Efficacy of Simultaneous Intradermal Vaccination of Swine against Porcine Circovirus 2, Porcine Reproductive and Respiratory Syndrome Virus, Mycoplasma hyopneumoniae and Lawsonia intracellularis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Vaccines
2.3. Study Groups
2.4. PCV Study Design and Sample Analysis
2.5. PRRSV Study Design and Sample Analysis
2.6. M. hyo Study Design and Sample Analysis
2.7. Lawsonia Study Design and Sample Analysis
2.8. Statistical Analysis
2.8.1. PCV2
2.8.2. PRRSV
2.8.3. M Hyo
2.8.4. LI
3. Results
3.1. Protection against PCV Challenge
3.2. Protection against PRRSV Challenge
3.3. Protection against M. Hyo Challenge
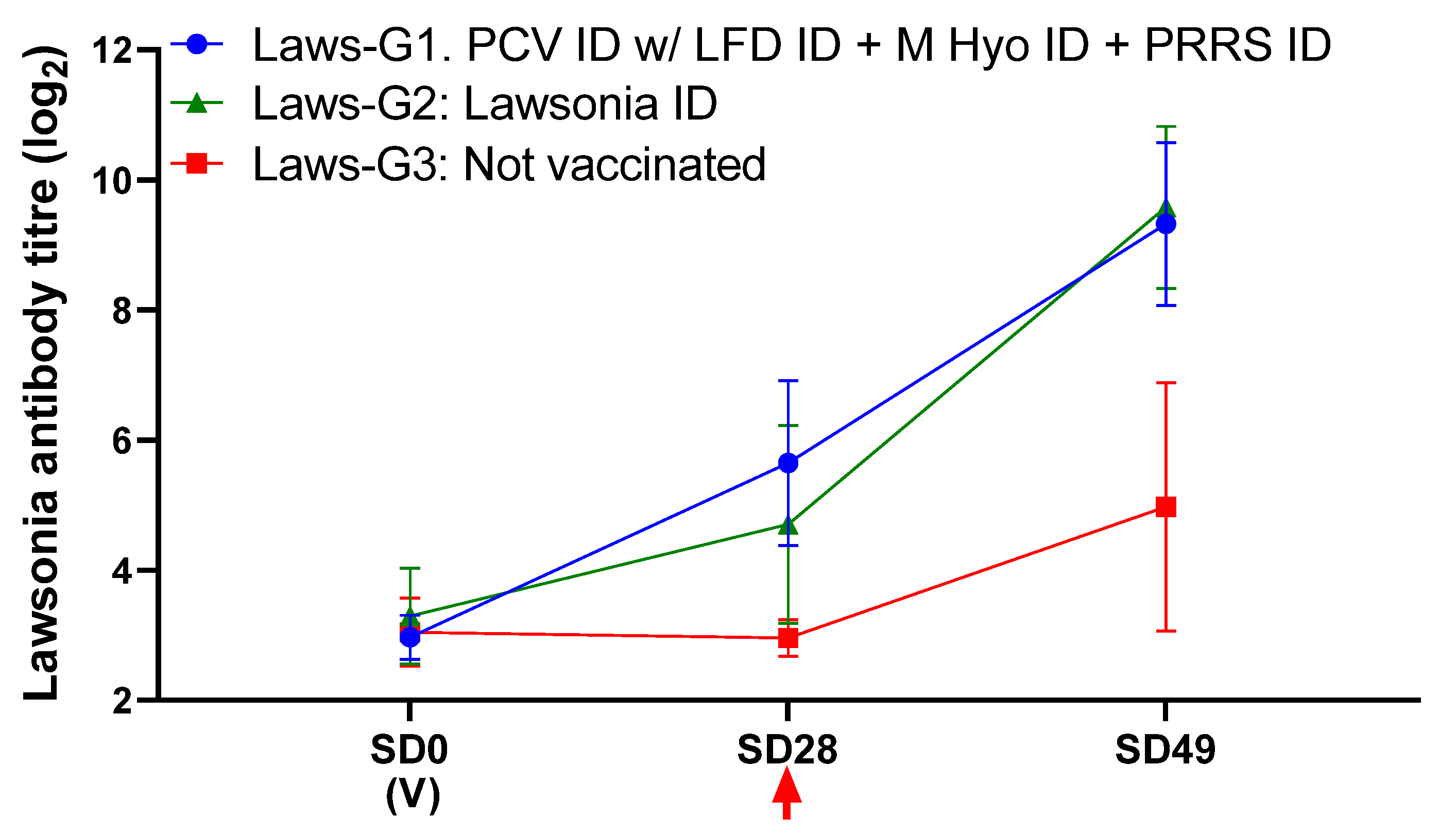
3.4. Protection against LI Challenge
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thomann, B.; Rushton, J.; Schuepbach-Regula, G.; Nathues, H. Modeling Economic Effects of Vaccination Against Porcine Reproductive and Respiratory Syndrome: Impact of Vaccination Effectiveness, Vaccine Price, and Vaccination Coverage. Front. Vet. Sci. 2020, 7, 500. [Google Scholar] [CrossRef]
- Helm, E.T.; Outhouse, A.C.; Schwartz, K.J.; Dekkers, J.C.M.; Lonergan, S.M.; Rauw, W.M.; Gabler, N.K. Impact of Mycoplasma hyopneumoniae and Lawsonia intracellularis on the performance of pigs divergently selected for feed efficiency. J. Anim. Sci. 2018, 96, 462–472. [Google Scholar] [CrossRef] [Green Version]
- VanderWaal, K.; Deen, J. Global trends in infectious diseases of swine. Proc. Natl. Acad. Sci. USA 2018, 115, 11495–11500. [Google Scholar] [CrossRef] [Green Version]
- Chae, C. Commercial porcine circovirus type 2 vaccines: Efficacy and clinical application. Vet. J. 2012, 194, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J. Porcine circovirus: A historical perspective. Vet. Pathol. 2014, 51, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Segales, J.; Kekarainen, T.; Cortey, M. The natural history of porcine circovirus type 2: From an inoffensive virus to a devastating swine disease? Vet. Microbiol. 2013, 165, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Sibila, M.; Pieters, M.; Molitor, T.; Maes, D.; Haesebrouck, F.; Segales, J. Current perspectives on the diagnosis and epidemiology of Mycoplasma hyopneumoniae infection. Vet. J. 2009, 181, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Kobisch, M.; Friis, N.F. Swine mycoplasmoses. Rev. Sci. Tech. 1996, 15, 1569–1605. [Google Scholar] [CrossRef]
