Evaluation of Factors Affecting Colostrum Quality and Quantity in Holstein Dairy Cattle
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Management
2.2. Clinical Examination
2.3. Colostrum Sample Collection
2.4. Cowside Determination of Colostrum Quality
2.5. Laboratory Determination of Colostrum Composition
2.6. Calculation of Colostrum Net Energy Content
2.7. Data Set
2.8. Statistical Analysis
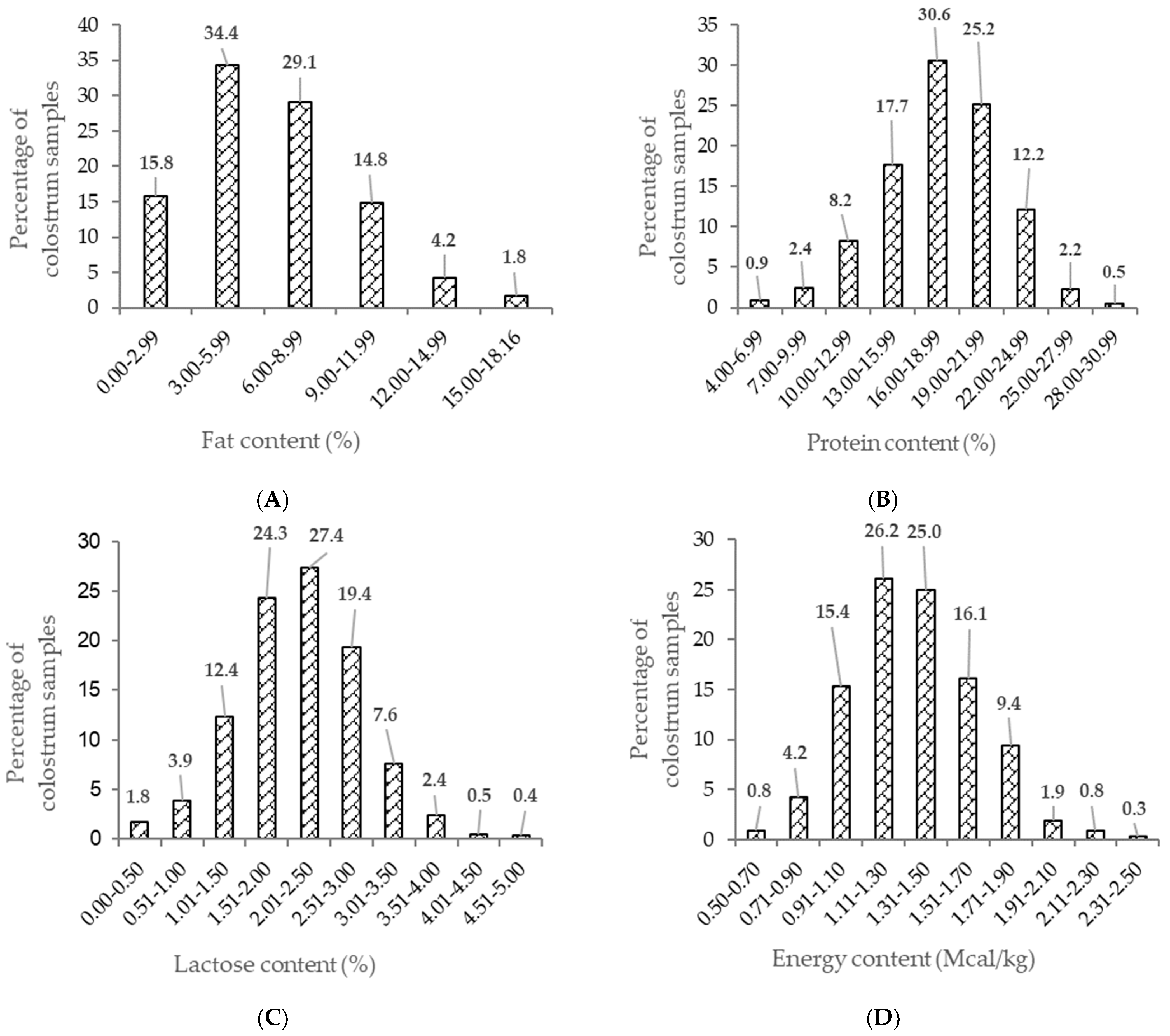
3. Results
Effects on Colostrum Quality and Yield
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weaver, D.M.; Tyler, J.W.; Van Metre, D.C.; Hostetler, D.E.; Barrington, G.M. Passive transfer of colostral immunoglobulins in calves. J. Vet. Intern. Med. 2000, 14, 569–577. [Google Scholar] [CrossRef]
- McGuirk, S.M.; Collins, M. Managing the production, storage, and delivery of colostrum. Vet. Clin. N. Am. Food Anim. Pract. 2004, 20, 593–603. [Google Scholar] [CrossRef]
- Reschke, C.; Schelling, E.; Michel, A.; Remy-Wohlfender, F.; Meylan, M. Factors Associated with Colostrum Quality and Effects on Serum Gamma Globulin Concentrations of Calves in Swiss Dairy Herds. J. Vet. Intern. Med. 2017, 31, 1563–1571. [Google Scholar] [CrossRef]
- Kehoe, S.I.; Jayarao, B.M.; Heinrichs, A.J. A survey of Bovine Colostrum Composition and Colostrum Management Practices on Pennsylvania Dairy Farms. J. Dairy Sci. 2007, 90, 4108–4116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGrath Brian, A.; Fox Patrick, F.; McSweeney Paul, L.H.; Kelly Alan, L. Composition and properties of bovine colostrum: A review. Dairy Sci. Technol. 2016, 96, 133–158. [Google Scholar] [CrossRef]
- Swan, H.; Godden, S.; Bey, R.; Wells, S.; Fetrow, J.; Chester-Jones, H. Passive Transfer of Immunoglobulin G and Preweaning Health in Holstein Calves Fed a Commercial Colostrum Replacer. J. Dairy Sci. 2007, 90, 3857–3866. [Google Scholar] [CrossRef] [PubMed]
- Dunn, A.; Ashfield, A.; Earley, B.; Welsh, M.; Gordon, A.; Morrison, S.J. Evaluation of factors associated with immunoglobulin G, fat, protein, and lactose concentrations in bovine colostrum and colostrum management practices in grassland-based dairy systems in Northern Ireland. J. Dairy Sci. 2017, 100, 068–2079. [Google Scholar] [CrossRef] [Green Version]
- Gulliksen, S.M.; Lie, K.I.; Sølverød, L.; Østera, O. Risk Factors Associated with Colostrum Quality in Norwegian Dairy Cows. J. Dairy Sci. 2008, 91, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Conneely, M.; Berry, D.P.; Sayers, R.; Murphy, J.P.; Lorenz, I.; Doherty, M.L.; Kennedy, E. Factors associated with the concen-tration of immunoglobulin G in the colostrum of dairy cows. Animal 2013, 7, 1824–1832. [Google Scholar] [CrossRef] [Green Version]
- Shivley, C.B.; Lombard, J.E.; Urie, N.J.; Haines, D.M.; Sargent, R.; Kopral, C.A.; Earleywine, T.J.; Olson, J.D.; Garry, F.B. Preweaned heifer management on US dairy operations: Part II. Factors associated with colostrum quality and passive transfer status of dairy heifer calves. J. Dairy Sci. 2018, 101, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Rodewald, R. ph-dependent binding of immunoglobulins to intestinal cells of the neonatal rat. J. Cell Biol. 1976, 71, 666–670. [Google Scholar] [CrossRef] [Green Version]
