Effects of Bacillus subtilis on Production Performance, Bone Physiological Property, and Hematology Indexes in Laying Hens
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Housing, and Diets
2.2. Sample Collection
2.3. Colonization Efficiency of Bacillus subtilis
2.4. Egg Quality
2.5. Hematological Measurements
2.6. Bone Traits Analysis
2.7. Real-Time PCR
2.8. Ca and P Content Analysis
2.9. Statistical Analysis
3. Results
3.1. Colonization Efficiency of Bacillus subtilis
3.2. Egg Production and Egg Quality Parameters
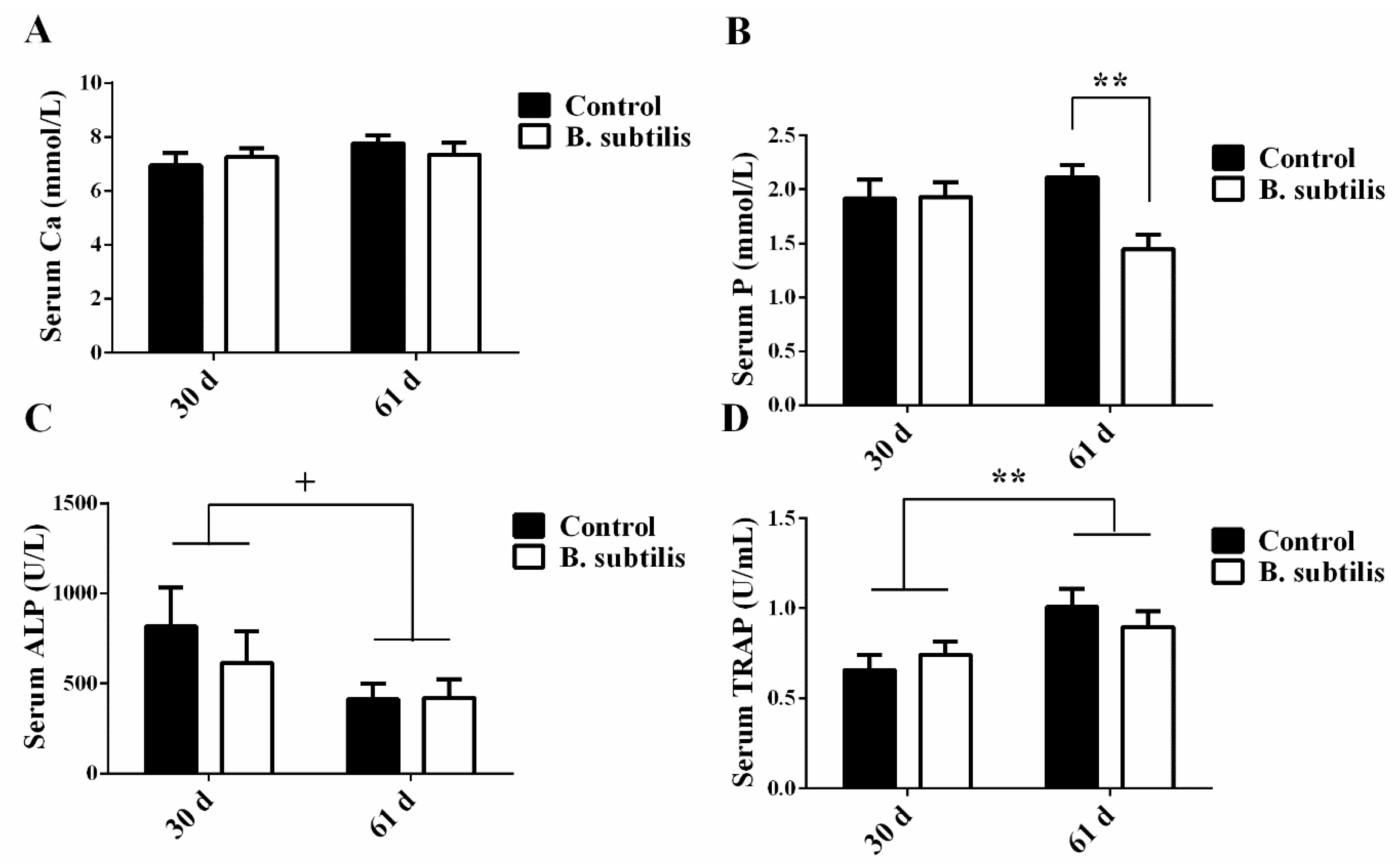
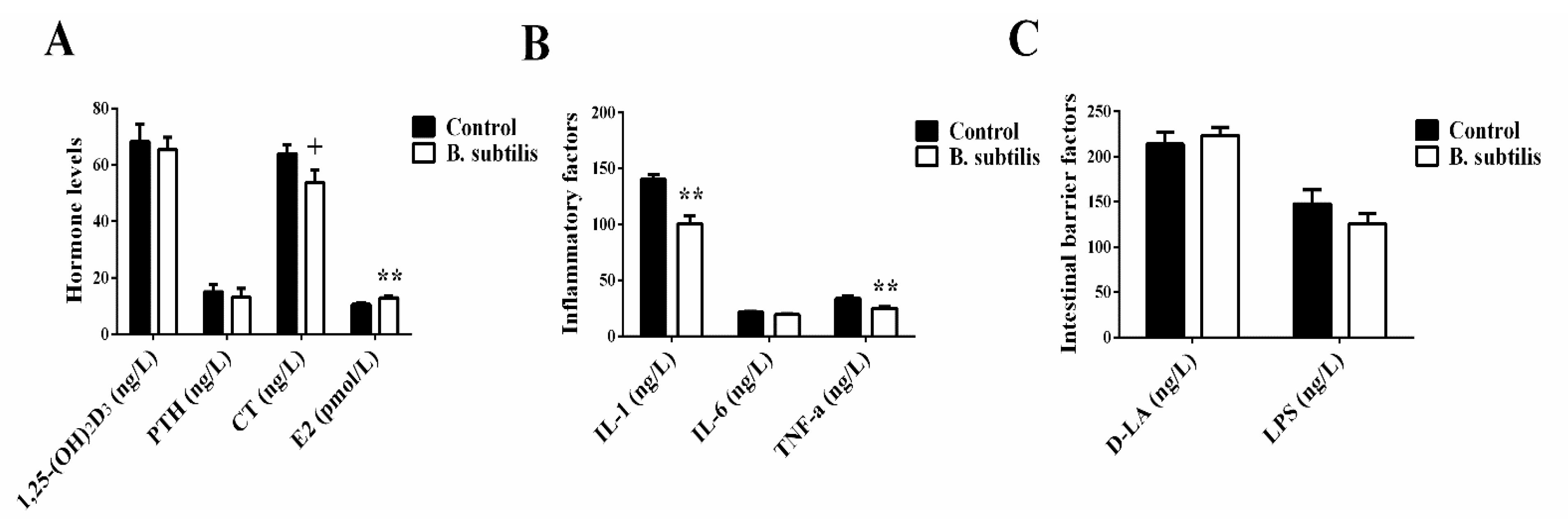
3.3. Hematological Analysis
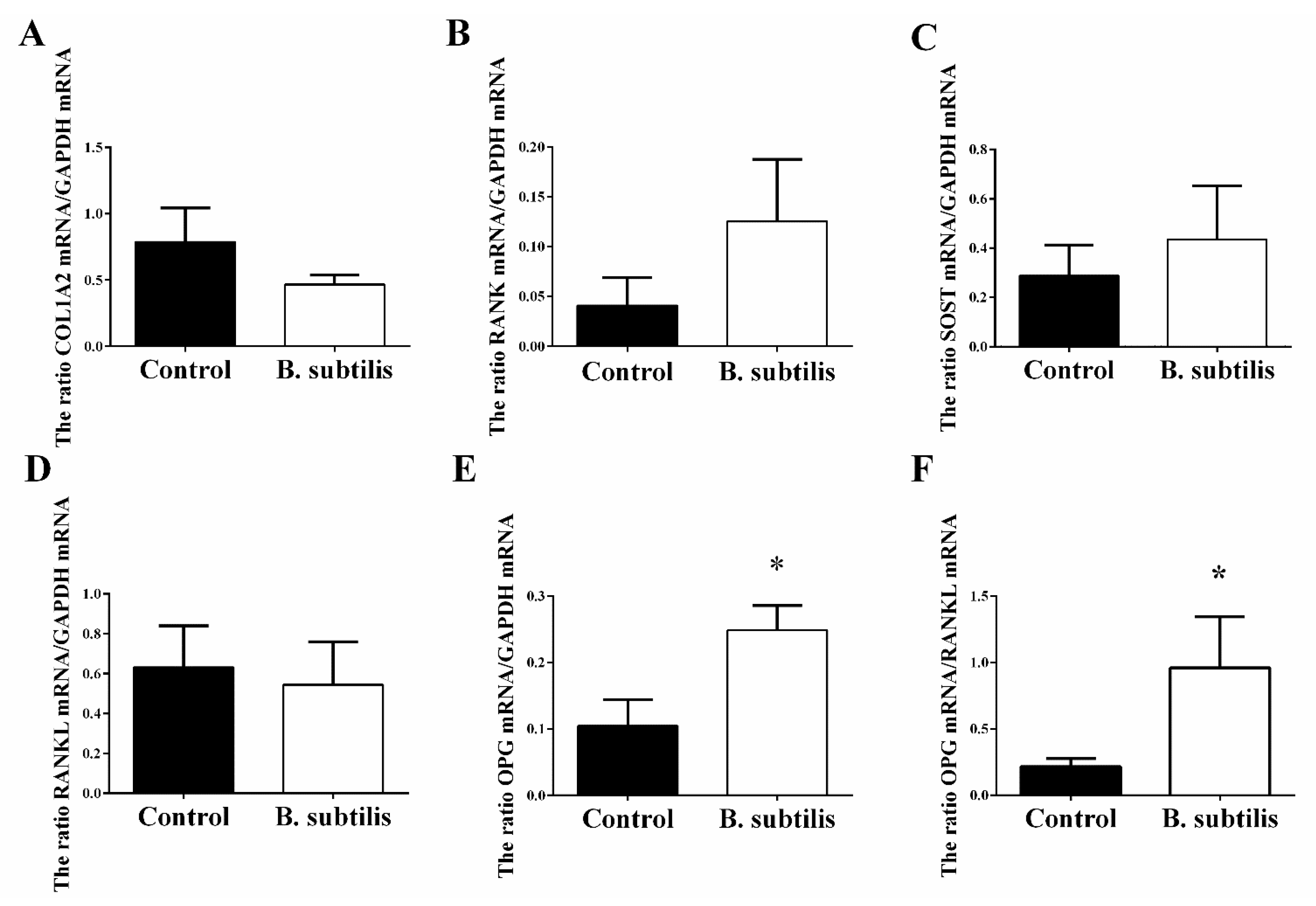
3.4. Bone Pathophysiological Parameters
3.5. Ca and P Content Analysis
3.6. Correlation between Ca or P and Bone Quality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mehring, A.L.; Titus, H.W. The effects of low levels of calcium in the diet of laying chickens. Poult. Sci. 1964, 43, 1405–1414. [Google Scholar] [CrossRef]
- Webster, A.B. Welfare implications of avian osteoporosis. Poult. Sci. 2004, 83, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Fleming, R.H. Nutritional factors affecting poultry bone health. Proc. Nutr. Soc. 2008, 67, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Leeson, S.; Summers, A. Commercial Poultry Nutrition, 3rd ed.; Nottingham University Press: Nottingham, UK, 2008; pp. 123–160. [Google Scholar]
- Whitehead, C.C.; Fleming, R.H. Osteoporosis in cage layers. Poult. Sci. 2000, 79, 1033–1041. [Google Scholar] [CrossRef] [PubMed]
- Khanal, T.; Widowski, T.; Bédécarrats, G.; Kiarie, E. Effects of pre-lay dietary calcium (2.5 vs. 4.0%) and pullet strain (Lohmann Brown vs. Selected Leghorn LSL-Lite) on calcium utilization and femur quality at 1st through to the 50th egg. Poult. Sci. 2019, 98, 4919–4928. [Google Scholar] [CrossRef]
- Sumano-Lopez, H.; Carrillo-Gonzalez, L.; Monroy-Barreto, M.; Tapia-Perez, G.; Olvera, L.G. Bioavailability of four calcium sources in the second-cycle egg-producing hens. J. Appl. Poult. Res. 2021, 30, 8. [Google Scholar]
- Rathnayaka, R.; Mutucumarana, R.K.; Andrew, M.S. Free-choice feeding of three different dietary calcium sources and their influence on egg quality parameters of commercial layers. J. Agric. Sci. Sri Lanka 2020, 15, 50–62. [Google Scholar] [CrossRef]
- Habibollahi, M.; Abousadi, M.A.; Nakhaee, P. The effect of phytase on production performance, egg quality, calcium and phosphorus excretion, and fatty acids and cholesterol concentration in hy-line layers fed diets containing rice bran. J. Appl. Poult. Res. 2019, 28, 688–698. [Google Scholar] [CrossRef]
- Shi, H.; Lee, K.Y.; Kim, I.H. Dietary supplementation with protected calcium effects production and egg quality of Hy-line brown laying hens. Anim. Prod. Sci. 2020, 60, 1793–1799. [Google Scholar] [CrossRef]
- Bernard, B.D.; Stagni, N.; Camerotto, R.; Vittur, F.; Zanetti, M.; Zallone, A.Z.; Teti, A. Influence of calcium depletion on medullary bone of laying hens. Calcif. Tissue Int. 1980, 32, 221–228. [Google Scholar] [CrossRef]
