Cultivated and Wild Juvenile Thick-Lipped Grey Mullet, Chelon labrosus: A Comparison from a Nutritional Point of View
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Rearing Conditions, Diets and Fish Sampling
2.2. Proximate Muscle Composition and Fatty Acid Profile
2.3. Indices of Lipid Metabolism and Quality
2.4. Amino Acid Content and Protein Quality Evaluation
2.5. Statistical Analysis
3. Results
3.1. Muscle Proximate Composition
3.2. Liver Fatty Acids
3.3. Muscle Fatty Acids
3.4. Amino Acid Content and Score
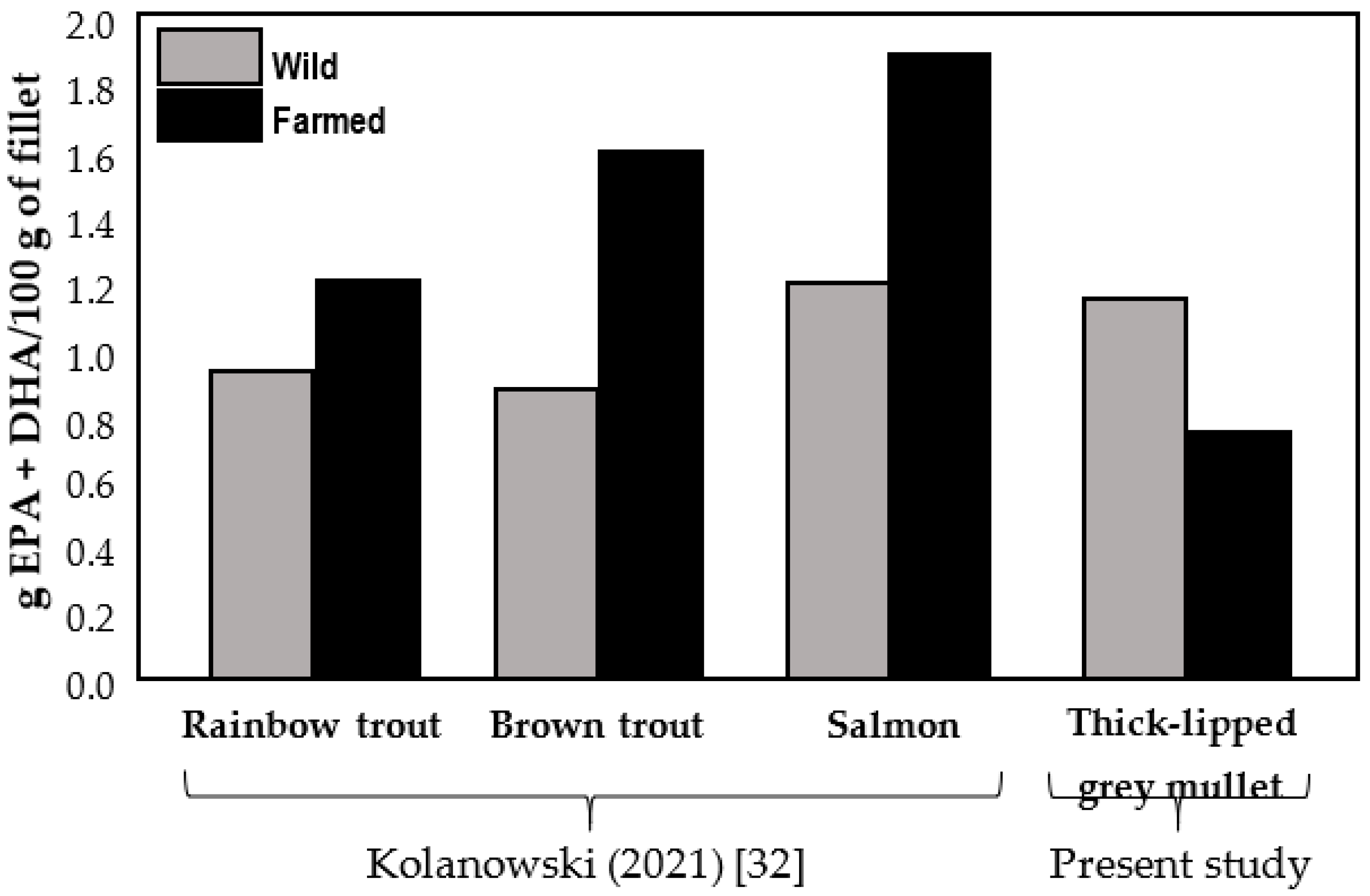
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- FAO (Food and Agricultural Organization). The State of World Fisheries and Aquaculture 2020. In Sustainability in Action; FAO: Rome, Italy, 2020. [Google Scholar]
- Jennings, S.; Stentiford, G.D.; Leocadio, A.M.; Jeffery, K.R.; Metcalfe, J.D.; Katsiadaki, I.; Auchterlonie, N.A.; Mangi, S.C.; Pinnegar, J.K.; Ellis, T.; et al. Aquatic food security: Insights into challenges and solutions from an analysis of interactions between fisheries, aquaculture, food safety, human health, fish and human welfare, economy and environment. Fish Fish. 2016, 17, 893–938. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Porchas, M.; Martinez-Cordova, L.R. World aquaculture: Environmental impacts and troubleshooting alternatives. Sci. World J. 2012, 2012, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Diana, J.S.; Egna, H.S.; Chopin, T.; Peterson, M.S.; Cao, L.; Pomeroy, R.; Verdegem, M.; Slack, W.T.; Bondad-Reantaso, M.G.; Cabello, F. Responsible Aquaculture in 2050: Valuing Local Conditions and Human Innovations Will Be Key to Success. Bioscience 2013, 63, 255–262. [Google Scholar] [CrossRef] [Green Version]
- Lazard, J. Les Paradoxes Et Les Questionnements Soulevés Par l’exploitation De La Biodiversité (autochtone Et Introduite) En Aquaculture. Potentiels Sci. Pour Avenir Agric. Aliment. Environ. 2013, 1, 1–13. [Google Scholar]
- FAO (Food and Agricultural Organization). Planning for Aquaculture Diversification: The Importance of Climate Change and Other Drivers; FAO Fisheries and Aquaculture Department: Rome, Italy, 2016. [Google Scholar]
- FAO (Food and Agricultural Organization). The State of World Fisheries and Aquaculture 2018—Meeting the Sustainable Development Goals; The State of World Fisheries and Aquaculture (SOFIA) Food and Agriculture Organization of the United Nations: Rome, Italy, 2018; pp. 1–227. [Google Scholar]
- Zouiten, D.; Ben Khemis, I.; Besbes, R.; Cahu, C. Ontogeny of the digestive tract of thick-lipped grey mullet (Chelon labrosus) larvae in “mesocosms”. Aquaculture 2008, 279, 166–172. [Google Scholar] [CrossRef] [Green Version]
- Ben Khemis, I.; Gisbert, E.; Alcaraz, C.; Zouiten, D.; Besbes, R.; Zouiten, A.; Masmoudi, A.S.; Cahu, C. Allometric growth patterns and development in larvae and juveniles of thick-lipped grey mullet Chelon labrosus reared in mesocosm conditions. Aquac. Res. 2013, 44, 1872–1888. [Google Scholar] [CrossRef] [Green Version]
