High β-Lactam and Quinolone Resistance of Enterobacteriaceae from the Respiratory Tract of Sheep and Goat with Respiratory Disease
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Sampling
2.2. Bacterial Isolation and Identification
2.3. Antimicrobial Sensitivity Testing
2.4. Detection of Carbapenemase Production
2.5. Detection of ESBL and AmpC production
2.6. DNA Preparation for PCR Experiments
2.7. Molecular Screening for ESBL and AmpC-Encoding Genes
2.8. Molecular Screening for Quinolone and Aminoglycoside Resistance
2.9. Sequencing and Sequence Data Analysis
2.10. Statistical Analysis
3. Results
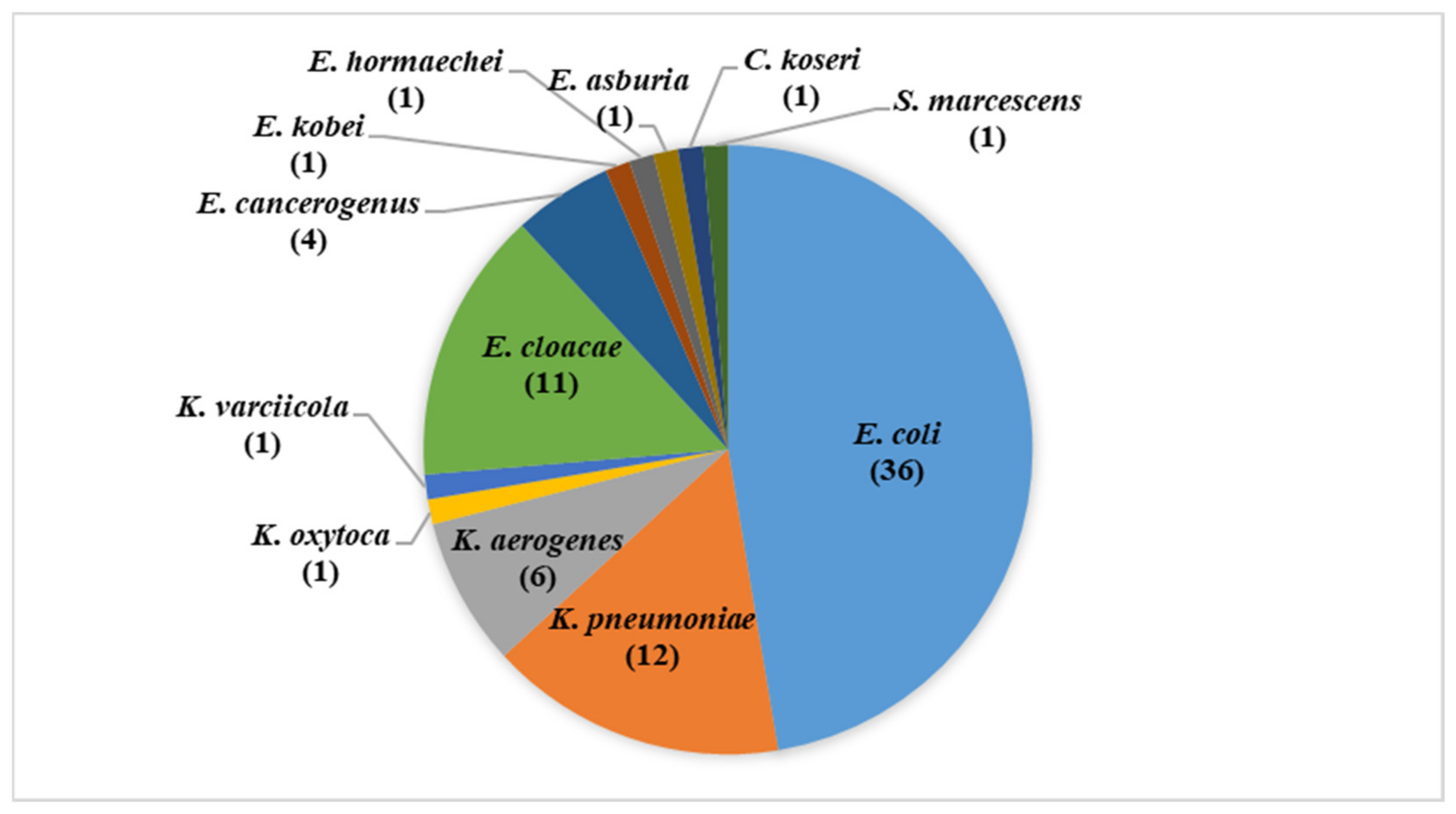
3.1. Isolates Identification
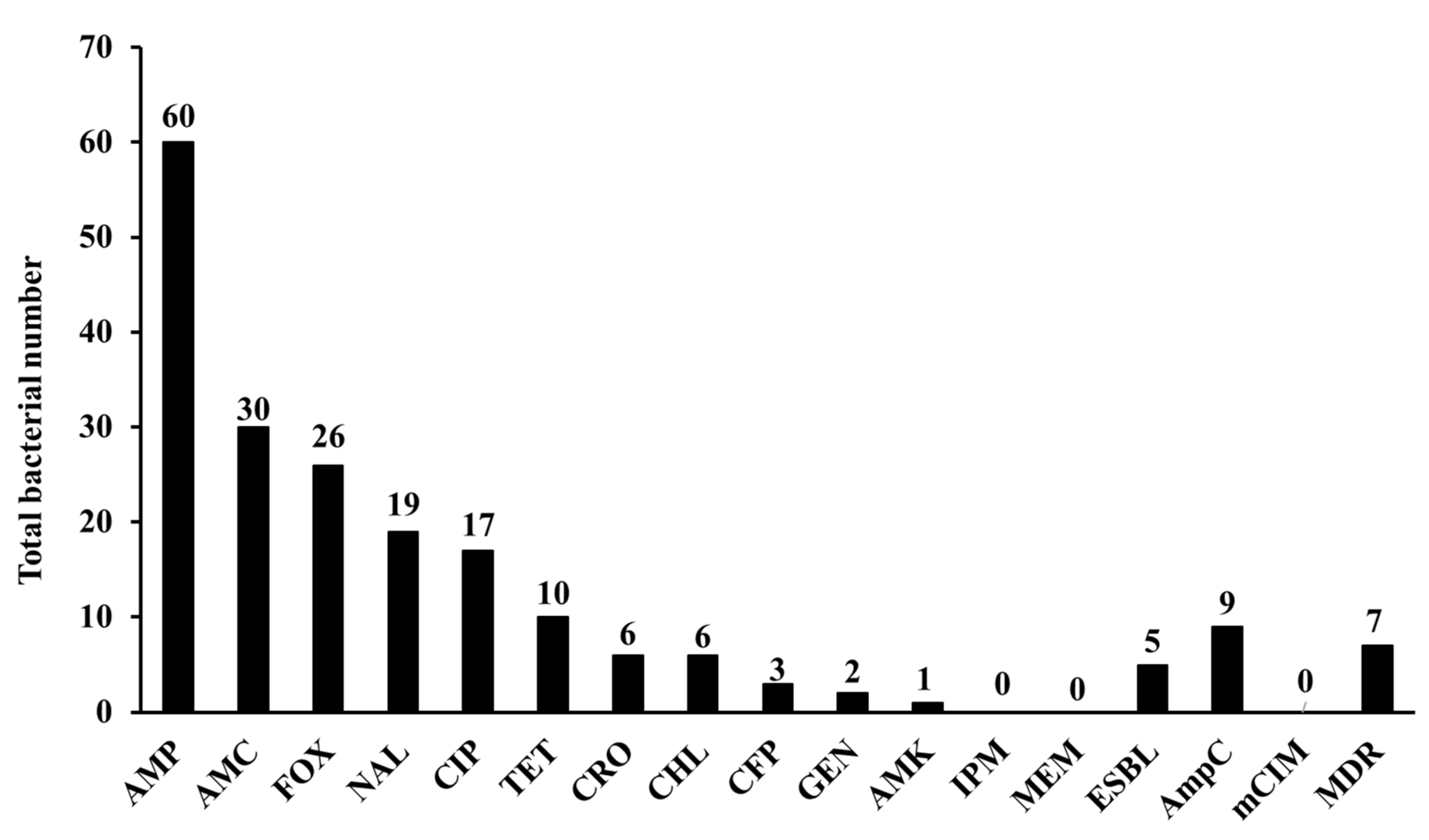
3.2. Phenotypic Characterization of Isolates
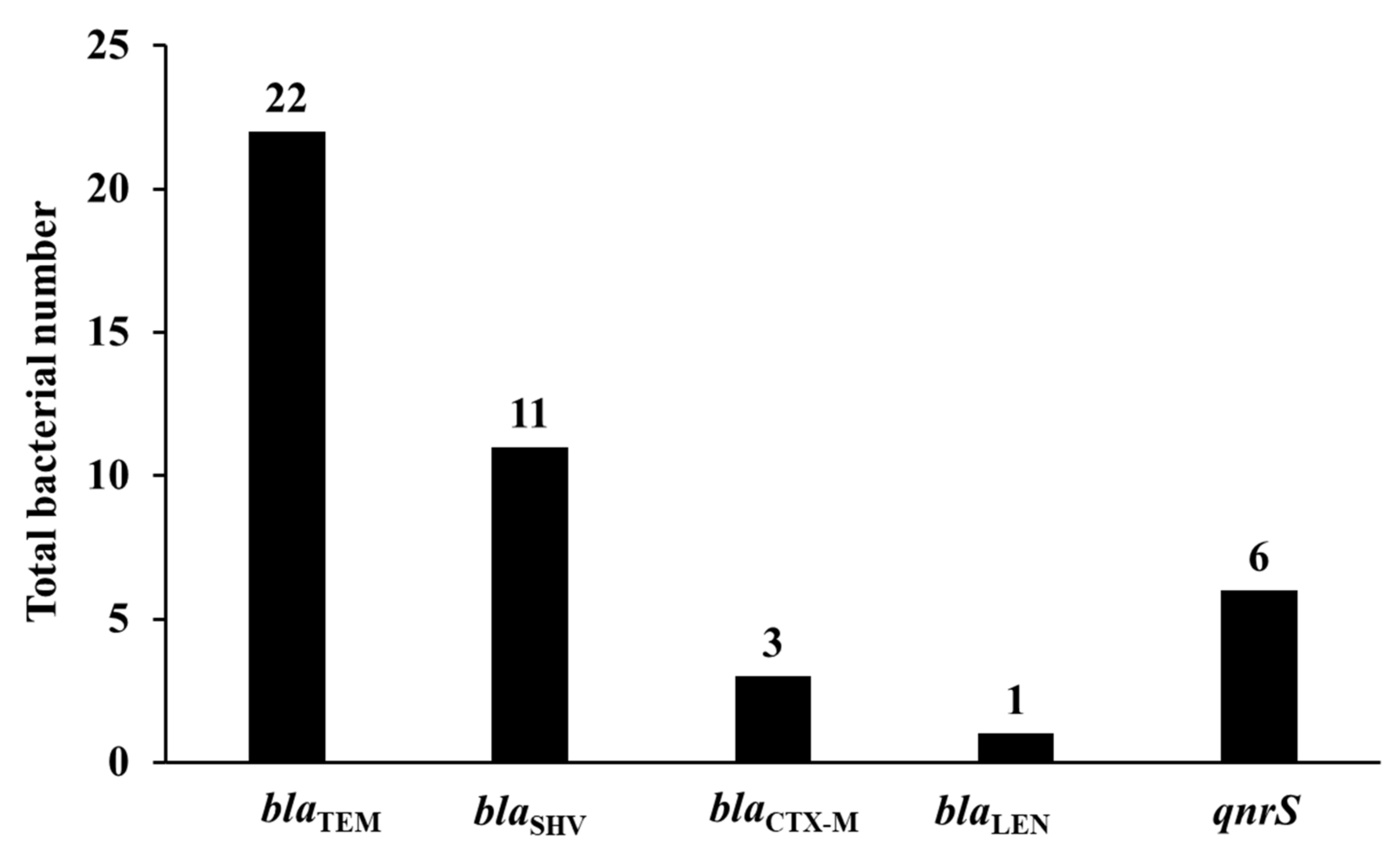
3.3. Prevalence of β-Lactamase Encoding Genes
3.4. Prevalence of Plasmid-Mediated Quinolone Resistance Genes and Other Resistance Genes
3.5. Relation between the Recovered Isolates and Resistant Determinants
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wall, B.A.; Mateus, A.L.P.; Marshall, L.; Pfeiffer, D.U.; Lubroth, J.; Ormel, H.J.; Otto, P.; Patriarchi, A. Drivers, Dynamics and Epidemiology of Antimicrobial Resistance in Animal Production; Food and Agriculture Organization of the United Nations: Rome, Italy, 2016. [Google Scholar]