- Rossow, K.D.; Bautista, E.M.; Goyal, S.M.; Molitor, T.W.; Murtaugh, M.P.; Morrison, R.B.; Benfield, D.A.; Collins, J.E. Experimental porcine reproductive and respiratory syndrome virus infection in one-, four-, and 10-week-old pigs. J. Vet. Diagn. Investig. 1994, 6, 3–12. [Google Scholar] [CrossRef] [Green Version]
- Terpstra, C.; Wensvoort, G.; Pol, J.M. Experimental reproduction of porcine epidemic abortion and respiratory syndrome (mystery swine disease) by infection with Lelystad virus: Koch’s postulates fulfilled. Vet. Q. 1991, 13, 131–136. [Google Scholar] [CrossRef]
- Hansen, M.S.; Pors, S.E.; Jensen, H.E.; Bille-Hansen, V.; Bisgaard, M.; Flachs, E.M.; Nielsen, O.L. An investigation of the pathology and pathogens associated with porcine respiratory disease complex in Denmark. J. Comp. Pathol. 2010, 143, 120–131. [Google Scholar] [CrossRef]
- Thacker, E.L. Immunology of the porcine respiratory disease complex. Vet. Clin. N. Am. Food Anim. Pract. 2001, 17, 551–565. [Google Scholar] [CrossRef]
- Vannucci, F.A.; Gebhart, C.J. Recent advances in understanding the pathogenesis of Lawsonia intracellularis infections. Vet. Pathol. 2014, 51, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Lawson, G.H.; Gebhart, C.J. Proliferative enteropathy. J. Comp. Pathol. 2000, 122, 77–100. [Google Scholar] [CrossRef]
- Chouet, S.; Prieto, C.; Mieli, L.; Veenhuizen, M.F.; McOrist, S. Patterns of exposure to Lawsonia intracellularis infection on European pig farms. Vet. Rec. 2003, 152, 14–17. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, M.; Hård af Segerstad, C.; Gunnarsson, A.; Fellstrom, C.; De Verdier Klingenberg, K.; Wallgren, P.; Jensen-Waern, M. Diarrhoea in the growing pig-a comparison of clinical, morphological and microbial findings between animals from good and poor performance herds. Res. Vet. Sci. 2003, 74, 163–169. [Google Scholar] [CrossRef]
- Teunissen, M.B.; Haniffa, M.; Collin, M.P. Insight into the immunobiology of human skin and functional specialization of skin dendritic cell subsets to innovate intradermal vaccination design. Curr. Top. Microbiol. Immunol. 2012, 351, 25–76. [Google Scholar] [PubMed]
