Effect of Niacin on Growth Performance, Intestinal Morphology, Mucosal Immunity and Microbiota Composition in Weaned Piglets
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Treatments
2.2. Feed Analysis
2.3. Intestinal Morphology
2.4. Protein Chip
2.5. Quantitative Real-Time PCR
2.6. Gut Microbiota
2.7. Gas Chromatographic Analysis
2.8. Performance Liquid Chromatography—Mass Spectrometry
2.9. Statistical Analysis
3. Results
3.1. Effect of Niacin on Growth Performance of Weaned Piglets
3.2. Niacin Content
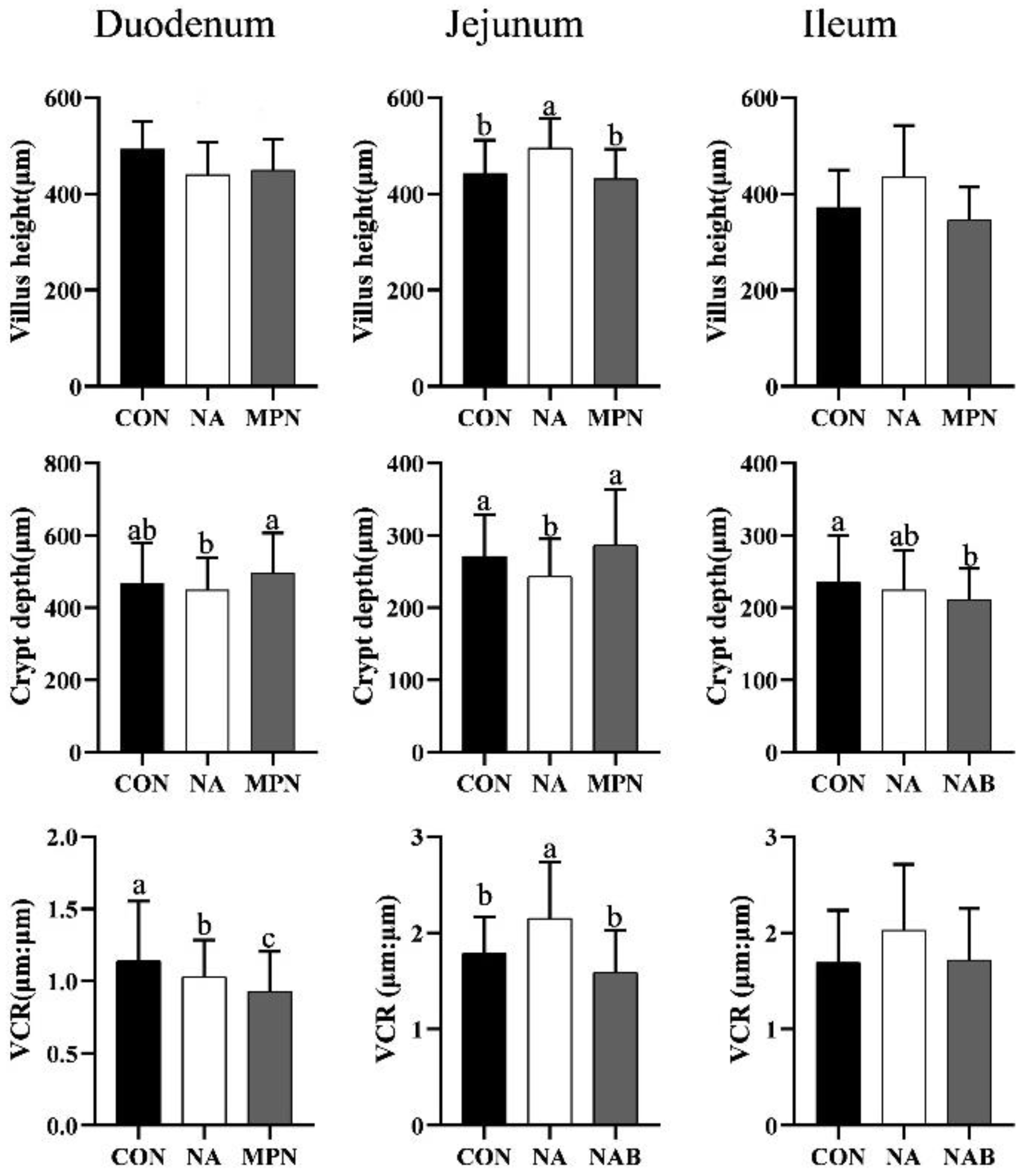
3.3. Effect of Niacin on Intestinal Morphology of Weaned Piglets
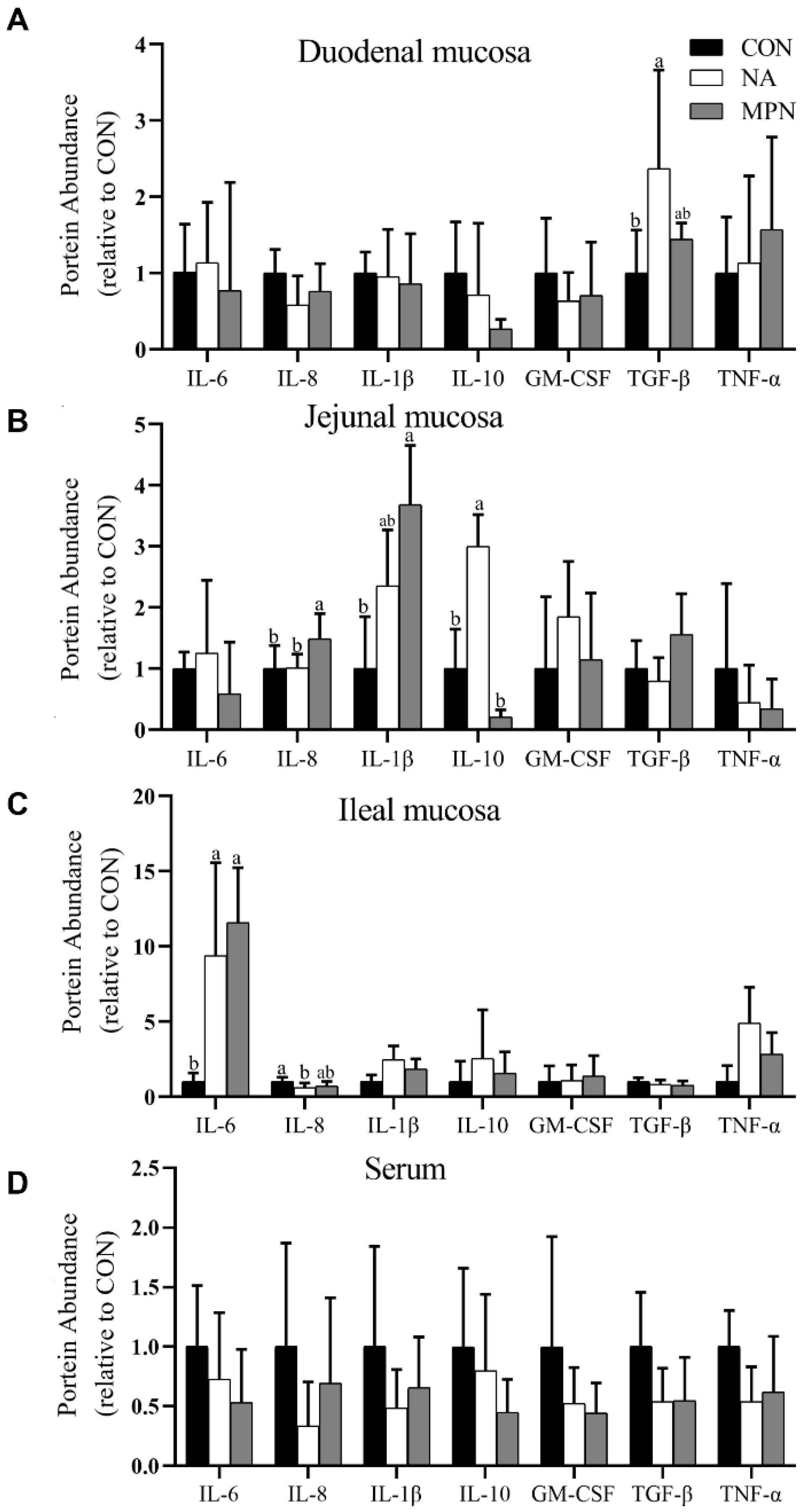
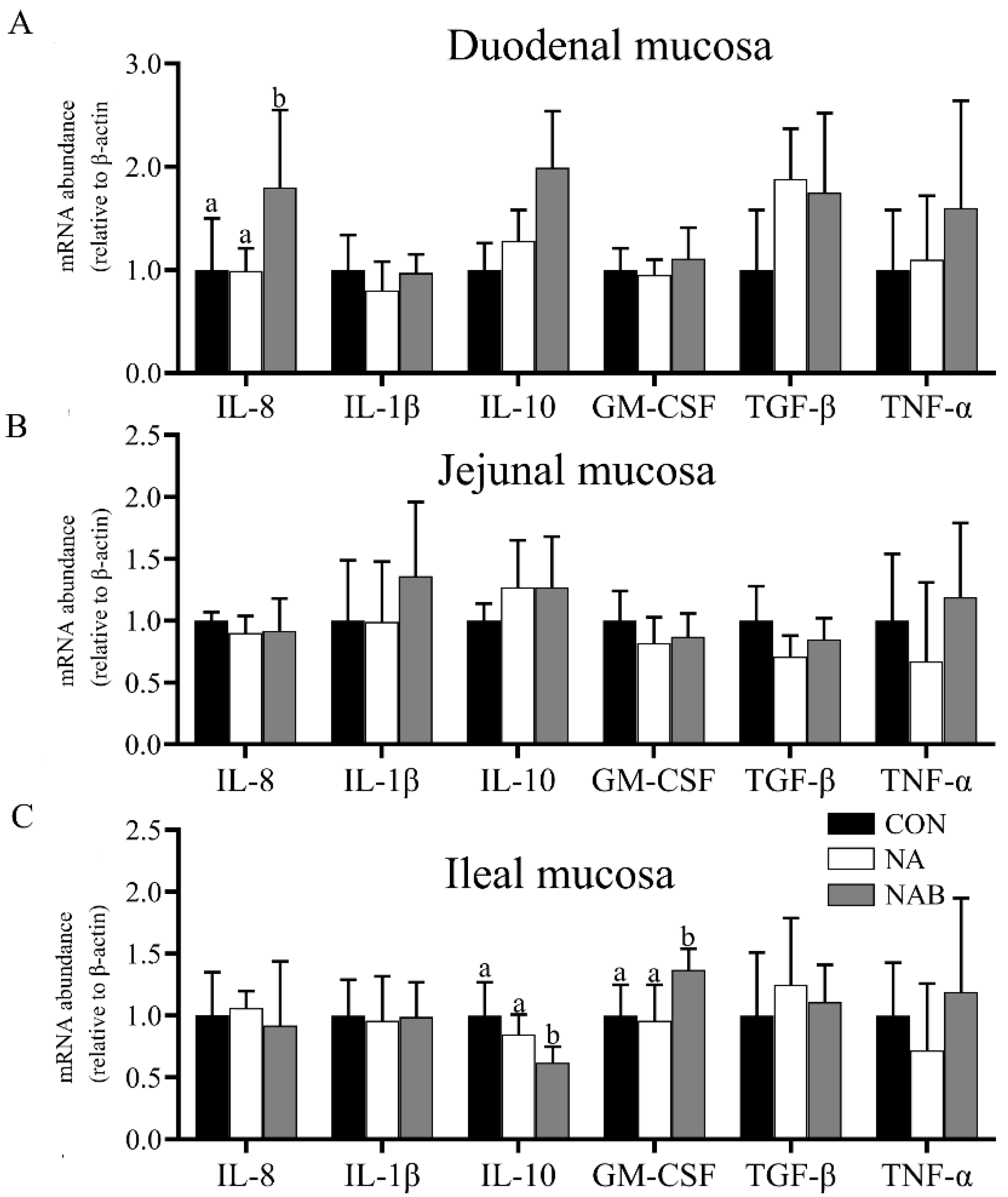
3.4. Effect of Niacin on Intestinal Immunity of Weaned Piglets
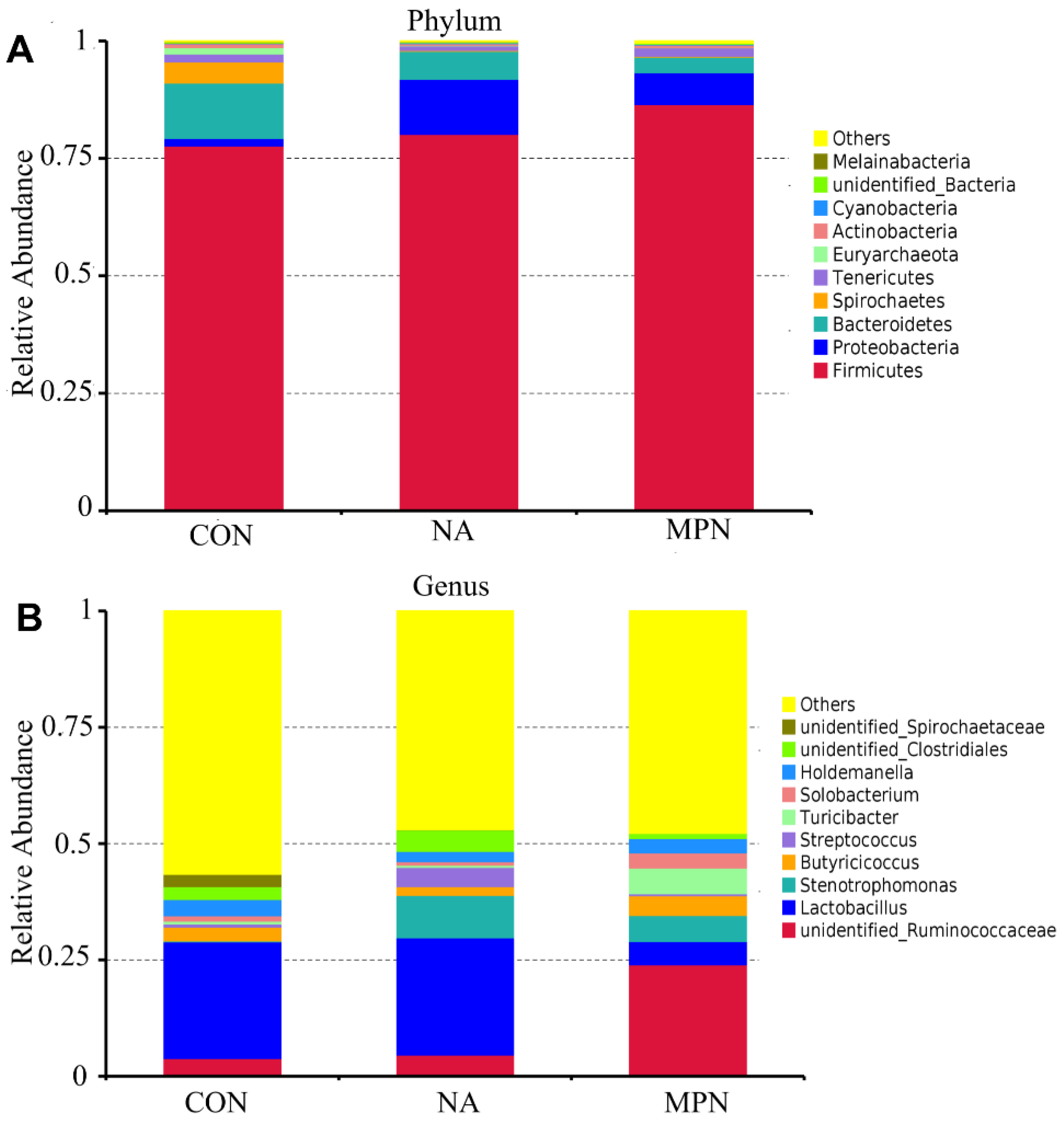
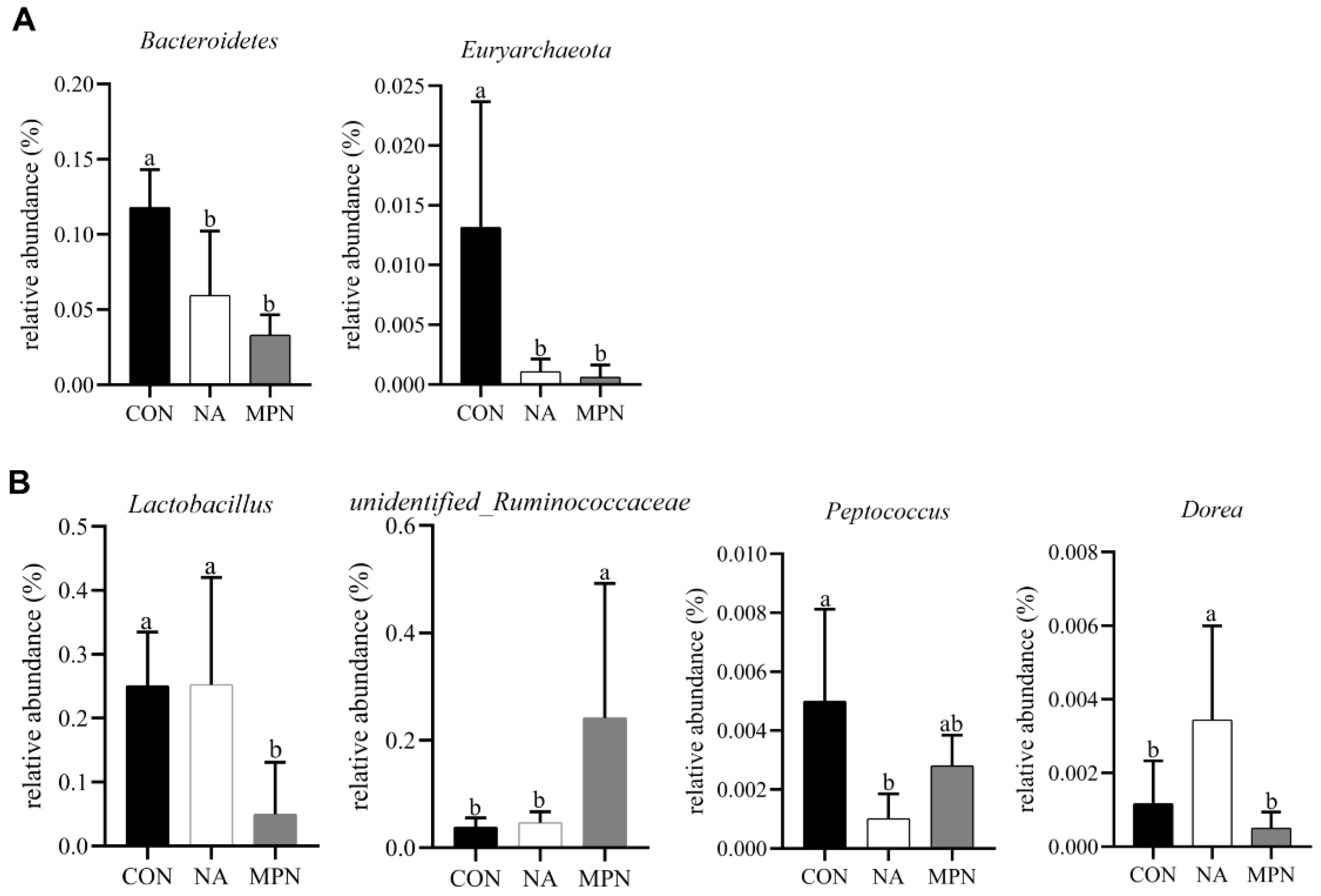
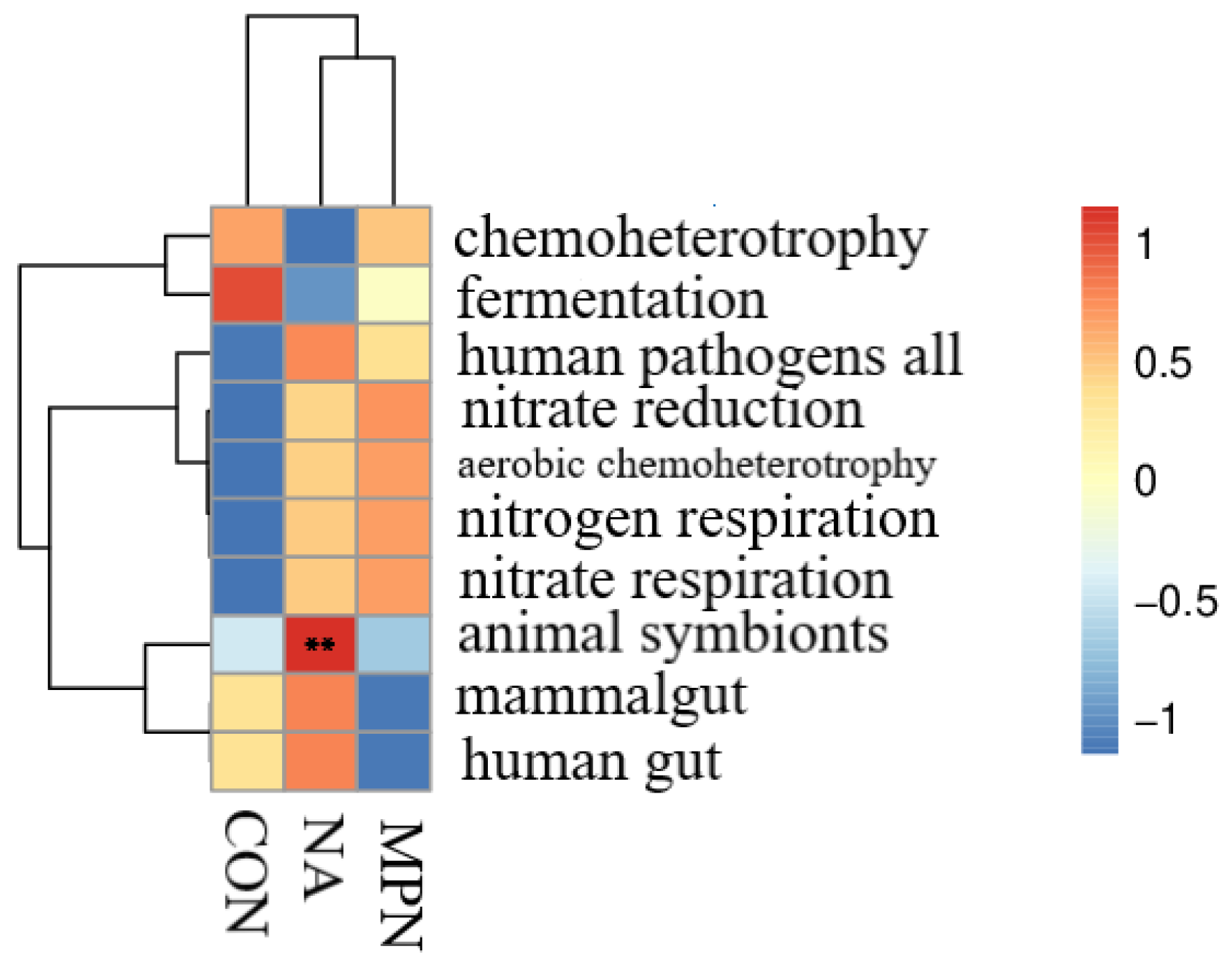
3.5. Effect of Niacin on Colonic Microbiota and Short-Chain Fatty Acids of Weaned Piglets
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Campbell, J.M.; Crenshaw, J.D.; Polo, J. The biological stress of early weaned piglets. J. Anim. Sci. Biotechnol. 2013, 4, 19. [Google Scholar] [CrossRef] [Green Version]
- Pan, L.; Zhao, P.F.; Ma, X.K.; Shang, Q.H.; Xu, Y.T.; Long, S.F.; Wu, Y.; Yuan, F.M.; Piao, X.S. Probiotic supplementation protects weaned pigs against enterotoxigenic Escherichia coli K88 challenge and improves performance similar to antibiotics. J. Anim. Sci. 2017, 95, 2627–2639. [Google Scholar] [CrossRef] [Green Version]
- Spreeuwenberg, M.A.; Verdonk, J.M.; Gaskins, H.R.; Verstegen, M.W. Small intestine epithelial barrier function is compromised in pigs with low feed intake at weaning. J. Nutr. 2001, 131, 1520–1527. [Google Scholar] [CrossRef] [Green Version]
- Boudry, G.; Péron, V.; Le Huërou-Luron, I.; Lallès, J.P.; Sève, B. Weaning induces both transient and long-lasting modifications of absorptive, secretory, and barrier properties of piglet intestine. J. Nutr. 2004, 134, 2256–2262. [Google Scholar] [CrossRef]
- Moeser, A.J.; Klok, C.V.; Ryan, K.A.; Wooten, J.G.; Little, D.; Cook, V.L.; Blikslager, A.T. Stress signaling pathways activated by weaning mediate intestinal dysfunction in the pig. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, G173–G181. [Google Scholar] [CrossRef]