- Wozniak, T.M.; Barnsbee, L.; Lee, X.J.; Pacella, R.E. Using the best available data to estimate the cost of antimicrobial resistance: A systematic review. Antimicrob. Resist. Infect. Control. 2019, 8, 26. [Google Scholar] [CrossRef] [PubMed]
- Ecdc/Efsa/EmEa. ECDC/EFSA/EMA First joint report on the integrated analysis of the consumption of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from humans and food-producing animals. EFSA J. 2015, 13, 114. [Google Scholar]
- Ejaz, H.; Younas, S.; Abosalif, K.O.; Junaid, K.; Alzahrani, B.; Alsrhani, A.; Abdalla, A.E.; Ullah, M.I.; Qamar, M.U.; Hamam, S.S. Molecular analysis of blaSHV, blaTEM, and blaCTX-M in extended-spectrum β-lactamase producing Enterobacteriaceae recovered from fecal specimens of animals. PLoS ONE 2021, 16, e0245126. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, H.O.; Oreiby, A.F.; Abd El-Hafeez, A.A.; Okanda, T.; Haque, A.; Anwar, K.S.; Tanaka, M.; Miyako, K.; Tsuji, S.; Kato, Y.; et al. First report of multidrug-resistant carbapenemase-producing bacteria co-harboring mcr-9 associated with pets’ respiratory disease complex: A potential of animal–human transmission. Antimicrob. Agents Chemother. 