- Mountzouris, K.C.; Tsitrsikos, P.; Palamidi, I.; Arvaniti, A.; Mohnl, M.; Schatzmayr, G.; Fegeros, K. Effects of probiotic inclusion levels in broiler nutrition on growth performance, nutrient digestibility, plasma immunoglobulins, and cecal microflora composition. Poult. Sci. 2010, 89, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Forte, C.; Acuti, G.; Manuali, E.; Casagrande Proietti, P.; Pavone, S.; Trabalza-Marinucci, M.; Moscati, L.; Onofri, A. Effects of two different probiotics on microflora, morphology, and morphometry of gut in organic laying hens. Poult. Sci. 2016, 95, 2528–2535. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Yan, T.; Wang, X.; Kuang, S.; Xiao, Y.C.; Lu, W.W.; Bi, D.R. Probiotic mixture ameliorates heat stress of laying hens by enhancing intestinal barrier function and improving gut microbiota. Ital. J. Anim. Sci. 2017, 16, 292–300. [Google Scholar] [CrossRef]
- Song, J.; Xiao, K.; Ke, Y.L.; Jiao, L.F.; Hu, C.H.; Diao, Q.Y.; Shi, B.; Zou, X.T. Effect of a probiotic mixture on intestinal microflora, morphology, and barrier integrity of broilers subjected to heat stress. Poult. Sci. 2014, 93, 581–588. [Google Scholar] [CrossRef]
- Panda, A.K.; Reddy, M.R.; Rama Rao, S.V.; Praharaj, N.K. Production performance, serum/yolk cholesterol and immune competence of white leghorn layers as influenced by dietary supplementation with probiotic. Trop. Anim. Health Prod. 2003, 35, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.P.; Wu, A.M.; Ding, X.M.; Lei, Y.; Bai, J.; Zhang, K.Y.; Chio, J.S. Effects of probiotic-supplemented diets on growth performance and intestinal immune characteristics of broiler chickens. Poult. Sci. 2013, 92, 663–670. [Google Scholar] [CrossRef]
- Mikulski, D.; Jankowski, J.; Naczmanski, J.; Mikulska, M.; Demey, V. Effects of dietary probiotic (Pediococcus acidilactici) supplementation on performance, nutrient digestibility, egg traits, egg yolk cholesterol, and fatty acid profile in laying hens. Poult. Sci. 2012, 91, 2691–2700. [Google Scholar] [CrossRef]
- Park, J.W.; Jeong, J.S.; Lee, S.I.; Kim, I.H. Effect of dietary supplementation with a probiotic (Enterococcus faecium) on production performance, excreta microflora, ammonia emission, and nutrient utilization in ISA brown laying hens. Poult. Sci. 2016, 95, 2829–2835. [Google Scholar] [CrossRef]
- Molnar, A.K.; Podmaniczky, B.; Kurti, P.; Glavits, R.; Virag, G.; Szabo, Z.; Farkas, Z. Effect of different concentrations of Bacillus subtilis on immune response of broiler chickens. Probiotics Antimicrob. Proteins 2011, 3, 8–14. [Google Scholar] [CrossRef]
- Park, J.H.; Yun, H.M.; Kim, I.H. The effect of dietary Bacillus subtilis supplementation on the growth performance, blood profile, nutrient retention, and caecal microflora in broiler chickens. J. Appl. Anim. Res. 2018, 46, 868–872. [Google Scholar] [CrossRef]
- Knap, I.; Kehlet, A.B.; Bennedsen, M.; Mathis, G.F.; Hofacre, C.L.; Lumpkins, B.S.; Jensen, M.M.; Raun, M.; Lay, A. Bacillus subtilis (DSM17299) significantly reduces Salmonella in broilers. Poult. Sci. 2011, 90, 1690–1694. [Google Scholar] [CrossRef]
- Lei, X.; Piao, X.; Ru, Y.; Zhang, H.; Peron, A.; Zhang, H. Effect of Bacillus amyloliquefaciens-based direct-fed microbial on performance, nutrient utilization, intestinal morphology and cecal microflora in broiler chickens. Asian Aust. J. Anim. Sci. 2015, 28, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, A.A. Bone mineralization of broiler chicks challenged with Salmonella enteritidis fed diet containing probiotic (Bacillus subtilis). Probiotics Antimicrob. Proteins 2014, 6, 136–140. [Google Scholar] [CrossRef]