- Zhang, M.; Hou, G.; Hu, P.; Feng, D.; Wang, J.; Zhu, W. Nano chitosan-zinc complex improves the growth performance and antioxidant capacity of the small intestine in weaned piglets. Br. J. Nutr. 2020, 1–12. [Google Scholar] [CrossRef]
- Holman, W.I.; De Lange, D.J. Significance of tryptophane in human nicotinic acid metabolism. Nature 1950, 165, 112. [Google Scholar] [CrossRef] [PubMed]
- Titcomb, T.J.; Tanumihardjo, S.A. Global Concerns with B Vitamin Statuses: Biofortification, Fortification, Hidden Hunger, Interactions, and Toxicity. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1968–1984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Real, D.E.; Nelssen, J.L.; Tokach, M.D.; Goodband, R.D.; Alonso, E. Influence of dietary niacin on starter pig performance. Kans. Agric. Exp. Stn. Res. Rep. 2001, 67–69. [Google Scholar] [CrossRef] [Green Version]
- Digby, J.E.; Martinez, F.; Jefferson, A.; Ruparelia, N.; Chai, J.; Wamil, M.; Greaves, D.R.; Choudhury, R.P. Anti-inflammatory effects of nicotinic acid in human monocytes are mediated by GPR109A dependent mechanisms. Arter. Thromb. Vasc. Biol. 2012, 32, 669–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chai, J.T.; Digby, J.E.; Ruparelia, N.; Jefferson, A.; Handa, A.; Choudhury, R.P. Nicotinic acid receptor GPR109A is down-regulated in human macrophage-derived foam cells. PLoS ONE 2013, 8, e62934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lipszyc, P.S.; Cremaschi, G.A.; Zorrilla-Zubilete, M.; Bertolino, M.L.; Capani, F.; Genaro, A.M.; Wald, M.R. Niacin Modulates Pro-inflammatory Cytokine Secretion. A Potential Mechanism Involved in its Anti-atherosclerotic Effect. Open Cardiovasc. Med. J. 2013, 7, 90–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, W.Y.; Suh, G.J.; Kim, K.S.; Kwak, Y.H. Niacin attenuates lung inflammation and improves survival during sepsis by downregulating the nuclear factor-κB pathway. Crit. Care Med. 2011, 39, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Li, S.Q.; Jiang, W.D.; Liu, Y.; Jiang, J.; Wu, P.; Zhao, J.; Kuang, S.Y.; Tang, L.; Tang, W.N.; et al. Deficiency of dietary niacin impaired intestinal mucosal immune function via regulating intestinal NF-κB, Nrf2 and MLCK signaling pathways in young grass carp (Ctenopharyngodon idella). Fish. Shellfish. Immunol. 2016, 49, 177–193. [Google Scholar] [CrossRef]
- Salem, H.A.; Wadie, W. Effect of Niacin on Inflammation and Angiogenesis in a Murine Model of Ulcerative Colitis. Sci. Rep. 2017, 7, 7139–7147. [Google Scholar] [CrossRef] [Green Version]