2020, 65, e01890-20. [Google Scholar] [CrossRef]
- Khalifa, H.O.; Oreiby, A.F.; Okanda, T.; Kato, Y.; Matsumoto, T. High β-lactam resistance in Gram-negative bacteria associated with kennel cough and cat flu in Egypt. Sci. Rep. 2021, 11, 1–9. [Google Scholar] [CrossRef]
- Khalifa, H.O.; Soliman, A.M.; Ahmed, A.M.; Shimamoto, T.; Nariya, H.; Matsumoto, T.; Shimamoto, T. High prevalence of antimicrobial resistance in gram-negative bacteria isolated from clinical settings in Egypt: Recalling for judicious use of conventional antimicrobials in developing nations. Microb. Drug Resist. 2019, 25, 371–385. [Google Scholar] [CrossRef]
- Khalifa, H.O.; Soliman, A.M.; Ahmed, A.M.; Shimamoto, T.; Hara, T.; Ikeda, M.; Kuroo, Y.; Kayama, S.; Sugai, M.; Shimamoto, T. High carbapenem resistance in clinical Gram-negative pathogens isolated in Egypt. Microb. Drug Resist. 2017, 23, 838–844. [Google Scholar] [CrossRef]
- Khalifa, H.O.; Soliman, A.M.; Ahmed, A.M.; Shimamoto, T.; Shimamoto, T. NDM-4-and NDM-5-producing Klebsiella pneumoniae coinfection in a 6-month-old infant. Antimicrob. Agents Chemother. 2016, 60, 4416–4417. [Google Scholar] [CrossRef]