- Romani, N.; Flacher, V.; Tripp, C.H.; Sparber, F.; Ebner, S.; Stoitzner, P. Targeting skin dendritic cells to improve intradermal vaccination. Curr. Top. Microbiol. Immunol. 2012, 351, 113–138. [Google Scholar]
- Scollo, A.; Minervini, S.; Galli, M.C.; Cevidalli, A.; Bortoletto, G.; Romano, G.; Gottardo, F. Evaluation of pain and stress in three-week old piglets in relation to route ofvaccine administration. Livest. Sci. 2020, 233, 103939. [Google Scholar] [CrossRef]
- Temple, D.; Escribano, D.; Jimenez, M.; Mainau, E.; Ceron, J.J.; Manteca, X. Effect of the needle-free “intra dermal application of liquids” vaccination on the welfare of pregnant sows. Porc. Health Manag. 2017, 3, 9. [Google Scholar] [CrossRef] [Green Version]
- Jacobs, A.A.C.; Harks, F.; Pauwels, R.; Cao, Q.; Holtslag, H.; Pel, S.; Segers, R. Efficacy of a novel intradermal Lawsonia intracellularis vaccine in pigs against experimental infection and under field conditions. Porc. Health Manag. 2020, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Sno, M.; Cox, E.; Holtslag, H.; Nell, T.; Pel, S.; Segers, R.; Fachinger, V.; Witvliet, M. Efficacy and safety of a new intradermal PCV2 vaccine in pigs. Trials Vaccinol. 2016, 5, 24–31. [Google Scholar] [CrossRef] [Green Version]
- Stadler, J.; Naderer, L.; Beffort, L.; Ritzmann, M.; Emrich, D.; Hermanns, W.; Fiebig, K.; Saalmuller, A.; Gerner, W.; Glatthaar-Saalmuller, B.; et al. Safety and immune responses after intradermal application of Porcilis PRRS in either the neck or the perianal region. PLoS ONE 2018, 13, e0203560. [Google Scholar] [CrossRef] [PubMed]
- Tassis, P.D.; Papatsiros, V.G.; Nell, T.; Maes, D.; Alexopoulos, C.; Kyriakis, S.C.; Tzika, E.D. Clinical evaluation of intradermal vaccination against porcine enzootic pneumonia (Mycoplasma hyopneumoniae). Vet. Rec. 2012, 170, 261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haake, M.; Palzer, A.; Rist, B.; Weissenbacher-Lang, C.; Fachinger, V.; Eggen, A.; Ritzmann, M.; Eddicks, M. Influence of age on the effectiveness of PCV2 vaccination in piglets with high levels of maternally derived antibodies. Vet. Microbiol. 2014, 168, 272–280. [Google Scholar] [CrossRef]