- Taggart, A.K.; Kero, J.; Gan, X.; Cai, T.Q.; Cheng, K.; Ippolito, M.; Ren, N.; Kaplan, R.; Wu, K.; Wu, T.J.; et al. (D)-beta-Hydroxybutyrate inhibits adipocyte lipolysis via the nicotinic acid receptor PUMA-G. J. Biol. Chem. 2005, 280, 26649–26652. [Google Scholar] [CrossRef] [Green Version]
- Soga, T.; Kamohara, M.; Takasaki, J.; Matsumoto, S.; Saito, T.; Ohishi, T.; Hiyama, H.; Matsuo, A.; Matsushime, H.; Furuichi, K. Molecular identification of nicotinic acid receptor. Biochem. Biophys. Res. Commun. 2003, 303, 364–369. [Google Scholar] [CrossRef]
- Maciejewski-Lenoir, D.; Richman, J.G.; Hakak, Y.; Gaidarov, I.; Behan, D.P.; Connolly, D.T. Langerhans cells release prostaglandin D2 in response to nicotinic acid. J. Investig. Dermatol. 2006, 126, 2637–2646. [Google Scholar] [CrossRef] [Green Version]
- Schaub, A.; Fütterer, A.; Pfeffer, K. PUMA-G, an IFN-gamma-inducible gene in macrophages is a novel member of the seven transmembrane spanning receptor superfamily. Eur. J. Immunol. 2001, 31, 3714–3725. [Google Scholar] [CrossRef]
- Macia, L.; Tan, J.; Vieira, A.T.; Leach, K.; Stanley, D.; Luong, S.; Maruya, M.; Ian McKenzie, C.; Hijikata, A.; Wong, C.; et al. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat. Commun. 2015, 6, 6734–6749. [Google Scholar] [CrossRef] [Green Version]
- Singh, N.; Gurav, A.; Sivaprakasam, S.; Brady, E.; Padia, R.; Shi, H.; Thangaraju, M.; Prasad, P.D.; Manicassamy, S.; Munn, D.H.; et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity 2014, 40, 128–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, E.; Li, Y.; Yao, M.; Wei, Z.; Fu, Y.; Yang, Z. Niacin attenuates the production of pro-inflammatory cytokines in LPS-induced mouse alveolar macrophages by HCA2 dependent mechanisms. Int. Immunopharmacol. 2014, 23, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Huang, B.; Fu, S.; Li, B.; Ran, X.; He, D.; Jiang, L.; Li, Y.; Liu, B.; Xie, L.; et al. G Protein-Coupled Receptor 109A and Host Microbiota Modulate Intestinal Epithelial Integrity during Sepsis. Front. Immunol. 2018, 9, 2079. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, B.; Zeng, P.; Zhu, H.; Sivaprakasam, S.; Li, S.; Xiao, H.; Dong, L.; Shiao, P.; Kolhe, R.; Patel, N.; et al. Gpr109a Limits Microbiota-Induced IL-23 Production To Constrain ILC3-Mediated Colonic Inflammation. J. Immunol. 2018, 200, 2905–2914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rask-Andersen, M.; Almén, M.S.; Schiöth, H.B. Trends in the exploitation of novel drug targets. Nat. Rev. Drug Discov. 2011, 10, 579–590. [Google Scholar] [CrossRef]