- Mutuş, R.; Kocabagli, N.; Alp, M.; Acar, N.; Eren, M.; Gezen, S.S. The effect of dietary probiotic supplementation on tibial bone characteristics and strength in broilers. Poult. Sci. 2006, 85, 1621–1625. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.F.; Wang, W.C.; Cheng, H.W. Bacillus subtilis based probiotic improved bone mass and altered brain serotoninergic and dopaminergic systems in broiler chickens. J. Funct. Foods. 2018, 49, 501–509. [Google Scholar] [CrossRef]
- Newberry, R.C.; Tarazona, A.M. Behavior and welfare of laying hens and broiler chickens. Rev. Colomb Cienc. Pecu. 2011, 24, 301–302. [Google Scholar]
- Guo, J.R.; Dong, X.F.; Liu, S.; Tong, J.M. High-throughput sequencing reveals the effect of Bacillus subtilis CGMCC 1.921 on the cecal microbiota and gene expression in ileum mucosa of laying hens. Poult. Sci. 2018, 97, 2543–2556. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Wang, H.; Zhang, J.; Tang, X.; Raheem, A.; Wang, M.; Lin, W.; Liang, L.; Qi, Y.; Zhu, Y. Modulatory effects of Bacillus subtilis on the performance, morphology, cecal microbiota and gut barrier function of laying hens. Animals 2021, 11, 1523. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.R.; Dong, X.F.; Liu, S.; Tong, J.M. Effects of long-term Bacillus subtilis CGMCC 1.921 supplementation on performance, egg quality, and fecal and cecal microbiota of laying hens. Poult. Sci. 2017, 96, 1280–1289. [Google Scholar] [CrossRef] [PubMed]
- Neijat, M.; Shirley, R.B.; Barton, J.; Thiery, P.; Welsher, A.; Kiarie, E. Effect of dietary supplementation of Bacillus subtilis DSM29784 on hen performance, egg quality indices, and apparent retention of dietary components in laying hens from 19 to 48 weeks of age. Poult. Sci. 2019, 98, 5622–5635. [Google Scholar] [CrossRef]
- Wang, J.; Wang, W.W.; Qi, G.H.; Cui, C.F.; Wu, S.G.; Zhang, H.J.; Xu, L.; Wang, J. Effects of dietary Bacillus subtilis supplementation and calcium levels on performance and eggshell quality of laying hens in the late phase of production. Poult. Sci. 2021, 100, 100970. [Google Scholar] [CrossRef] [PubMed]
- Juzaitis-Boelter, C.P.; Benson, A.P.; Ahammad, M.U.; Jones, M.K.; Ferrel, J.; Davis, A.J. Dietary inclusion of AZOMITE improves feed efficiency in broilers and egg production in laying and broiler breeder hens. Poult. Sci. 2021, 100, 101144. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, C.L.N.; Barreto, S.L.T.; Reis, R.S.; Muniz, J.C.L.; Viana, G.S.; Ribeiro, V.; Mendonca, M.O.; Ferreira, R.C.; DeGroot, A.A. The effect of calcium and available phosphorus levels on performance, egg quality and bone characteristics of japanese quails at end of the egg-production phase. Braz. J. Poult. Sci. 2016, 18, 33–39. [Google Scholar] [CrossRef]
- Jiang, S.; Cheng, H.W.; Cui, L.Y.; Zhou, Z.L.; Hou, J.F. Changes of blood parameters associated with bone remodeling following experimentally induced fatty liver disorder in laying hens. Poult. Sci. 2013, 92, 1443–1453. [Google Scholar] [CrossRef]
- Guo, X.H.; Li, D.F.; Lu, W.Q.; Piao, X.S.; Chen, X.L. Screening of Bacillus strains as potential probiotics and subsequent confirmation of the in vivo effectiveness of Bacillus subtilis MA139 in pigs. Antonie Van Leeuwenhoek 2006, 90, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Naghii, M.R.; Torkaman, G.; Mofid, M. Effects of boron and calcium supplementation on mechanical properties of bone in rats. Biofactors 2006, 28, 195–201. [Google Scholar] [CrossRef]
- Cole, J.H.; van der Meulen, M.C. Whole bone mechanics and bone quality. Clin. Orthop. Relat. Res. 2011, 469, 2139–2149. [Google Scholar] [CrossRef]
- Elaroussi, M.A.; Forte, L.R.; Eber, S.L.; Biellier, H.V. Calcium homeostasis in the laying hen. 1. Age and dietary calcium effects. Poult. Sci. 1994, 73, 1581–1589. [Google Scholar] [CrossRef] [PubMed]