- Khalifa, H.O.; Ahmed, A.M.; Oreiby, A.F.; Eid, A.M.; Shimamoto, T.; Shimamoto, T. Characterization of the plasmid-mediated colistin resistance gene mcr-1 in Escherichia coli isolated from animals in Egypt. Int. J. Antimicrob. Agent 2016, 5, 413–414. [Google Scholar] [CrossRef]
- Elnahriry, S.S.; Khalifa, H.O.; Soliman, A.M.; Ahmed, A.M.; Hussein, A.M.; Shimamoto, T.; Shimamoto, T. Emergence of plasmid-mediated colistin resistance gene mcr-1 in a clinical Escherichia coli isolate from Egypt. Antimicrob. Agents Chemother. 2016, 60, 3249–3250. [Google Scholar] [CrossRef]
- Oreiby, A.; Khalifa, H.; Eid, A.; Ahmed, A.; Shimamoto, T. Staphylococcus aureus and bovine mastitis: Molecular typing of methicillin resistance and clinical description of infected quarters. J. Hell. Vet. Med. Soc. 2019, 70, 1511–1516. [Google Scholar] [CrossRef]
- World Health Organization. Critically Important Antimicrobials for Human Medicine, 6th ed.; World Health Organization: Geneva, Switzerland, 2018; Available online: https://apps.who.int/iris/bitstream/handle/10665/312266/9789241515528-eng.pdf (accessed on 20 May 2021).