- Singh, V.; Jamwal, S.; Jain, R.; Verma, P.; Gokhale, R.; Rao, K.V. Mycobacterium tuberculosis-driven targeted recalibration of macrophage lipid homeostasis promotes the foamy phenotype. Cell Host Microbe 2012, 12, 669–681. [Google Scholar] [CrossRef] [Green Version]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, Research0034. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Langille, M.G.; Zaneveld, J.; Caporaso, J.G.; McDonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Vega Thurber, R.L.; Knight, R.; et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 2013, 31, 814–821. [Google Scholar] [CrossRef]
- Marquardt, R.R.; Jin, L.Z.; Kim, J.W.; Fang, L.; Frohlich, A.A.; Baidoo, S.K. Passive protective effect of egg-yolk antibodies against enterotoxigenic Escherichia coli K88+ infection in neonatal and early-weaned piglets. FEMS Immunol. Med. Microbiol. 1999, 23, 283–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dowarah, R.; Verma, A.K.; Agarwal, N. The use of Lactobacillus as an alternative of antibiotic growth promoters in pigs: A review. Anim. Nutr. 2017, 3, 1–6. [Google Scholar] [CrossRef]
- Kreuzer-Redmer, S.; Bekurtz, J.C.; Arends, D.; Bortfeldt, R.; Kutz-Lohroff, B.; Sharbati, S.; Einspanier, R.; Brockmann, G.A. Feeding of Enterococcus faecium NCIMB 10415 Leads to Intestinal miRNA-423-5p-Induced Regulation of Immune-Relevant Genes. Appl. Environ. Microbiol. 2016, 82, 2263–2269. [Google Scholar] [CrossRef] [Green Version]
- Shin, D.; Chang, S.Y.; Bogere, P.; Won, K.; Choi, J.Y.; Choi, Y.J.; Lee, H.K.; Hur, J.; Park, B.Y.; Kim, Y.; et al. Beneficial roles of probiotics on the modulation of gut microbiota and immune response in pigs. PLoS ONE 2019, 14, e0220843. [Google Scholar] [CrossRef] [Green Version]
- Real, D.E.; Nelssen, J.L.; Tokach, M.; Goodband, R.D.; Alonso, E. Effects of Increasing Dietary Niacin on Weanling Pig Performance. Prof. Anim. Sci. 2004, 20, 353–357. [Google Scholar] [CrossRef]