- Besser, T.E.; Gay, C.C. Septicemic colibacillosis and failure of passive transfer of colostral immunoglobulin in calves. Vet. Clin. N. Am. Food Anim. Pract. 1985, 1, 445–459. [Google Scholar] [CrossRef]
- Muller, L.; Ellinger, D. Colostral Immunoglobulin Concentrations Among Breeds of Dairy Cattle. J. Dairy Sci. 1981, 64, 1727–1730. [Google Scholar] [CrossRef]
- Guy, M.; McFadden, T.; Cockrell, D.; Besser, T. Regulation of Colostrum Formation in Beef and Dairy Cows. J. Dairy Sci. 1994, 77, 3002–3007. [Google Scholar] [CrossRef]
- Pritchett, L.C.; Gay, C.C.; Besser, T.E.; Hancock, D.D. Management and Production Factors Influencing Immunoglobulin G1 Concentration in Colostrum from Holstein Cows. J. Dairy Sci. 1991, 74, 2336–2341. [Google Scholar] [CrossRef]
- Hodgins Douglas, C.; Shewen Patricia, E. Preparturient vaccination to enhance passive immunity to the capsular polysac-charide of Pasteurella haemolytica AVet. Immun. Immunopathol. 1996, 50, 67–77. [Google Scholar] [CrossRef]
- Morin, D.E.; Nelson, S.V.; Reid, E.D.; Nagy, D.W.; Dahl, G.E.; Constable, P.D. Effect of colostral volume, interval between calving and first milking, and photoperiod on colostral IgG concentrations in dairy cows. J. Am. Vet. Med. Assoc. 2010, 237, 420–428. [Google Scholar] [CrossRef]
- Fleenor, W.A.; Stott, G.H. Single radial immunodiffusion analysis for quantitation of colostral immunoglobulin concen-tration. J. Dairy Sci. 1981, 64, 740–747. [Google Scholar] [CrossRef]
- Chelack, B.J.; Morley, P.; Haines, D.M. Evaluation of methods for dehydration of bovine colostrum for total replacement of normal colostrum in calves. Can. Vet. J. Rev. Vet. Can. 1993, 34, 407–412. [Google Scholar]
- Bielmann, V.; Gillan, J.N.R.; Perkins, A.L.; Skidmore, L.; Godden, S.; Leslie, K.E. An evaluation of Brix refractometry instruments for measurement of colostrum quality in dairy cattle. J. Dairy Sci. 2010, 93, 3713–3721. [Google Scholar] [CrossRef]
- Bartier, A.L.; Windeyer, M.C.; Doepel, L. Evaluation of on-farm tools for colostrum quality measurement. J. Dairy Sci. 2015, 98, 1878–1884. [Google Scholar] [CrossRef]
- Hammon, H.M.; Steinhoff-Wagner, J.; Flor, J.; Schönhusen, U.; Metges, C.C. Lactation Biology Symposium: Role of colostrum and colostrum components on glucose metabolism in neonatal calves1. J. Anim. Sci. 2013, 91, 685–695. [Google Scholar] [CrossRef] [Green Version]
- Contarini, G.; Povolo, M.; Pelizzola, V.; Monti, L.; Bruni, A.; Passolungo, L.; Abeni, F.; Degano, L. Bovine colostrum: Changes in lipid constitutes in the first 5 days after parturition. J. Dairy Sci. 2014, 97, 5065–5072. [Google Scholar] [CrossRef] [Green Version]