- Wassef, E.A.; El Masry, M.H.; Mikhail, F.R. Growth enhancement and muscle structure 34 of striped mullet, Mugil cephalus L., fingerlings by feeding algal meal based diets. Aquac. Res. 2001, 32, 315–322. [Google Scholar] [CrossRef]
- Pujante, I.; Mancera, J.M.; Moyano, F.J. Caracterización de las principales enzimas digestivas presentes en la liseta (Chelon labrosus) durante su desarrollo [Characterization of the main digestive enzymes present in the thick-lipped grey mullet (Chelon labrosus) during its development]. In Extended Abstract of XIII National Congress of Aquaculture; Universidad Politécnica de Catalunya: Barcelona, Spain, 2011; pp. 13–14. [Google Scholar]
- de las Heras, V.; Martos-Sitcha, J.A.; Mata, J.A.; Dias, J.; Conceiçâo, L.E.C.; Martínez Rodríguez, G.; Yúfera, M. La liseta (Chelon labrosus): Crecimiento a partir de proteína vegetal en estadios juveniles [The thick-lipped grey mullet (Chelon labrosus): Growth from vegetal protein during juveniles stages of development]. In Foro Iberoamericano de los Recursos Marinos y la Acuicultura; Cárdenas, S., Mancera, J.M., Rey-Méndez, M., Lodeiros, C., Eds.; Santiago de Compostela: La Coruña, Spain, 2013; Volume 5, pp. 555–564. [Google Scholar]
- Pujante, I.M.; Moyano, F.J.; Martos-Sitcha, J.A.; Mancera, J.M.; Martínez-Rodríguez, G. Effect of different salinities on gene expression and activity of digestive enzymes in the thick-lipped grey mullet (Chelon labrosus). Fish Physiol. Biochem. 2018, 44, 349–373. [Google Scholar] [CrossRef]
- Besbes, R.; Benseddik, A.B.; Kokokiris, L.; Changeux, T.; Hamza, A.; Kammoun, F.; Missaoui, H. Thicklip (Chelon labrosus) and flathead (Mugil cephalus) grey mullets fry production in Tunisian aquaculture. Aquac. Rep. 2020, 17, 100380. [Google Scholar] [CrossRef]
- Pal, J.; Shukla, B.; Maurya, A.K.; Verma, H.O.; Pandey, G.; Amitha, A. A review on role of fish in human nutrition with special emphasis to essential fatty acid. Int. J. Fish. Aquat. Stud. 2018, 6, 427–430. [Google Scholar]
- Fiorella, K.J.; Okronipa, H.; Baker, K.; Heilpern, S. Contemporary aquaculture: Implications for human nutrition. Curr. Opin. Biotechnol. 2021, 70, 83–90. [Google Scholar] [CrossRef]
- Hicks, C.C.; Cohen, P.J.; Graham, N.A.J.; Nash, K.L.; Allison, E.H.; D’Lima, C.; Mills, D.J.; Roscher, M.; Thilsted, S.H.; Thorne-Lyman, A.L.; et al. Harnessing global fisheries to tackle micronutrient deficiencies. Nature 2019, 574, 95–98. [Google Scholar] [CrossRef] [Green Version]
- Mohanty, B.P.; Mahanty, A.; Ganguly, S.; Mitra, T.; Karunakaran, D.; Anandan, R. Nutritional composition of food fishes and their importance in providing food and nutritional security. Food Chem. 2019, 293, 561–570. [Google Scholar] [CrossRef]