- Ivers, D.J.; Veum, T.L. Effect of niacin additions to corn-soybean meal diets on performance of pigs from weaning to finishing. J. Anim. Sci. 1993, 71, 3383–3388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biviano, A.B.; Martínez del Rio, C.; Phillips, D.L. Ontogenesis of intestine morphology and intestinal disaccharidases in chickens (Gallus gallus) fed contrasting purified diets. J. Comp. Physiol. B 1993, 163, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Malo, C.; Boyle, C.R.; Buddington, R.K. Diet influences development of the pig (Sus scrofa) intestine during the first 6 hours after birth. J. Nutr. 1998, 128, 1302–1310. [Google Scholar] [CrossRef] [Green Version]
- Adeola, O.; King, D.E. Developmental changes in morphometry of the small intestine and jejunal sucrase activity during the first nine weeks of postnatal growth in pigs. J. Anim. Sci. 2006, 84, 112–118. [Google Scholar] [CrossRef]
- Wang, M.; Yang, C.; Wang, Q.Y.; Li, J.Z.; Li, Y.L.; Ding, X.Q.; Yin, J.; Yang, H.S.; Yin, Y.L. The growth performance, intestinal digestive and absorptive capabilities in piglets with different lengths of small intestines. Animal 2020, 14, 1196–1203. [Google Scholar] [CrossRef]
- Pluske, J.; Williams, I.; Aherne, F. Maintenance of villous height and crypt depth in piglets by providing continuous nutrition after weaning. Anim. Sci. 1996, 62, 131–144. [Google Scholar] [CrossRef] [Green Version]
- Yu, M.; Li, Z.; Chen, W.; Rong, T.; Wang, G.; Wang, F.; Ma, X. Evaluation of full-fat Hermetia illucens larvae meal as a fishmeal replacement for weanling piglets: Effects on the growth performance, apparent nutrient digestibility, blood parameters and gut morphology. Anim. Feed. Sci. Technol. 2020, 264, 114431. [Google Scholar] [CrossRef]
- Gong, Y.; Jin, X.; Yuan, B.; Lv, Y.; Yan, G.; Liu, M.; Xie, C.; Liu, J.; Tang, Y.; Gao, H.; et al. G Protein-Coupled Receptor 109A Maintains the Intestinal Integrity and Protects Against ETEC Mucosal Infection by Promoting IgA Secretion. Front. Immunol. 2020, 11, 583652. [Google Scholar] [CrossRef] [PubMed]
- Doreau, M.; Ottou, J.F. Influence of niacin supplementation on in vivo digestibility and ruminal digestion in dairy cows. J. Dairy Sci. 1996, 79, 2247–2254. [Google Scholar] [CrossRef]
- Horner, J.L.; Coppock, C.E.; Moya, J.R.; Labore, J.M.; Lanham, J.K. Effects of niacin and whole cottonseed on ruminal fermentation, protein degradability, and nutrient digestibility. J. Dairy Sci. 1988, 71, 1239–1247. [Google Scholar] [CrossRef]