- Agresti, A. Categorical Data Analysis, 2nd ed.; John Wiley & Sons, Inc.: New Jersey, NY, USA, 2002. [Google Scholar]
- Pagot, E.; Rigaut, M.; Roudaut, D.; Panzavolta, L.; Jolie, R.; Duivon, D. Field efficacy of Porcilis(R) PCV M Hyo versus a licensed commercially available vaccine and placebo in the prevention of PRDC in pigs on a French farm: A randomized controlled trial. Porc. Health Manag. 2017, 3, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thacker, E.L.; Thacker, B.J.; Young, T.F.; Halbur, P.G. Effect of vaccination on the potentiation of porcine reproductive and respiratory syndrome virus (PRRSV)-induced pneumonia by Mycoplasma hyopneumoniae. Vaccine 2000, 18, 1244–1252. [Google Scholar] [CrossRef]
- Garcia-Morante, B.; Friedrich, R.; Kaiser, T.; Kraft, C.; Bridger, P.; Noguera, M. Gilt Vaccination with a Mixed Administration of a PRRS MLV and a PPV1 Subunit Vaccine Protects against Heterologous PRRSV1 Infection and Prevents Detrimental Effects on Piglet Performance. Viruses 2020, 12, 789. [Google Scholar] [CrossRef]
- Garcia-Morante, B.; Noguera, M.; Kraft, C.; Bridger, P. Field evaluation of the safety and compatibility of a combined vaccine against porcine parvovirus 1 and porcine reproductive and respiratory syndrome virus in breeding animals. Porc. Health Manag. 2019, 5, 28. [Google Scholar] [CrossRef]
- Martinez-Miro, S.; Tecles, F.; Ramon, M.; Escribano, D.; Hernandez, F.; Madrid, J.; Orengo, J.; Martinez-Subiela, S.; Manteca, X.; Ceron, J.J. Causes, consequences and biomarkers of stress in swine: An update. BMC Vet. Res. 2016, 12, 171. [Google Scholar] [CrossRef] [Green Version]
- Campbell, J.M.; Crenshaw, J.D.; Polo, J. The biological stress of early weaned piglets. J. Anim. Sci. Biotechnol. 2013, 4, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]

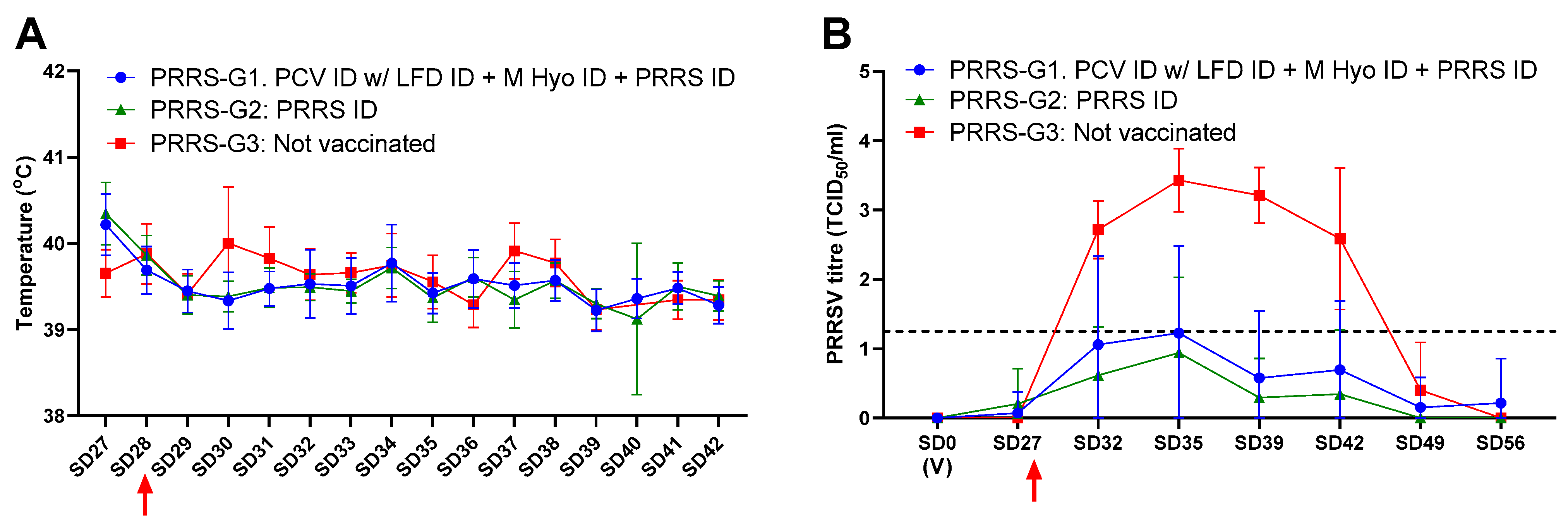
| Group | % Seropositive to PRRSV Antibodies | ADWG (g) [st.dev.] * | ||
|---|---|---|---|---|
| SD0 | SD28 | SD56 | ||
| PRRS-G1: PCV ID/LFD ID + M Hyo ID + PRRS ID | 0.0 | 87.0 | 100.0 | 818 a [±89] |
| PRRS-G2: Porcilis PRRS | 0.0 | 100.0 | 100.0 | 812 a [±129] |
| PRRS-G3: Not vaccinated | 0.0 | 0.0 | 93.0 | 655 b [±60] |
| Group | Treatment | Lung Lesion Score | |
