol/L) | 12.7 | 0.5 | ||
| FE sodium | 0.004–1.040 | <1 | 2.21 | 0.04 |
| Potassium (mmol/L) | 170.5 | 53.5 | ||
| FE potassium (%) | 11–118 | 50–120 | 982.49 | 122.41 |
| FE water (%) | 0.14–2.16 | 23.91 | 10.64 | |
| GGT (U/L) | 0–29 | 125.2 | 45.3 | |
| GGT (urine)/crea (urine) | 0.1–0.64 | 13.67 | 4.93 |
| Plasma | Urine | ||||
|---|---|---|---|---|---|
| Reference | Cria 1 | Cria 2 | Cria 1 | Cria 2 | |
| Total protein (g/L) | 57–72 | 41.5 | 31.5 | 40.5 | 6.8 |
| Albumin (g/L) | 29–40 | 19.7 | 12.2 | 29.5 | 3.6 |
| Alpha-1-globulin (g/L) | 1–5 | 1.1 | 1.2 | 2.9 | 2.3 |
| Alpha-2-globulin (g/L) | 3–7 | 3.6 | 3.4 | 3.4 | 0.6 |
| Beta-globulin (g/L) | 9–16 | 9.4 | 4.8 | 2.0 | 0.3 |
| Gamma-globulin (g/L) | 5–15 | 11.7 | 9.9 | 2.7 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wagener, M.G.; Helmer, C.; Kammeyer, P.; Kleinschmidt, S.; Punsmann, T.M.; Meilwes, J.M.; Schwennen, C.; von Altrock, A.; Wilkens, M.; Schwert, B.; et al. Calcinosis in Alpaca Crias (Vicugna pacos) Due to Vitamin D Intoxication—Clinical, Laboratory and Pathological Findings with a Focus on Kidney Function. Animals 2021, 11, 2332. https://doi.org/10.3390/ani11082332
Wagener MG, Helmer C, Kammeyer P, Kleinschmidt S, Punsmann TM, Meilwes JM, Schwennen C, von Altrock A, Wilkens M, Schwert B, et al. Calcinosis in Alpaca Crias (Vicugna pacos) Due to Vitamin D Intoxication—Clinical, Laboratory and Pathological Findings with a Focus on Kidney Function. Animals. 2021; 11(8):2332. https://doi.org/10.3390/ani11082332
Chicago/Turabian StyleWagener, Matthias Gerhard, Carina Helmer, Patricia Kammeyer, Sven Kleinschmidt, Teresa Maria Punsmann, Johanna Maria Meilwes, Cornelia Schwennen, Alexandra von Altrock, Mirja Wilkens, Barbara Schwert, and et al. 2021. "Calcinosis in Alpaca Crias (Vicugna pacos) Due to Vitamin D Intoxication—Clinical, Laboratory and Pathological Findings with a Focus on Kidney Function" Animals 11, no. 8: 2332. https://doi.org/10.3390/ani11082332
APA StyleWagener, M. G., Helmer, C., Kammeyer, P., Kleinschmidt, S., Punsmann, T. M., Meilwes, J. M., Schwennen, C., von Altrock, A., Wilkens, M., Schwert, B., von Keyserlingk-Eberius, N., & Ganter, M. (2021). Calcinosis in Alpaca Crias (Vicugna pacos) Due to Vitamin D Intoxication—Clinical, Laboratory and Pathological Findings with a Focus on Kidney Function. Animals, 11(8), 2332. https://doi.org/10.3390/ani11082332