- Christensen, R.A.; Overton, T.R.; Clark, J.H.; Drackley, J.K.; Nelson, D.R.; Blum, S.A. Effects of dietary fat with or without nicotinic acid on nutrient flow to the duodenum of dairy cows. J. Dairy Sci. 1996, 79, 1410–1424. [Google Scholar] [CrossRef]
- Feng, J.; Wang, L.; Chen, Y.; Xiong, Y.; Wu, Q.; Jiang, Z.; Yi, H. Effects of niacin on intestinal immunity, microbial community and intestinal barrier in weaned piglets during starvation. Int. Immunopharmacol. 2021, 95, 107584. [Google Scholar] [CrossRef]
- Bhardwaj, S.B. Gut flora and its modification as a therapy. Rev. Med. Microbiol. 2013, 24, 52–54. [Google Scholar] [CrossRef]
- Bourgault, A.M.; Rosenblatt, J.E.; Fitzgerald, R.H. Peptococcus magnus: A significant human pathogen. Ann. Intern. Med. 1980, 93, 244–248. [Google Scholar] [CrossRef]
- Matsuda, H.; Fujiyama, Y.; Andoh, A.; Ushijima, T.; Kajinami, T.; Bamba, T. Characterization of antibody responses against rectal mucosa-associated bacterial flora in patients with ulcerative colitis. J. Gastroenterol. Hepatol. 2000, 15, 61–68. [Google Scholar] [CrossRef]
- Azad, M.A.K.; Sarker, M.; Li, T.; Yin, J. Probiotic Species in the Modulation of Gut Microbiota: An Overview. Biomed. Res. Int. 2018, 2018, 9478630. [Google Scholar] [CrossRef] [Green Version]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bajaj, J.S.; Hylemon, P.B.; Ridlon, J.M.; Heuman, D.M.; Daita, K.; White, M.B.; Monteith, P.; Noble, N.A.; Sikaroodi, M.; Gillevet, P.M. Colonic mucosal microbiome differs from stool microbiome in cirrhosis and hepatic encephalopathy and is linked to cognition and inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G675–G685. [Google Scholar] [CrossRef] [PubMed]
- Jenq, R.R.; Ubeda, C.; Taur, Y.; Menezes, C.C.; Khanin, R.; Dudakov, J.A.; Liu, C.; West, M.L.; Singer, N.V.; Equinda, M.J.; et al. Regulation of intestinal inflammation by microbiota following allogeneic bone marrow transplantation. J. Exp. Med. 2012, 209, 903–911. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chia, N.; Kalari, K.R.; Yao, J.Z.; Novotna, M.; Paz Soldan, M.M.; Luckey, D.H.; Marietta, E.V.; Jeraldo, P.R.; Chen, X.; et al. Multiple sclerosis patients have a distinct gut microbiota compared to healthy controls. Sci. Rep. 2016, 6, 28484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chambers, E.S.; Morrison, D.J.; Frost, G. Control of appetite and energy intake by SCFA: What are the potential underlying mechanisms? Proc. Nutr. Soc. 2015, 74, 328–336. [Google Scholar] [CrossRef]