- Byrd, K.A.; Thilsted, S.H.; Fiorella, K.J. Fish nutrient composition: A review of global data from poorly assessed inland and marine species. Public Health Nutr. 2021, 24, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Pleadin, J.; Lešić, T.; Krešić, G.; Barić, R.; Bogdanović, T.; Oraić, D.; Vulić, A.; Legac, A.; ZrnčIć, S. Nutritional quality of different fish species farmed in the Adriatic sea. Ital. J. Food Sci. 2017, 29, 537–549. [Google Scholar] [CrossRef]
- Saavedra, M.; Pereira, T.G.; Carvalho, L.M.; Pousão-Ferreira, P.; Grade, A.; Teixeira, B.; Quental-Ferreira, H.; Mendes, R.; Bandarra, N.; Gonçalves, A. Wild and farmed meagre, Argyrosomus regius: A nutritional, sensory and histological assessment of quality differences. J. Food Compos. Anal. 2017, 63, 8–14. [Google Scholar] [CrossRef]
- Baki, B.; Ozturk, D.K.; Kerim, M. Comaprative fatty acid composition of European seabass Dicentrarchus labrax (Linnaeus, 1758) farmed in cages in the Aegean Sea and the Black Sea coasts of Turkey. Indian J. Fish. 2019, 66, 54–61. [Google Scholar] [CrossRef] [Green Version]
- Parma, L.; Badiani, A.; Bonaldo, A.; Viroli, C.; Farabegoli, F.; Silvi, M.; Bonvini, E.; Pirini, M.; Gatta, P.P. Farmed and wild common sole (Solea solea L.): Comparative assessment of morphometric parameters, processing yields, selected nutritional traits and sensory profile. Aquaculture 2019, 502, 63–71. [Google Scholar] [CrossRef]
- Nefedova, Z.A.; Murzina, S.A.; Pekkoeva, S.N.; Voronin, V.P.; Nemova, N.N. Comparative Characteristics of the Fatty-Acid Composition of Lipids in Factory and Wild Juveniles of Atlantic Salmon Salmo salar L. Contemp. Probl. Ecol. 2020, 13, 156–161. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 17th ed.; The Association of Official Analytical Chemists: Rockville, MD, USA, 2000. [Google Scholar]
- Folch, J. A simple method for the isolation and purification of the total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Rodríguez-Ruíz, J.; Belarbi, E.H.; García, J.L.; López, D. Rapid simultaneous lipid extraction and transesterification for fatty acid analyses. Biotechnol. Tech. 1998, 12, 689–691. [Google Scholar] [CrossRef]
- Lepage, G.; Roy, C.C. Improved recovery of fatty acid through direct transesterification without prior extraction of purification. J. Lipid Res. 1984, 25, 1391–1396. [Google Scholar] [CrossRef]
- Arakawa, K.; Sagai, M. Species differences in lipid peroxide levels in lung tissue and investigation of their determining factors. Lipids 1986, 21, 769–775. [Google Scholar] [CrossRef]
- Senso, L.; Suarez, M.D.; Ruiz-Cara, T.; Garcıa-Gallego, M. On the possible effects of harvesting season and chilled storage on the fatty acid profile of the fillet of farmed gilthead sea bream (Sparus aurata). Food Chem. 2007, 101, 298–307. [Google Scholar] [CrossRef]
- FAO/WHO (Food and Agriculture Organization of the United Nations/World Health Organization). Dietary Protein Quality Evaluation in Human Nutrition. In FAO Food and Nutrition Paper; FAO: Rome, Italy, 2013; Volume 92. [Google Scholar]
- Kolanowski, W. Salmonids as Natural Functional Food Rich in Omega-3 PUFA. Appl. Sci. 2021, 11, 2409. [Google Scholar] [CrossRef]