- Hooper, D.C.; Jacoby, G.A. Mechanisms of drug resistance: Quinolone resistance. Ann. N. Y. Acad. Sci. 2015, 1354, 12–31. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standard Institute. Performance Standard for Antimicrobial Susceptibility Testing; Twenty Second Informational Supplement; Clinical and Laboratory Standard Institute: Wayne, PA, USA, 2012; Volume 32, M100-S22. [Google Scholar]
- Khalifa, H.O.; Okanda, T.; Abd El-Hafeez, A.A.; Abd El Latif, A.; Habib, A.G.; Yano, H.; Kato, Y.; Matsumoto, T. Comparative evaluation of five assays for detection of carbapenemases with a proposed scheme for their precise application. J. Mol. Diagn. 2020, 22, 1129–1138. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Ensor, V.; Gossain, S.; Nye, K.; Hawkey, P. Rapid and simple detection of blaCTX-M genes by multiplex PCR assay. J. Med. Microbiol. 2005, 54, 1183–1187. [Google Scholar] [CrossRef]
- Caroline, D.; Anaelle, D.C.; Dominique, D.; Christine, F.; Guillaume, A. Development of a set of multiplex PCR assays for the detection of genes encoding important β-lactamases in Enterobacteriaceae. J. Antimicrob. Chemother. 2010, 65, 490–495. [Google Scholar]
- Perez-Perez, F.J.; Hanson, N.D. Detection of plasmid-mediated AmpC beta-lactamase genes in clinical isolates by using multiplex PCR. J. Clin. Microbiol. 2002, 40, 2153–2162. [Google Scholar] [CrossRef]
- Jacoby, G.A.; Gacharna, N.; Black, T.A.; Miller, G.H.; Hooper, D.C. Temporal appearance of plasmid-mediated quinolone resistance genes. Antimicrob. Agents Chemother. 2009, 53, 1665–1666. [Google Scholar] [CrossRef]
- Kim, H.B.; Park, C.H.; Kim, C.J.; Kim, E.C.; Jacoby, G.A.; Hooper, D.C. Prevalence of plasmid-mediated quinolone resistance determinants over a 9-year period. Antimicrob. Agents Chemother. 2009, 53, 639–645. [Google Scholar] [CrossRef]
- Wangkheimayum, J.; Paul, D.; Dhar, D.; Nepram, R.; Chetri, S.; Bhowmik, D.; Chakravarty, A.; Bhattacharjee, A. Occurrence of acquired 16S rRNA methyltransferase-mediated aminoglycoside resistance in clinical isolates of Enterobacteriaceae within a tertiary referral hospital of Northeast India. Antimicrob. Agents Chemother. 2017, 61, e01037-16. [Google Scholar] [CrossRef]
- Khalifa, H.O.; Arai, T.; Majima, H.; Watanabe, A.; Kamei, K. Genetic basis of azole and echinocandin resistance in clinical Candida glabrata in Japan. Antimicrob. Agents Chemother. 2020, 64, e00783-20. [Google Scholar] [CrossRef]
- Khalifa, H.O.; Arai, T.; Majima, H.; Watanabe, A.; Kamei, K. Evaluation of Surveyor nuclease for rapid identification of FKS genes mutations in Candida glabrata. J. Infect. Chemother. 2021, 27, 834–839. [Google Scholar] [CrossRef]