- Kles, K.A.; Chang, E.B. Short-chain fatty acids impact on intestinal adaptation, inflammation, carcinoma, and failure. Gastroenterology 2006, 130, S100–S105. [Google Scholar] [CrossRef] [PubMed]
- Alam, R.; Abdolmaleky, H.M.; Zhou, J.R. Microbiome, inflammation, epigenetic alterations, and mental diseases. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2017, 174, 651–660. [Google Scholar] [CrossRef]
- Felizardo, R.J.F.; de Almeida, D.C.; Pereira, R.L.; Watanabe, I.K.M.; Doimo, N.T.S.; Ribeiro, W.R.; Cenedeze, M.A.; Hiyane, M.I.; Amano, M.T.; Braga, T.T.; et al. Gut microbial metabolite butyrate protects against proteinuric kidney disease through epigenetic- and GPR109a-mediated mechanisms. FASEB J. 2019, 33, 11894–11908. [Google Scholar] [CrossRef] [Green Version]


| Item | Diets 1 | |
|---|---|---|
| CON/MPN | NA | |
| Ingredient, % | ||
| Corn | 34.00 | 34.00 |
| Expanded corn | 18.00 | 18.00 |
| Soybean meal | 9.50 | 9.50 |
| Expanded soybean | 15.00 | 15.00 |
| Lactose | 2.00 | 2.00 |
| Whey powder | 8.00 | 8.00 |
| Fish meal | 5.00 | 5.00 |
| Soybean oil | 2.00 | 2.00 |
| Soybean hull | 0.31 | 0.31 |
| Limestone | 0.60 | 0.598 |
| Lysine 98.5% | 0.80 | 0.80 |
| Methionine | 0.30 | 0.30 |
| L-Threonine | 0.30 | 0.30 |
| Valine | 0.13 | 0.13 |
| L-Tryptophan | 0.10 | 0.10 |
| Monocalcium phosphate | 1.50 | 1.50 |
| Salt | 0.25 | 0.25 |
| 60% choline chloride | 0.15 | 0.15 |
| Acidifier | 0.05 | 0.05 |
| Phytase | 0.01 | 0.01 |
| Niacin | 0 | 0.002 |
| Premix 2 | 2.00 | 2.00 |
| Total | 100.00 | 100.00 |
| Estimated energy and nutrient composition- | ||
| GE, kcal/kg | 3526.00 | 3526.00 |
| ME, kcal/kg | 3395.01 | 3395.01 |
| NE, kcal/kg | 2610.93 | 2610.93 |
| Crude protein, % | 19.00 | 19.00 |
| Crude fat, % | 8.00 | 8.00 |
| SID 3 lys, % | 1.49 | 1.49 |
| SID thr, % | 0.86 | 0.86 |
| SID try, % | 0.27 | 0.27 |
| SID met + cys, % | 0.83 | 0.83 |
| Calcium, % | 0.88 | 0.88 |
| Total phosphorus, % | 0.68 | 0.68 |
| STTD 4 phosphorus, % | 0.47 | 0.47 |
| Analyzed energy and nutrient composition | ||
| GE, kcal/kg | 3524.72 | 3524.72 |
| Crude protein, % | 19.30 | 19.30 |
| Crude fat, % | 8.74 | 8.74 |
| Crude fiber, % | 3.39 | 3.39 |
| Crude ash, % | 1.48 | 1.48 |
| The content of niacin in diets, mg/kg | 9.92 | 30.32 |
| Primer | Sequence (5′–3′) |
|---|---|