- Lenas, D.S.; Triantafillou, D.J.; Chatziantoniou, S.; Nathanailides, C. Fatty acid profile of wild and farmed gilthead sea bream (Sparus aurata). J. Verbrauch. Lebensm. 2011, 6, 435–440. [Google Scholar] [CrossRef]
- Baki, B.; Gönener, S.; Kaya, D. Comparison of Food, Amino Acid and Fatty Acid Compositions of Wild and Cultivated Sea Bass (Dicentrarchus labrax L., 1758). Turk. J. Fish. Aquat. Sci. 2015, 15, 175–179. [Google Scholar] [CrossRef]
- Ning, J.; Hu, P.; Li, B.; Jiang, C. Nutritional comparison in muscle of wild, pond and factory cultured Japanese flounder (Paralichthys olivaceus) adults. Aquac. Res. 2018, 49, 2572–2578. [Google Scholar] [CrossRef]
- Alexi, N.; Luca, A.; Nanou, E.; Byrne, D.V.; Grigorakis, K. Investigation of the proximate composition, lipid quality, volatile and sensory profiles of wild vs. reared Greater amberjack (Seriola dumerili, Risso). Aquac. Res. 2020, 51, 2443–2455. [Google Scholar] [CrossRef]
- Łuczyńska, J.; Borejszo, Z.; Łuczyński, M.J. The composition of fatty acids in muscles of six freshwater fish species from the Mazurian Great Lakes (northeastern Poland). Arch. Pol. Fish. 2008, 16, 167–178. [Google Scholar] [CrossRef] [Green Version]
- Żarski, D.; Krejszeff, S.; Horváth, Á.; Bokor, Z.; Palińska, K.; Szentes, K.; Łuczyńska, J.; Targońska, K.; Kupren, K. Dynamics of composition and morphology in oocytes of Eurasian perch, Perca fluviatilis L., during induced spawning. Aquaculture 2012, 364, 103–110. [Google Scholar] [CrossRef]
- Stepanowska, K.; Biernaczyk, M.; Opanowski, A.; Neja, Z. Selected morphometric characteristics, condition, and body chemical composition of perch (Perca fluviatilis L.) from Lake Miedwie, Poland. Ecol. Chem. Eng. A 2012, 19, 145–153. [Google Scholar] [CrossRef]
- Johnston, I.A.; Li, X.; Vieira, V.L.A.; Nickell, D.; Dingwall, A.; Alderson, R.; Campbell, P.; Bickerdike, R. Muscle and flesh quality traits in wild and farmed Atlantic salmon. Aquaculture 2006, 256, 323–336. [Google Scholar] [CrossRef]
- Grigorakis, K.; Alexis, M.N.; Taylor, K.D.A.; Hole, M. Comparison of wild and cultured gilthead sea bream (Sparus aurata); composition, appearance and seasonal variations. Int. J. Food Sci. Technol. 2002, 37, 477–484. [Google Scholar] [CrossRef]
- Rincón, L.; Castro, P.L.; Álvarez, B.; Hernández, M.D.; Álvarez, A.; Claret, A.; Guerrero, L.; Ginés, R. Differences in proximal and fatty acid profiles, sensory characteristics, texture, colour and muscle cellularity between wild and farmed blackspot seabream (Pagellus bogaraveo). Aquaculture 2016, 451, 195–204. [Google Scholar] [CrossRef]
- Izquierdo, M.S.; Obach, A.; Arantzamendi, L.; Montero, D.; Robaina, L.; Rosenlund, G. Dietary lipid sources for sea bream and sea bass: Growth performance, tissue composition and flesh quality. Aquac. Nutr. 2003, 9, 397–407. [Google Scholar] [CrossRef]