- Abdelqader, A.; Al-Fataftah, A.-R.; Daş, G. Effects of dietary Bacillus subtilis and inulin supplementation on performance, eggshell quality, intestinal morphology and microflora composition of laying hens in the late phase of production. Anim. Feed Sci. Technol. 2013, 179, 103–111. [Google Scholar] [CrossRef]
- Fischer, A.; Malara, P.; Wiechula, D. The study of barium concentration in deciduous teeth, impacted teeth, and facial bones of polish residents. Biol. Trace Elem. Res. 2014, 161, 32–37. [Google Scholar] [CrossRef]
- Abe, E.; Hiroshi, H.; Masumura, T.; Sugahara, M.; Kubota, M.; Suda, T. Disorders of cholecaiciferol metabolism in old egg-laying hens. J. Nutr. 1982, 112, 436–446. [Google Scholar] [CrossRef]
- Al-Batshan, H.A.; Scheideler, S.E.; Black, B.L.; Garlich, J.D.; Anderson, K.E. Duodenal calcium uptake, femur ash, and eggshell quality decline with age and increase following molt. Poult. Sci. 1994, 73, 1590–1596. [Google Scholar] [CrossRef] [PubMed]
- Beck, M.M.; Hansen, K.K. Role of estrogen in avian osteoporosis. Poult. Sci. 2004, 83, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.Y.; Wu, K.F.; Li, X.; Luo, M.; Liu, H.C.; Zhang, S.C.; Hu, Y. Age-related changes in small intestinal mucosa epithelium architecture and epithelial tight junction in rat models. Aging Clin. Exp. Res. 2014, 26, 183–191. [Google Scholar] [CrossRef]
- Mueller, W.J.; Schraer, R.; Schraer, H. Calcium metabolism and skeletal dynamics of laying pullets. J. Nutr. 1964, 84, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Irie, S.; Hayashida, N.; Shinkawa, T.; Taira, Y.; Sekitani, Y.; Teraoka, S.; Hashiguchi, K.; Yoshida, K.; Morishita, M.; Takamura, N. Suitability of tartrate-resistant acid phosphatase type 5b as a screening marker for bone mineral density in community-dwelling elderly individuals. Tohoku J. Exp. Med. 2011, 224, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Wu, X.L.; Jin, M.L.; Wang, X.Z.; Tang, Q.; Sun, Y.X.; Cheng, H.W. Pathophysiological characteristics and gene transcriptional profiling of bone microstructure in a low calcium diet fed laying hens. Poult. Sci. 2019, 98, 4359–4368. [Google Scholar] [CrossRef]
- Morgan, C.L.; Mitchell, J.H. The calcium and phosphorus balance of laying hens. Poult. Sci. 1938, 17, 99–104. [Google Scholar] [CrossRef]
- Hurwitz, S.; Griminger, P. Observations on the calcium balance of laying hens. J. Agric. Sci. 1960, 54, 373–377. [Google Scholar] [CrossRef]
- An, S.H.; Kim, D.W.; An, B.K. Effects of dietary calcium levels on productive performance, eggshell quality and overall calcium status in aged laying hens. Asian Aust. J. Anim. Sci. 2016, 29, 1477–1482. [Google Scholar] [CrossRef]
- Reynard, M.; Savory, C.J. Stress-induced oviposition delays in laying hens: Duration and consequences for eggshell quality. Br. Poult. Sci. 1999, 40, 585–591. [Google Scholar] [CrossRef]
- Zhu, Y.Z.; Cheng, J.L.; Ren, M.; Yin, L.; Piao, X.S. Effect of gamma-aminobutyric acid-producing Lactobacillus strain on laying performance, egg quality and serum enzyme activity in hy-line brown hens under heat stress. Asian Aust. J. Anim. Sci. 2015, 28, 1006–1013. [Google Scholar] [CrossRef][Green Version]
- Alfonso-Carrillo, C.; Benavides-Reyes, C.; de Los Mozos, J.; Dominguez-Gasca, N.; Sanchez-Rodríguez, E.; Garcia-Ruiz, A.I.; Rodriguez-Navarro, A.B. Relationship between bone quality, egg production and eggshell quality in laying hens at the end of an extended production cycle (105 weeks). Animals 2021, 11, 623. [Google Scholar] [CrossRef] [PubMed]