|---|---|---|---|
| Median * | Reduction | ||
| Mhyo-G1 | PCV ID/LFD ID + M Hyo ID + PRRS ID | 2.6 a | 50% |
| Mhyo-G2 | M Hyo ID + PRRS ID | 0.9 a | 83% |
| Mhyo-G3 | PRRS ID | 5.2 b | - |
| Group | Mean Diarrhoea Score 13–21 dpc | ADWG g/day 13–20 dpc | qPCR Faeces (Mean log10 pg DNA/μL) | qPCR Ileum Mucosa 21 dpc (Mean log10 pg DNA/μL) | Mean Macroscopic Ileum Score 21 dpc | Mean Microscopic Ileum Score (IHC) 21 dpc | |
|---|---|---|---|---|---|---|---|
| AUC | 21 dpc | ||||||
| Laws-G1 | 0.12 ± 0.44 a | 1074 ± 240 a | 0.14 ± 0.59 a | 0.10 ± 0.34 a | 0.04 ± 0.11 a | 0.0 ± 0.0 a | 0.3 ± 0.9 a |
| Laws-G2 | 0.08 ± 0.28 a | 1002 ± 303 a | 0.69 ± 1.38 a | 0.11 ± 0.40 a | 0.07 ± 0.21 a | 8 ± 26 a | 0.7 ± 1.9 a |
| Laws-G3 | 2.00 ± 4.34 b | 668 | 2.90 ± 1.75 b | 1.18 ± 1.00 b | 0.43 ± 0.38 b | 109 ± 153 b | 4.1 ± 3.4 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horsington, J.; Witvliet, M.; Jacobs, A.A.C.; Segers, R.P.A.M. Efficacy of Simultaneous Intradermal Vaccination of Swine against Porcine Circovirus 2, Porcine Reproductive and Respiratory Syndrome Virus, Mycoplasma hyopneumoniae and Lawsonia intracellularis. Animals 2021, 11, 2225. https://doi.org/10.3390/ani11082225
Horsington J, Witvliet M, Jacobs AAC, Segers RPAM. Efficacy of Simultaneous Intradermal Vaccination of Swine against Porcine Circovirus 2, Porcine Reproductive and Respiratory Syndrome Virus, Mycoplasma hyopneumoniae and Lawsonia intracellularis. Animals. 2021; 11(8):2225. https://doi.org/10.3390/ani11082225
Chicago/Turabian StyleHorsington, Jacquelyn, Maarten Witvliet, Antonius A. C. Jacobs, and Ruud P. A. M. Segers. 2021. "Efficacy of Simultaneous Intradermal Vaccination of Swine against Porcine Circovirus 2, Porcine Reproductive and Respiratory Syndrome Virus, Mycoplasma hyopneumoniae and Lawsonia intracellularis" Animals 11, no. 8: 2225. https://doi.org/10.3390/ani11082225
APA StyleHorsington, J., Witvliet, M., Jacobs, A. A. C., & Segers, R. P. A. M. (2021). Efficacy of Simultaneous Intradermal Vaccination of Swine against Porcine Circovirus 2, Porcine Reproductive and Respiratory Syndrome Virus, Mycoplasma hyopneumoniae and Lawsonia intracellularis. Animals, 11(8), 2225. https://doi.org/10.3390/ani11082225