- Fountoulaki, E.; Vasilaki, A.; Hurtado, R.; Grigorakis, K.; Karacostas, I.; Nengas, I.; Rigos, G.; Kotzamanis, Y.; Venou, B.; Alexis, M.N. Fish oil substitution by vegetable oils in commercial diets for gilthead sea bream (Sparus aurata L.); effects on growth performance, flesh quality and fillet fatty acid profile: Recovery of fatty acid profiles by a fish oil finishing diet under fluctuating water temperatures. Aquaculture 2009, 289, 317–326. [Google Scholar] [CrossRef]
- Guler, G.O.; Zengin, G.; Selim Çakmak, Y.; Aktumsek, A. Comparison of Fatty Acid Compositions and ω3/ω6 Ratios of Wild Brown Trout and Cultured Rainbow Trout. Turk. J. Fish. Aquat. Sci. 2017, 17, 1179–1187. [Google Scholar] [CrossRef]
- Syama Dayal, J.; Ambasankar, K.; Jannathulla, R.; Kumuraguruvasagam, K.P.; Kailasam, M. Nutrient and fatty acid composition of cultured and wild caught gold-spot mullet Liza parsia (Hamilton, 1822). Indian J. Fish. 2019, 66, 62–70. [Google Scholar] [CrossRef] [Green Version]
- Akpinar, N.; Akpinar, M.A.; Gorgun, S.; Akpinar, A.E. Fatty acid composition and ω3/ω6 ratios in the muscle of wild and reared Oncorhynchus mykiss. Chem. Nat. Compd. 2015, 51, 22–25. [Google Scholar] [CrossRef]
- Łuczyńska, J.; Tońska, E.; Krejszeff, S.; Żarski, D. Comparison of Fatty Acids in the Muscles and Liver of Pond-Cultured and Wild Perch, Perca fluviatilis (L.), in Poland. Turk. J. Fish. Aquat. Sci. 2016, 16, 19–27. [Google Scholar] [CrossRef]
- Tocher, D.R. Fatty acid requirements in ontogeny of marine and freshwater fish. Aquac. Res. 2010, 41, 717–732. [Google Scholar] [CrossRef]
- Drake, P.; Arias, A.M.; Gallego, L. Biología de los Mugílidos (Osteichthyes, Mugilidae) en los esteros de las Salinas de San Fernando (Cádiz). III. Hábitos alimentarios y su relación con la morfometría del aparato digestivo. Investig. Pesq. 1984, 48, 337–367. [Google Scholar]
- Fernández-Delgado, C.; Drake, P.; Arias, A.; García, D. Peces de Doñana y su Entorno, Serie Técnica; Organismo Autónomo Parques Nacionales, Secretaría General de Medio Ambiente, Ministerio de Medio Ambiente: Madrid, Spain, 2000; 272p.
- Shah, M.R.; Lutzu, G.A.; Alam, A.; Sarker, P.; Kabir Chowdhury, M.A.; Parsaeimehr, A.; Liang, Y.; Daroch, M. Microalgae in aquafeeds for a sustainable aquaculture industry. J. Appl. Phycol. 2018, 30, 197–213. [Google Scholar] [CrossRef]
- Han, P.; Lu, Q.; Fan, L.; Zhou, W. A review on the use of microalgae for sustainable aquaculture. Appl. Sci. 2019, 9, 2377. [Google Scholar] [CrossRef] [Green Version]
- Brown, M.R.; Jeffrey, S.W.; Volkman, J.K.; Dunstan, G.A. Nutritional properties of microalgae for mariculture. Aquaculture 1997, 151, 315–331. [Google Scholar] [CrossRef]
- Sinclair, A.J.; Begg, D.; Mathai, M.; Weisinger, R.S. Omega 3 fatty acids and the brain: Review of studies in depression. Asia Pac. J. Clin. Nutr. 2007, 16, 391–397. [Google Scholar]
- Simopoulos, A.P. Dietary Omega-3 Fatty Acid Deficiency and High Fructose Intake in the Development of Metabolic Syndrome, Brain Metabolic Abnormalities, and Non-Alcoholic Fatty Liver Disease. Nutrients 2013, 5, 2901–2923. [Google Scholar] [CrossRef] [Green Version]