- Min, Y.N.; Liu, F.X.; Qi, X.; Ji, S.; Ma, S.X.; Liu, X.; Wang, Z.P.; Gao, Y.P. Effects of methionine hydroxyl analog chelated zinc on laying performance, eggshell quality, eggshell mineral deposition, and activities of Zn-containing enzymes in aged laying hens. Poult. Sci. 2018, 97, 3587–3593. [Google Scholar] [CrossRef]
- Chen, J.F.; Kuang, Y.H.; Qu, X.Y.; Guo, S.C.; Kang, K.L.; He, C.Q. The effects and combinational effects of Bacillus subtilis and montmorillonite supplementation on performance, egg quality, oxidation status, and immune response in laying hens. Livest. Sci. 2019, 227, 114–119. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; Mahgoub, S.A.; Alagawany, M.; Ashour, E.A. Improving productive performance and mitigating harmful emissions from laying hen excreta via feeding on graded levels of corn DDGS with or without Bacillus subtilis probiotic. J. Anim. Physiol. Anim. Nutr. 2017, 101, 904–913. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Peng, C.Y.; Qu, X.Y.; Guo, S.C.; Chen, J.F.; He, C.Q.; Zhou, X.B.; Zhu, S.W. Effects of Bacillus subtilis C-3102 on production, hatching performance, egg quality, serum antioxidant capacity and immune response of laying breeders. J. Anim. Physiol. Anim. Nutr. 2019, 103, 182–190. [Google Scholar] [CrossRef]
- Fathi, M.; Al-Homidan, I.; Al-Dokhail, A.; Ebeid, T.; Abou-Emera, O.; Alsagan, A. Effects of dietary probiotic (Bacillus subtilis) supplementation on productive performance, immune response and egg quality characteristics in laying hens under high ambient temperature. Ital. J. Anim. Sci. 2018, 17, 804–814. [Google Scholar] [CrossRef]
- Ferretti, J.L.; Vázquez, S.O.; Delgado, C.J.; Capozza, R.; Cointry, G. Biphasic dose-response curves of cortisol effects on rat diaphyseal bone biomechanics. Calcif. Tissue Int. 1992, 50, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Van Ruijven, L.J.; Mulder, L.; van Eijden, T.M. Variations in mineralization affect the stress and strain distributions in cortical and trabecular bone. J. Biomech. 2007, 40, 1211–1218. [Google Scholar] [CrossRef]
- Heaney, R.P. Phosphorus nutrition and the treatment of osteoporosis. Mayo Clin. Proc. 2004, 79, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Addison, W.N.; Azari, F.; Sørensen, E.S.; Kaartinen, M.T.; McKee, M.D. Pyrophosphate inhibits mineralization of osteoblast cultures by binding to mineral, up-regulating osteopontin, and inhibiting alkaline phosphatase activity. J. Biol. Chem. 2007, 282, 15872–15883. [Google Scholar] [CrossRef] [PubMed]
- Miles, R.D.; Junqueira, O.M.; Harms, R.H. Plasma phosphorus at 0, 6, and 21 hours postoviposition in hens laying in the morning or afternoon. Poult. Sci. 1984, 63, 354–359. [Google Scholar] [CrossRef]
- Shao, Y.; Sun, G.; Cao, S.; Lu, L.; Zhang, L.; Liao, X.; Luo, X. Bone phosphorus retention and bone development of broilers at different ages. Poult. Sci. 2019, 98, 2114–2121. [Google Scholar] [CrossRef]
- Taylor, W. The excretion of steroid hormone metabolites in bile and feces. Vitam. Horm. 1971, 29, 201–285. [Google Scholar]
- Adlercreutz, H.; Martin, F. Biliary excretion and intestinal metabolism of progesterone and estrogens in man. J. Steroid Biochem. 1980, 13, 231–244. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, S.; Pang, Q.; Miao, Z. Bacillus amyloliquefaciens BLCC1-0238 can effectively improve laying performance and egg quality via enhancing immunity and regulating reproductive hormones of laying hens. Probiotics Antimicrob. Proteins 2020, 12, 246–252. [Google Scholar] [CrossRef]