| β-actin-F | CCTGAACCTCTCATTGCCA |
| β-actin-R | AGGGCCGTGATCTCCTTCTG |
| IL1-β-F | GAIAGTGCTTCGTGCTGGAGT |
| IL1-β-R | ACTGGCATCTGCCCAGTTC |
| IL-8-F | ATGAGTCTTAGAGGTCTGGGT |
| IL-8-R | ACAGTGAGGGCTAGGAGGG |
| IL-10-F | GCATCCACTTCCCAACCA |
| IL-10-R | GCAACAAGTCGCCCATCT |
| TNF-α-F | GAAGCAGCGTTTGGGAGTG |
| TNF-α-R | GTTGTGGGACAGGGTAGGG |
| GM-SCF-F | GCAATTTCACCAAACTCAAGG |
| GM-SCF-R | CTCATTACGCAGGCACAAAAG |
| TGF-β-F | TTGGGACTTGTGCTCTAT |
| TGF-β-R | AGTTCTGCTGGGATGTTT |
| Item | CON | NA | MPN | p-Value |
|---|---|---|---|---|
| ADG (g/d) | 101.13 ± 9.41 ab | 116.80 ± 9.12 a | 83.26 ± 6.26 b | 0.042 |
| ADFI (g/d) | 192.34 ± 7.18 | 203.79 ± 7.90 | 189.96 ± 4.28 | 0.328 |
| G/F | 0.52 ± 0.04 | 0.57 ± 0.03 | 0.46 ± 0.02 | 0.055 |
| Diarrhea incidence (%) | 29.68 ± 10.05 | 24.74 ± 9.42 | 31.04± 11.09 | 0.468 |
| Item | CON | NA | MPN | p-Value |
|---|---|---|---|---|
| Niacin content | ||||
| Jejunum, ng/mL | 65.84 ± 19.01 ab | 99.70 ± 15.48 a | 38.93 ± 4.39 b | 0.03 |
| Ileum, ng/mL | 103.04 ± 18.84 | 114.07 ± 23.94 | 74.39 ± 18.97 | 0.44 |
| Colon, ng/mL | 821.95 ± 68.20 | 963.29 ± 164.83 | 618.74 ± 76.06 | 0.09 |
| Nicotinamide content | ||||
| Serum, ng/mL | 29.54 ± 9.29 b | 41.38 ± 15.90 a | 18.36 ± 6.17 b | <0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Zhu, X.; Qiu, Y.; Wang, L.; Shang, X.; Gao, K.; Yang, X.; Jiang, Z. Effect of Niacin on Growth Performance, Intestinal Morphology, Mucosal Immunity and Microbiota Composition in Weaned Piglets. Animals 2021, 11, 2186. https://doi.org/10.3390/ani11082186
Liu S, Zhu X, Qiu Y, Wang L, Shang X, Gao K, Yang X, Jiang Z. Effect of Niacin on Growth Performance, Intestinal Morphology, Mucosal Immunity and Microbiota Composition in Weaned Piglets. Animals. 2021; 11(8):2186. https://doi.org/10.3390/ani11082186
Chicago/Turabian StyleLiu, Shilong, Xiaoping Zhu, Yueqin Qiu, Li Wang, Xiuguo Shang, Kaiguo Gao, Xuefen Yang, and Zongyong Jiang. 2021. "Effect of Niacin on Growth Performance, Intestinal Morphology, Mucosal Immunity and Microbiota Composition in Weaned Piglets" Animals 11, no. 8: 2186. https://doi.org/10.3390/ani11082186
APA StyleLiu, S., Zhu, X., Qiu, Y., Wang, L., Shang, X., Gao, K., Yang, X., & Jiang, Z. (2021). Effect of Niacin on Growth Performance, Intestinal Morphology, Mucosal Immunity and Microbiota Composition in Weaned Piglets. Animals, 11(8), 2186. https://doi.org/10.3390/ani11082186