- Simopoulos, A.P.; DiNicolantonio, J.J. The importance of a balanced ω-6 to ω-3 ratio in the prevention and management of obesity. Open Heart 2016, 3, e000385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Román, G.C.; Jackson, R.E.; Gadhia, R.; Román, A.N.; Reis, J. Mediterranean diet: The role of long-chain ω-3 fatty acids in fish; polyphenols in fruits, vegetables, cereals, coffee, tea, cacao and wine; probiotics and vitamins in prevention of stroke, age-related cognitive decline, and Alzheimer disease. Rev. Neurol. 2019, 175, 724–741. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition, and Allergies (NDA). Scientific Opinion on Dietary Reference Values for fats, including saturated fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids, trans fatty acids, and cholesterol. EFSA J. 2010, 8, 1461. [Google Scholar] [CrossRef] [Green Version]
- FAO (Food and Agricultural Organization). Fats and Fatty Acids in Human Nutrition. In Report of an Expert Consultation; Food and Agriculture Organization of the United Nations: Rome, Italy, 2010; pp. 1–166. [Google Scholar]
- Jiang, H.; Cheng, X.; Geng, L.; Tang, S.; Tong, G.; Xu, W. Comparative study of the nutritional composition and toxic elements of farmed and wild Chanodichthys mongolicus. Chin. J. Oceanol. Limnol. 2017, 35, 737–744. [Google Scholar] [CrossRef]
- Liao, S.M.; Du, Q.S.; Meng, J.Z.; Pang, Z.W.; Huang, R.B. The multiple roles of histidine in protein interactions. Chem. Cent. J. 2013, 7, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohanty, B.; Mahanty, A.; Ganguly, S.; Sankar, T.V.; Chakraborty, K.; Rangasamy, A.; Paul, B.; Sarma, D.; Mathew, S.; Asha, K.K.; et al. Amino Acid Compositions of 27 Food Fishes and Their Importance in Clinical Nutrition. J. Amino Acids 2014, 2014, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.Z.; Yang, S.; Wu, G. Free radicals, antioxidants, and nutrition. Nutrition 2002, 18, 872–879. [Google Scholar] [CrossRef]
- Fuentes, A.; Fernandez-Segovia, I.; Serra, J.A.; Barat, J.M. Comparison of wild and cultured sea bass (Dicentrarchus labrax) quality. Food Chem. 2010, 119, 1514–1518. [Google Scholar] [CrossRef]
- Oztekin, A.; Yigit, M.; Kizilkaya, B.; Ucyol, N.; Tan, E.; Yilmaz, S.; Bulut, M.; Ayaz, A.; Ergun, S. Nutritional quality of amino acid in farmed, farm-aggregated and wild Axillary seabream (Pagellus acarne) with implications to Human Health. Aquac. Res. 2020, 51, 1844–1853. [Google Scholar] [CrossRef]
- FAO/WHO (Food and Agriculture Organization of the United Nations/World Health Organization). Protein Quality Evaluation. Report of Joint FAO/WHO Expert Consultation. In FAO Food and Nutrition Paper; Food and Agriculture Organization of the United Nations: Rome, Italy, 1991; pp. 1–66. [Google Scholar]
| Fatty Acids | % |
|---|---|
| 14:0 | 2.5 |
| 16:0 | 21.3 |
| 18:0 | 4.7 |
| 16:1 | 3.0 |
| 18:1n-9 | 18.6 |
| 20:1n-9 | 1.8 |
| 18:2n-6 | 22.1 |
| 20:4n-6, ARA | 0.9 |
| 18:3n-3 | 2.3 |
| 20:5n-3, EPA | 5.7 |
| 22:5n-3 | 1.3 |