- Tobias, J.H.; Compston, J.E. Does estrogen stimulate osteoblast function in postmenopausal women? Bone 1999, 24, 121–124. [Google Scholar] [CrossRef]
- Amonkar, M.M.; Mody, R. Developing profiles of postmenopausal women being prescribed estrogen therapy to prevent osteoporosis. J. Community Health 2002, 27, 335–350. [Google Scholar] [CrossRef] [PubMed]
- Clowes, J.A.; Riggs, B.L.; Khosla, S. The role of the immune system in the pathophysiology of osteoporosis. Immunol. Rev. 2005, 208, 207–227. [Google Scholar] [CrossRef]
- Li, J.Y.; Chassaing, B.; Tyagi, A.M.; Vaccaro, C.; Luo, T.; Adams, J.; Darby, T.M.; Weitzmann, M.N.; Mulle, J.G.; Gewirtz, A.T.; et al. Sex steroid deficiency-associated bone loss is microbiota dependent and prevented by probiotics. J. Clin. Investig. 2016, 126, 2049–2063. [Google Scholar] [CrossRef]
- Jones, R.M.; Mulle, J.G.; Pacifici, R. Osteomicrobiology: The influence of gut microbiota on bone in health and disease. Bone 2018, 115, 59–67. [Google Scholar] [CrossRef]
- Britton, R.A.; Irwin, R.; Quach, D.; Schaefer, L.; Zhang, J.; Lee, T.; Parameswaran, N.; McCabe, L.R. Probiotic L. reuteri treatment prevents bone loss in a menopausal ovariectomized mouse model. J. Cell. Physiol. 2014, 229, 1822–1830. [Google Scholar] [CrossRef]
- Ohlsson, C.; Engdahl, C.; Fak, F.; Andersson, A.; Windahl, S.H.; Farman, H.H.; Moverare-Skrtic, S.; Islander, U.; Sjogren, K. Probiotics protect mice from ovariectomy-induced cortical bone loss. PLoS ONE 2014, 9, 8. [Google Scholar] [CrossRef]
- Wigg, A.J.; Roberts-Thomson, I.C.; Dymock, R.B.; McCarthy, P.J.; Grose, R.H.; Cummins, A.G. The role of small intestinal bacterial overgrowth, intestinal permeability, endotoxaemia, and tumour necrosis factor alpha in the pathogenesis of non-alcoholic steatohepatitis. Gut 2001, 48, 206–211. [Google Scholar] [CrossRef]
- Lee, H.S.; Han, S.Y.; Bae, E.A.; Huh, C.S.; Ahn, Y.T.; Lee, J.H.; Kim, D.H. Lactic acid bacteria inhibit proinflammatory cytokine expression and bacterial glycosaminoglycan degradation activity in dextran sulfate sodium-induced colitic mice. Int. Immunopharmacol. 2008, 8, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Jurado, S.; Garcia-Giralt, N.; Díez-Pérez, A.; Esbrit, P.; Yoskovitz, G.; Agueda, L.; Urreizti, R.; Pérez-Edo, L.; Saló, G.; Mellibovsky, L. Effect of IL-1beta, PGE(2), and TGF-beta1 on the expression of OPG and RANKL in normal and osteoporotic primary human osteoblasts. J. Cell. Biochem. 2010, 110, 304–310. [Google Scholar]
- Liu, X.H.; Kirschenbaum, A.; Yao, S.; Levine, A.C. Cross-talk between the interleukin-6 and prostaglandin E-2 signaling systems results in enhancement of osteoclastogenesis through effects on the osteoprotegerin/receptor activator of nuclear factor-kappa B (RANK) ligand/RANK system. Endocrinology 2005, 146, 1991–1998. [Google Scholar] [CrossRef] [PubMed]
- Liao, E.Y.; Luo, X.H.; Su, X. Comparison of the effects of 17 beta-E2 and progesterone on the expression of osteoprotegerin in normal human osteoblast-like cells. J. Endocrinol. Invest. 2002, 25, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Trouvin, A.P.; Goëb, V. Receptor activator of nuclear factor-κB ligand and osteoprotegerin: Maintaining the balance to prevent bone loss. Clin. Interv. Aging 2010, 5, 345–354. [Google Scholar]