- Khalifa, H.O.; Soliman, A.M.; Saito, T.; Kayama, S.; Yu, L.; Hisatsune, J.; Sugai, M.; Nariya, H.; Ahmed, A.M.; Shimamoto, T.; et al. First report of foodborne Klebsiella pneumoniae co-harboring blaVIM-1, blaNDM-1, and mcr-9. Antimicrob. Agents Chemother. 2020, 64, e00882-20. [Google Scholar] [CrossRef]
- Soliman, A.M.; Khalifa, H.O.; Ahmed, A.M.; Shimamoto, T.; Shimamoto, T. Emergence of an NDM-5-producing clinical Escherichia coli isolate in Egypt. Int. J. Infect. Dis. 2016, 48, 46–48. [Google Scholar] [CrossRef] [PubMed]
- Awosile, B.B.; Heider, L.C.; Saab, M.E.; McClure, J.T. Antimicrobial resistance in mastitis, respiratory and enteric bacteria isolated from ruminant animals from the Atlantic Provinces of Canada from 1994–2013. Can. Vet. J. 2018, 59, 1099–1104. [Google Scholar]
- Berge, A.C.; Sischo, W.M.; Craigmill, A.L. Antimicrobial susceptibility patterns of respiratory tract pathogens from sheep and goats. J. Am. Vet. Med. Assoc. 2006, 229, 1279–1281. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.K.; Saddique, U.; Ahmad, S.; Hayat, Y.; ur Rahman, S.; Hassan, M.F.; Ali, T. Isolation rate and antimicrobial susceptibility profiles of Mycoplasma mycoides subspecies capri field isolates from sheep and goats in Pakistan. Small Rumin. Res. 2017, 153, 118–122. [Google Scholar] [CrossRef]
- Emikpe, B.O.; Oyero, O.G.; Akpavie, S.O. Isolation and antibiogram of aerobic nasal bacterial flora of apparently healthy West African dwarf goats. Rev. D’elevage Med. Vet. Pays Trop. 2009, 62, 17–21. [Google Scholar] [CrossRef][Green Version]
- Trott, D. β-lactam resistance in Gram-negative pathogens isolated from animals. Curr. Pharm. Des. 2013, 19, 239–249. [Google Scholar] [CrossRef]
- Pehlivanoglu, F.; Turutoglu, H.; Ozturk, D.; Yardimci, H. Molecular characterization of ESBL-producing Escherichia coli isolated from healthy cattle and sheep. Acta Vet. 2016, 66, 520–533. [Google Scholar] [CrossRef]
- Seni, J.; Falgenhauer, L.; Simeo, N.; Mirambo, M.M.; Imirzalioglu, C.; Matee, M.; Rweyemamu, M.; Chakraborty, T.; Mshana, S.E. Multiple ESBL-producing Escherichia coli sequence types carrying quinolone and aminoglycoside resistance genes circulating in companion and domestic farm animals in Mwanza, Tanzania, harbor commonly occurring plasmids. Front. Microbiol. 2016, 7, 142. [Google Scholar] [CrossRef]
- Elafify, M.; Khalifa, H.O.; Al-Ashmawy, M.; Elsherbini, M.; El Latif, A.A.; Okanda, T.; Matsumoto, T.; Koseki, S.; Abdelkhalek, A. Prevalence and antimicrobial resistance of Shiga toxin-producing Escherichia coli in milk and dairy products in Egypt. J. Environ. Sci. Health B 2020, 55, 265–272. [Google Scholar] [CrossRef]