| 22:6n-3, DHA | 14.2 |
| ƩSFA | 28.5 |
| ƩMUFA | 23.3 |
| ƩPUFA | 46.5 |
| Other FA | 1.7 |
| Ʃn-3 | 23.5 |
| Ʃn-6 | 23 |
| n-3/n-6 | 1.02 |
| Cultivated | Wild | p | |
|---|---|---|---|
| Total protein | 20.16 ± 0.02 | 22.06 ± 0.01 * | <0.001 |
| Tota lipid | 3.91 ± 0.66 * | 1.96 ± 0.17 | 0.007 |
| Ash | 1.38 ± 0.00 | 1.52 ± 0.00 * | <0.001 |
| Moisture | 73.01 ± 0.41 | 72.89 ± 0.91 | n.s. |
| Fatty Acids | Cultivated | Wild | p |
|---|---|---|---|
| 14:0 | 12.60 ± 3.71 | 25.74 ± 1.05 * | 0.040 |
| 16:0 | 25.41 ± 1.34 * | 10.84 ± 0.06 | 0.003 |
| 18:0 | 5.27 ± 0.11 * | 2.26 ± 0.00 | 0.013 |
| 16:1 | 7.56 ± 0.53 * | 1.25 ± 0.07 | 0.003 |
| 18:1n-9 | 20.65 ± 1.90 * | 8.22 ± 0.24 | 0.011 |
| 18:2n-6 | 4.58 ± 0.54 * | 2.98 ± 0.01 | 0.023 |
| 18:3n-3 | 0.69 ± 0.12 | 0.81 ± 0.04 | n.s. |
| 20:1n-9 | 0.03 ± 0.00 | 2.77 ± 0.38 * | 0.030 |
| 20:4n-6, ARA | 0.39 ± 0.09 | 3.26 ± 0.06 * | 0.018 |
| 20:4n-3 | 0.85 ± 0.06 | 1.50 ± 0.35 | n.s. |
| 20:5n-3, EPA | 1.86 ± 0.12 | 10.36 ± 0.09 * | <0.001 |
| 22:5n-3 | 4.73 ± 0.13 * | 2.64 ± 0.09 | 0.002 |
| 22:6n-3, DHA | 8.48 ± 0.53 | 15.49 ± 0.07 * | 0.004 |
| ƩSFA | 43.27 ± 2.48 | 38.85 ± 1.11 | n.s. |
| ƩMUFA | 32.15 ± 2.75 * | 14.14 ± 0.34 | 0.011 |
| ƩPUFA | 21.58 ± 0.48 | 33.24 ± 0.67 * | 0.002 |
| Other FA | 2.99 ± 0.76 | 12.74 ± 0.43 * | 0.004 |
| Ʃn-3 | 16.61 ± 0.60 | 31.03 ± 0.56 * | 0.001 |
| Ʃn-6 | 4.97 ± 1.09 | 6.24 ± 0.06 | n.s. |
| Ʃn-9 | 20.68 ± 1.90 * | 10.99 ± 0.14 | 0.018 |
| n-3/n-6 | 3.44 ± 0.87 | 4.97 ± 0.04 | n.s. |
| EPA/DHA | 0.22 ± 0.00 | 0.67 ± 0.00 * | <0.001 |
| Fatty Acids | Cultivated | Wild | p |
|---|---|---|---|
| 14:0 | 3.59 ± 0.17 * | 0.91 ± 0.19 | <0.001 |
| 16:0 | 27.34 ± 1.93 * | 12.86 ± 3.12 | 0.002 |
| 18:0 | 8.43 ± 1.30 | 6.39 ± 2.34 | n.s. |
| 16:1 | 5.86 ± 0.96 * | 2.59 ± 0.87 | 0.011 |
| 18:1n-9 | 18.40 ± 2.86 * | 3.62 ± 0.46 | <0.001 |
| 18:2n-6 | 7.04 ± 2.73 * | 1.41 ± 0.88 | 0.027 |
| 18:3n-6 | 0.24 ± 0.05 | 0.46 ± 0.22 | n.s. |
| 18:3n-3 | 0.81 ± 0.10 | 0.90 ± 0.46 | n.s. |
| 20:1 | 1.24 ± 0.15 * | 0.14 ± 0.04 | <0.001 |
| 20:3n-6 | 0.27 ± 0.05 | 0.72 ± 0.24 * | 0.030 |
| 20:4n-6, ARA | 2.83 ± 0.59 | 6.12 ± 1.89 * | 0.044 |
| 20:3n-3 | 0.05 ± 0.01 | 0.12 ± 0.05 | n.s. |
| 20:5n-3, EPA | 7.53 ± 2.03 | 29.46 ± 3.91 * | 0.002 |
| 22:6n-3, DHA | 12.05 ± 3.55 | 29.88 ± 4.15 * | 0.015 |
| Total lipids (mg g−1 d.w.) | 144.87 ± 1.48 * | 72.30 ± 0.74 | <0.001 |
| Other FA | 3.22 ± 0.78 | 4.41± 1.15 | n.s. |
| ƩSFA | 39.36 ± 3.31 * | 20.16 ± 5.49 | 0.019 |
| ƩMUFA | 25.50 ± 3.91 * | 6.35 ± 1.03 | 0.001 |
| ƩPUFA | 30.80 ± 7.83 | 72.69 ± 6.87 * | 0.015 |
| Ʃn-3 | 20.41 ± 9.74 | 60.36 ± 3.85 * | 0.002 |
| Ʃn-6 | 10.39 ± 2.23 | 8.71 ± 3.05 | n.s. |
| Ʃn-9 | 18.40 ± 2.86 * | 3.62 ± 0.46 | <0.001 |
| n-3/n-6 | 2.17 ± 1.55 | 7.41 ± 2.19 * | 0.027 |
| EPA/DHA | 0.62 ± 0.05 | 1.02 ± 0.31 | n.s. |
| PI 1 | 163.67 ± 69.14 | 447.07 ± 34.62 * | 0.003 |
| IT 2 | 0.46 ± 0.15 * | 0.10 ± 0.03 | 0.016 |
| IA 3 | 0.74 ± 0.10 * | 0.22 ± 0.07 | 0.001 |
| FLQ 4 | 14.01 ± 1.72 | 57.51 ± 1.57 * | 0.002 |