- Lindberg, M.K.; Erlandsson, M.; Alatalo, S.L.; Windahl, S.; Andersson, G.; Halleen, J.M.; Carlsten, H.; Gustafsson, J.A.; Ohlsson, C. Estrogen receptor alpha, but not estrogen receptor beta, is involved in the regulation of the OPG/RANKL (osteoprotegerin/receptor activator of NF-kappa B ligand) ratio and serum interleukin-6 in male mice. J. Endocrinol. 2001, 171, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Kuehn, M.C.; Willenberg, H.S.; Schott, M.; Papewalis, C.; Stumpf, U.; Flohe, S.; Scherbaum, W.A.; Schinner, S. Adipocyte-secreted factors increase osteoblast proliferation and the OPG/RANKL ratio to influence osteoclast formation. Mol. Cell. Endocrinol. 2012, 349, 180–188. [Google Scholar] [CrossRef]
- Abrahamsen, B.; Shalhoub, V.; Larson, E.K.; Eriksen, E.F.; Beck-Nielsen, H.; Marks, S.C. Cytokine RNA levels in transiliac bone biopsies from healthy early postmenopausal women. Bone 2000, 26, 137–145. [Google Scholar] [CrossRef]
- Suda, K.; Udagawa, N.; Sato, N.; Takami, M.; Itoh, K.; Woo, J.T.; Takahashi, N.; Nagai, K. Suppression of osteoprotegerin expression by prostaglandin E-2 is crucially involved in lipopolysaccharide-induced osteoclast formation. J. Immunol. 2004, 172, 2504–2510. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, N.; Maeno, M.; Suzuki, N.; Fujisaki, K.; Tanaka, H.; Ogiso, B.; Ito, K. IL-1 alpha stimulates the formation of osteoclast-like cells by increasing M-CSF and PGE2 production and decreasing OPG production by osteoblasts. Life Sci. 2005, 77, 615–626. [Google Scholar] [CrossRef] [PubMed]
| Item | Factor | Control Diet | B. subtilis Diet |
|---|---|---|---|
| Ingredient | Corn (%) | 65 | 65 |
| Soybean (%) | 22 | 22 | |
| Shell powder (%) | 8.9 | 8.9 | |
| Zeolite powder (%) | 1.1 | 1.1 | |
| 3% premix 1 (%) | 3 | 3 | |
| Bacillus subtilis (g/kg) | 0 | 0.5 | |
| Nutrient composition | Crude protein (%) | 15.05 | 15.05 |
| Calcium (%) | 3.7 | 3.7 | |
| Energy (MJ/kg) | 11.57 | 11.57 |
| Gene | GenBank ID | PCR Primers Sequence (5′ to 3′) | PCR Products (bp) |
|---|---|---|---|
| OPG | NM_001033641.1 | F: GTTCCTACTCGTTCCACACC R: GCTCTTGTGAACTGTGCCTTTG | 115 |
| RANKL | XM_015275777.2 | F: CTGGAACTCGCAAAGTGAACCT R: TTTCCCATCACTGAACGTCATATTT | 86 |
| SOST | XM_025144077.1 | F: TTGTCTGTATTCGTCTCGCTAT R: AACGTCCTTTCTGAGTCACCT | 180 |
| COL1A2 | NM_001079714.2 | F: GGCTTTGATGCAGAATACTACCG R: GTTGTTCAATGTTTTCAGAGTGGC | 90 |
| RANK | XM_004939689.3 | F: GCCATGTCCCAGAGGATACT | 87 |
| R: GCCAATCCCAGAGCTGAACA | |||
| GAPDH | NM_204305.1 | F: TTGACGTGCAGCAGGAACAC R: ATGGCCACCACTTGGACTTT | 124 |
| Time | Lg (Control) | Lg (B. subtilis) | SEM | p-Value |
|---|---|---|---|---|
| D 30 | 2.67 | 6.41 ** | 0.98 | 0.007 |
| D 60 | 3.31 | 6.08 ** | 0.75 | 0.007 |
| Parameters | Control | B. subtilis | SEM | p-Value |
|---|---|---|---|---|
| 48 week body weight (kg) | 1.69 | 1.70 | 0.08 | 0.32 |
| 57 week body weight (kg) | 1.72 | 1.73 | 0.06 | 0.76 |
| Feed intake (g) | 120.33 | 119.28 | 10.95 | 0.93 |
| Egg production, % | 80.64 | 78.26 | 1.71 | 0.18 |
| Marketable eggs, % | 76.19 | 88.74 ** | 2.29 | <0.01 |
| Egg weight (g) | 62.08 | 62.8 | 1.80 | 0.69 |
| Egg shape index | 1.35 | 1.34 | 0.02 | 0.64 |
| Height of albumen (mm) | 6.55 | 6.48 | 0.34 | 0.84 |
| Yolk color | 5.08 | 4.58 | 0.35 | 0.16 |
| Haugh unit | 79.75 | 79.05 | 2.46 | 0.78 |
| Eggshell thickness (mm) | 0.47 | 0.48 | 0.00 | 0.45 |
| Eggshell strength (kg) | 3.54 | 3.47 | 0.41 | 0.87 |