- Li, X.Z.; Mehrotra, M.; Ghimire, S.; Adewoye, L. β-Lactam resistance and β-lactamases in bacteria of animal origin. Vet. Microbiol. 2007, 121, 197–214. [Google Scholar] [CrossRef]
- Rensing, K.L.; Abdallah, H.M.; Koek, A.; Elmowalid, G.A.; Vandenbroucke-Grauls, C.M.; Al Naiemi, N.; van Dijk, K. Prevalence of plasmid-mediated AmpC in Enterobacteriaceae isolated from humans and from retail meat in Zagazig, Egypt. Antimicrob. Resist. Infect. Control. 2019, 8, 45. [Google Scholar] [CrossRef] [PubMed]
- Veldman, K.; Cavaco, L.M.; Mevius, D.; Battisti, A.; Franco, A.; Botteldoorn, N.; Bruneau, M.; Perrin-Guyomard, A.; Cerny, T.; De Frutos Escobar, C.; et al. International collaborative study on the occurrence of plasmid-mediated quinolone resistance in Salmonella enterica and Escherichia coli isolated from animals, humans, food and the environment in 13 European countries. J. Antimicrob. Chemother. 2011, 66, 1278–1286. [Google Scholar] [CrossRef] [PubMed]
- Baguy, O.M.; Nathalie, G.K.; David, C.N.G.; Daniel, S.N.G.; Julien, C.K.; Rose, K.N.; Djénéba, O.G.; Valérie, G.; Bertin, T.K.; Mireille, D. First report of qnr genes in multidrugs resistant (ESBL) enterobacteria isolated from different ecosystems in Abidjan, Ivory Coast. Int. J. Biol. Sci. Appl. 2014, 1, 170–175. [Google Scholar]
- Shabana, I.I.; Al-Enazi, A.T. Investigation of plasmid-mediated resistance in E. coli isolated from healthy and diarrheic sheep and goats. Saudi. J. Biol. Sci. 2020, 27, 788–796. [Google Scholar] [CrossRef]
- Kilani, H.; Ferjani, S.; Mansouri, R.; Boutiba-Benboubaker, I.; Abbassi, M.S. Occurrence of plasmid-mediated quinolone resistance determinants among Escherichia coli strains isolated from animals in Tunisia: Specific pathovars acquired qnr genes. J. Glob. Antimicrobe. Resist. 2020, 20, 50–55. [Google Scholar] [CrossRef] [PubMed]
| Primer Name | Sequence (5’-3’) | Amplicon Size (bp) | Target | Reference |
|---|---|---|---|---|
| β-lactamases | ||||
| CTXM7 CTXM8 | GCGTGATACCACTTCACC TC TGAAGTAAGTGACCAGAA TC | 260 | blaCTX-M-1group | [17] |
| CTXM17 CTXM18 | TGATACCACCACGCCGCT C TATTGCATCAGAAACCGTGGG | 341 | blaCTX-M-2group | [17] |
| CTXM19 CTXM20 | CAATCTGACGTTGGGCAATG ATAACCGTCGGTGACAATT | 207 | blaCTX-M-8/25/26group | [17] |
| CTXM11 CTXM12 | ATCAAGCCTGCCGATCTGGTTA GTAAGCTGACGCAACGTCTGC | 293 | blaCTX-M-9group | [17] |
| SHV_F SHV_R | AGCCGCTTGAGCAAATTAAAC ATCCCGCAGATAAATCACCAC | 713 | blaSHV-1/variant | [18] |
| TEM-F TEM-R | CATTTCCGTGTCGCCCTTATTC CGTTCATCCATAGTTGCCTGAC | 800 | blaTEM-1/-2/variant | [18] |
| MOXMF MOXMR | GCT GCTCAAGGAGCACAG GAT CAC ATT GAC ATA GGT GTG GTG C | 520 | blaMOX-1,blaMOX-2, and blaCMY-1, CMY-8 to CMY-11 | [19] |
| CITMF CITMR | TGGCCAGAACTGACAGGCAAA TTTCTCCTGAACGTGGCTGGC | 462 | blaLAT-1 to LAT-4,blaCMY-2 to CMY-7, and blaBIL-1 | [19] |