| Amino Acids | Cultivated | Wild | p |
|---|---|---|---|
| Essential Amino Acids (EAAs) | |||
| Valine | 3.45 ± 0.02 | 3.31 ± 0.12 | n.s. |
| Methionine | 1.84 ± 0.01 | 1.91 ± 0.03 | n.s. |
| Isoleucine | 3.06 ± 0.03 | 2.97 ± 0.10 | n.s. |
| Leucine | 5.28 ± 0.01 | 5.46 ± 0.16 | n.s. |
| Threonine | 3.01 ± 0.01 | 3.11 ± 0.08 | n.s. |
| Phenylalanine | 2.38 ± 0.05 | 2.44 ± 0.02 | n.s. |
| Histidine | 1.26 ± 0.01 * | 1.04 ± 0.02 | 0.006 |
| Lysine | 6.74 ± 0.00 | 7.38 ± 0.18 | n.s. |
| Arginine | 3.99 ± 0.01 | 4.19 ± 0.11 | n.s. |
| ƩEAA | 29.17 ± 0.13 | 29.90 ± 0.80 | n.s. |
| Non-essential amino acids (NEAAs) | |||
| Aspartic acid | 6.95 ± 0.00 | 7.33 ± 0.16 | n.s. |
| Tyrosine | 2.09 ± 0.07 | 2.25 ± 0.03 | n.s. |
| Serine | 2.71 ± 0.01 | 2.95 ± 0.04 * | 0.011 |
| Glutamic acid | 9.89 ± 0.06 | 10.66 ± 0.24 * | 0.048 |
| Glycine | 2.85 ± 0.01 | 2.99 ± 0.09 | n.s. |
| Alanine | 5.13 ± 0.00 | 5.28 ± 0.13 | n.s. |
| Cysteine | 0.21 ± 0.03 | 0.19 ± 0.01 | n.s. |
| Proline | 4.29 ± 0.54 | 4.09 ± 0.24 | n.s. |
| ƩNEAA | 35.96 ± 0.63 | 37.66 ± 0.97 | n.s. |
| Ratio EAA/NEAA | 0.81 ± 0.01 * | 0.79 ± 0.00 | 0.014 |
| Amino Acids | mg g Protein−1 | 1 AA Score (%) | |||
|---|---|---|---|---|---|
| Cultivated | Wild | FAO/WHO Standard | Cultivated | Wild | |
| Valine | 46.2 | 40.5 | 39 | 118 | 104 |
| Isoleucine | 41.0 | 36.3 | 30 | 137 | 121 |
| Leucine | 70.7 | 66.8 | 59 | 120 | 113 |
| Threonine | 40.3 | 38.1 | 23 | 175 | 165 |
| Phenylalanine + Tyrosine | 59.9 | 57.4 | 38 | 158 | 151 |
| Histidine | 16.9 | 12.7 | 15 | 112 | 85 |
| Methionine + Cysteine | 27.5 | 25.7 | 22 | 125 | 117 |
| Lysine | 90.3 | 90.3 | 45 | 201 | 201 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Márquez, J.; Galafat, A.; Alarcón, F.J.; Figueroa, F.L.; Martínez-Manzanares, E.; Arijo, S.; Abdala-Díaz, R.T. Cultivated and Wild Juvenile Thick-Lipped Grey Mullet, Chelon labrosus: A Comparison from a Nutritional Point of View. Animals 2021, 11, 2112. https://doi.org/10.3390/ani11072112
García-Márquez J, Galafat A, Alarcón FJ, Figueroa FL, Martínez-Manzanares E, Arijo S, Abdala-Díaz RT. Cultivated and Wild Juvenile Thick-Lipped Grey Mullet, Chelon labrosus: A Comparison from a Nutritional Point of View. Animals. 2021; 11(7):2112. https://doi.org/10.3390/ani11072112
Chicago/Turabian StyleGarcía-Márquez, Jorge, Alba Galafat, Francisco Javier Alarcón, Félix L. Figueroa, Eduardo Martínez-Manzanares, Salvador Arijo, and Roberto Teófilo Abdala-Díaz. 2021. "Cultivated and Wild Juvenile Thick-Lipped Grey Mullet, Chelon labrosus: A Comparison from a Nutritional Point of View" Animals 11, no. 7: 2112. https://doi.org/10.3390/ani11072112
APA StyleGarcía-Márquez, J., Galafat, A., Alarcón, F. J., Figueroa, F. L., Martínez-Manzanares, E., Arijo, S., & Abdala-Díaz, R. T. (2021). Cultivated and Wild Juvenile Thick-Lipped Grey Mullet, Chelon labrosus: A Comparison from a Nutritional Point of View. Animals, 11(7), 2112. https://doi.org/10.3390/ani11072112