| Bone Strength Parameters | Control | B. subtilis | SEM | p-Value |
|---|---|---|---|---|
| - | Femur | - | ||
| Absolute mass (g) | 8.67 | 8.47 | 0.32 | 0.54 |
| Relative mass% | 50.63 | 49.31 | 1.85 | 0.48 |
| Maximum load (N) | 118.85 | 143.5 + | 12.1 | 0.06 |
| Maximum stress (MPa) | 115.63 | 211.90 ** | 32.4 | 0.01 |
| Maximum strain | 0.014 | 0.012 + | <0.0001 | 0.06 |
| Stiffness (N/m) | 148,607 | 205,039 ** | 13,906 | <0.01 |
| Young’s modulus (MPa) | 10,226.7 | 28,910.1 ** | 3586.1 | <0.01 |
| Density (g/cm2) | 0.49 | 0.43 | 0.11 | 0.57 |
| - | Tibia | - | ||
| Absolute mass (g) | 9.93 | 9.52 | 0.35 | 0.25 |
| Relative mass% | 58.10 | 55.05 | 1.91 | 0.13 |
| Maximum load (N) | 97.86 | 101.34 | 8.98 | 0.70 |
| Maximum stress (MPa) | 191.31 | 202.39 | 35.93 | 0.76 |
| Maximum strain | 0.040 | 0.037 | 0.001 | 0.31 |
| Stiffness (N/m) | 44,657.21 | 46,069.66 | 2705.6 | 0.61 |
| Young’s modulus (MPa) | 6372.69 | 6679.99 | 1299.8 | 0.82 |
| Density (g/cm2) | 0.28 | 0.38 + | 0.05 | 0.07 |
| Sample | Parameters | Control | B. subtilis | SEM | p-Value |
|---|---|---|---|---|---|
| Eggshell | Ca (mg/g) | 281.77 | 270.56 | 6.91 | 0.12 |
| - | P (mg/g) | 1.04 | 1.02 | 0.19 | 0.94 |
| - | Mg (mg/g) | 3.15 | 3.04 | 0.12 | 0.49 |
| Excretion | Ca (mg/g) | 53.76 | 75.54 | 12.46 | 0.10 |
| - | P (mg/g) | 13.26 | 12.81 | 1.64 | 0.79 |
| - | Mg (mg/g) | 3.51 | 4.26 + | 0.42 | 0.09 |
| Bone | Ca (mg/g) | 240.98 | 243.12 | 8.43 | 0.80 |
| - | P (mg/g) | 94.60 | 103.78 ** | 3.17 | <0.01 |
| - | Mg (mg/g) | 3.39 | 3.09 * | 0.14 | 0.04 |
| - | bone ash (%) | 58.41 | 59.87 | 3.20 | 0.65 |
| Femur | Correlation Analysis | Serum Ca | Serum P | Femoral Ca | Femoral P |
|---|---|---|---|---|---|
| Maximum load | r | 0.26 | −0.32 | 0.21 | 0.42 |
| P | 0.28 | 0.18 | 0.40 | 0.07 | |
| Maximum stress | r | 0.34 | −0.44 | 0.25 | 0.55 * |
| P | 0.16 | 0.06 | 0.30 | 0.02 | |
| Maximum strain | r | 0.13 | 0.28 | −0.08 | −0.26 |
| P | 0.59 | 0.24 | 0.75 | 0.28 | |
| Stiffness | r | −0.07 | −0.71 ** | 0.11 | 0.45 * |
| P | 0.77 | <0.01 | 0.67 | 0.05 | |
| Young’s modulus | r | 0.16 | −0.58 ** | 0.12 | 0.56 * |
| P | 0.51 | <0.01 | 0.63 | 0.01 | |
| Serum Ca | r | - | 0.35 | −0.13 | −0.11 |
| P | - | 0.15 | 0.59 | 0.67 | |
| Serum P | r | - | - | −0.31 | −0.59 ** |
| P | - | - | 0.20 | <0.01 | |
| Femoral Ca | r | - | - | - | 0.80 ** |
| P | - | - | - | <0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, X.; Jiang, S.; Zhang, M.; Hu, H.; Wu, X.; Liu, J.; Jin, M.; Cheng, H. Effects of Bacillus subtilis on Production Performance, Bone Physiological Property, and Hematology Indexes in Laying Hens. Animals 2021, 11, 2041. https://doi.org/10.3390/ani11072041
Zou X, Jiang S, Zhang M, Hu H, Wu X, Liu J, Jin M, Cheng H. Effects of Bacillus subtilis on Production Performance, Bone Physiological Property, and Hematology Indexes in Laying Hens. Animals. 2021; 11(7):2041. https://doi.org/10.3390/ani11072041
Chicago/Turabian StyleZou, Xinyu, Sha Jiang, Mi Zhang, Haiqiang Hu, Xiaoling Wu, Jianzhu Liu, Meilan Jin, and Hengwei Cheng. 2021. "Effects of Bacillus subtilis on Production Performance, Bone Physiological Property, and Hematology Indexes in Laying Hens" Animals 11, no. 7: 2041. https://doi.org/10.3390/ani11072041
APA StyleZou, X., Jiang, S., Zhang, M., Hu, H., Wu, X., Liu, J., Jin, M., & Cheng, H. (2021). Effects of Bacillus subtilis on Production Performance, Bone Physiological Property, and Hematology Indexes in Laying Hens. Animals, 11(7), 2041. https://doi.org/10.3390/ani11072041