| DHAMF DHAMR | AACTTTCACAGGTGTGCTGGGT CCGTACGCATACTGGCTTTGC | 405 | blaDHA | [19] |
| ACCMF ACCMR | AACAGCCTCAGCAGCCGGTTA TTCGCCGCAATCATCCCTAGC | 346 | blaACC | [19] |
| EBCMF EBCMR | TCGGTAAAGCCGATGTTGCGG CTTCCACTGCGGCTGCCAGTT | 302 | blaMIR-1 and blaACT-1 | [19] |
| FOXMF FOXMR | AACATGGGGTATCAGGGAGATG CAAAGCGCGTAACCGGATTGG | 190 | blaFOX-1 to FOX-5b | [19] |
| Plasmid-mediated quinolone resistance | ||||
| qnrA-F | ATTTCTCACGCCAGGATTTG | 468 | qnrA | [20] |
| qnrA-R | TGCCAGGCACAGATCTTGAC | |||
| qnrB-F | CGACCTKAGCGGCACTGAAT | 513 | qnrB | [20] |
| qnrB-R | GAGCAACGAYGCCTGGTAGYTG | |||
| qnrS-F | ACTGCAAGTTCATTGAACAG | 431 | qnrS | [20] |
| qnrS-R | GATCTAAACCGTCGAGTTCG | |||
| Quinolone efflux pump determinant | ||||
| qepA-F qepA-R | AACTGCTTGAGCCCGTAGAT GTCTACGCCATGGACCTCAC | 596 | qepA | [21] |
| 16S rRNA methylases | ||||
| armA-F armA-R | GGTGCGAAAACAGTCGTAGT TCCTCAAATATCCTCTATGT | 1153 | armA | [22] |
| npmA-F npmA-R | CGGGATCCAAGCACTTTCATACTGACG CGGAATTCCAATTTTGTTCTTATTAGC | 981 | npmA | [22] |
| rmtA-F rmtA-R | CTAGCGTCCATCCTTTCCTC TTTGCTTCCATGCCCTTGCC | 635 | rmtA | [22] |
| rmtB-F rmtB-R | GGAATTCCATATGAACATCAACGATGCC CCGCTCGAGTCCATTCTTTTTTATCAAGT | 756 | rmtB | [22] |
| rmtC-F rmtC-R | CGAAGAAGTAACAGCCAAAG GCTAGAGTCAAGCCAGAAAA | 1000 | rmtC | [22] |
| rmtD-F rmtD-R | TCATTTTCGTTTCAGCAC AAACATGAGCGAACTGAAGG | 744 | rmtD | [22] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalifa, H.O.; Oreiby, A.; Abd El-Hafeez, A.A.; Abd El Latif, A.; Okanda, T.; Kato, Y.; Matsumoto, T. High β-Lactam and Quinolone Resistance of Enterobacteriaceae from the Respiratory Tract of Sheep and Goat with Respiratory Disease. Animals 2021, 11, 2258. https://doi.org/10.3390/ani11082258
Khalifa HO, Oreiby A, Abd El-Hafeez AA, Abd El Latif A, Okanda T, Kato Y, Matsumoto T. High β-Lactam and Quinolone Resistance of Enterobacteriaceae from the Respiratory Tract of Sheep and Goat with Respiratory Disease. Animals. 2021; 11(8):2258. https://doi.org/10.3390/ani11082258
Chicago/Turabian StyleKhalifa, Hazim O., Atef Oreiby, Amer Ali Abd El-Hafeez, Amira Abd El Latif, Takashi Okanda, Yasuyuki Kato, and Tetsuya Matsumoto. 2021. "High β-Lactam and Quinolone Resistance of Enterobacteriaceae from the Respiratory Tract of Sheep and Goat with Respiratory Disease" Animals 11, no. 8: 2258. https://doi.org/10.3390/ani11082258
APA StyleKhalifa, H. O., Oreiby, A., Abd El-Hafeez, A. A., Abd El Latif, A., Okanda, T., Kato, Y., & Matsumoto, T. (2021). High β-Lactam and Quinolone Resistance of Enterobacteriaceae from the Respiratory Tract of Sheep and Goat with Respiratory Disease. Animals, 11(8), 2258. https://doi.org/10.3390/ani11082258