- Elfstrand, L.; Lindmark-Mansson, H.; Paulssona, M.; Nyberg, L.; Akesson, B. Immunoglobulins, growth factors and growth hormone in bovine colostrum and the effects of processing. Int. Dairy J. 2002, 12, 879–887. [Google Scholar] [CrossRef]
- Zarcula, S.; Cernescu, H.; Mircu, C.; Tulcan, C.; Morvay, A.; Baul, S.; Popovici, D. Influence of breed, parity and food intake on chemical composition of first colostrum in cow. Anim. Sci. Biotech. 2010, 43, 154–157. [Google Scholar]
- Morrill, K.M.; Conrad, E.M.; Lago, A.; Campbell, J.; Quigley, J.; Tyler, H. Nationwide evaluation of quality and composition of colostrum on dairy farms in the United States. J. Dairy Sci. 2012, 95, 3997–4005. [Google Scholar] [CrossRef] [Green Version]
- Ferguson, J.D.; Galligan, D.T.; Thomsen, N. Principal descriptors of body condition score in Holstein cows. J. Dairy Sci. 1994, 77, 2695–2703. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Dairy Cattle, 7th ed.; National Academies Press: Washington, DC, USA, 2001. [Google Scholar]
- Okamoto, M.; Robinson, J.B.; Christopherson, R.J.; Young, B.A. Summit metabolism of newborn calves with and without co-lostrum feeding. Can. J. Anim. Sci. 1986, 66, 937–944. [Google Scholar] [CrossRef]
- Van Amburgh, M.E. Nutrition of the preweaned calf. Large Dairy herd Management. J. Physiol. 2017, 244, 223–234. [Google Scholar]
- Zierler, K. Whole body glucose metabolism. Am. J. Physiol. 1999, 276, 409–426. [Google Scholar] [CrossRef] [PubMed]
- Kehoe, S.I.; Heinrichs, A.J.; Moody, M.L.; Jones, C.M.; Long, M.R. Comparison of immunoglobulin G concentrations in pri-miparous and multiparous bovine colostrum. PAS 2011, 27, 176–180. [Google Scholar]
- Quigley, J.; Lago, A.; Chapman, C.; Erickson, P.; Polo, J. Evaluation of the Brix refractometer to estimate immunoglobulin G concentration in bovine colostrum. J. Dairy Sci. 2013, 96, 1148–1155. [Google Scholar] [CrossRef] [PubMed]
- Mann, S.; Yepes, F.L.; Overton, T.; Lock, A.; Lamb, S.V.; Wakshlag, J.; Nydam, D. Effect of dry period dietary energy level in dairy cattle on volume, concentrations of immunoglobulin G, insulin, and fatty acid composition of colostrum. J. Dairy Sci. 2016, 99, 1515–1526. [Google Scholar] [CrossRef] [Green Version]
- Quigley, J.D. Calf Note 141–Further Thoughts on Colostrum Variation. 2009. Available online: https://www.calfnotes.com/ (accessed on 21 June 2009).
- Denholm, K.S.; McDougall, S.; Chambers, G.; Clough, W. Factors Associated with Colostrum Quality in Individual Cows from Dairy Herds in the Waikato region of New Zealand. N. Z. Vet. J. 2018, 66, 115–120. [Google Scholar] [CrossRef]
- Soufleri, A.; Banos, G.; Panousis, N.; Fletouris, D.; Arsenos, G.; Valergakis, G.E. Genetic parameters of colostrum traits in Holstein dairy cows. J. Dairy Sci. 2019, 102, 11225–11232. [Google Scholar] [CrossRef]
- Shearer, J.; Mohammed, H.; Brenneman, J.; Tran, T. Factors associated with concentrations of immunoglobulins in colostrum at the first milking post-calving. Prev. Vet. Med. 1992, 14, 143–154. [Google Scholar] [CrossRef]
- Nardone, A.; Lacetera, N.; Bernabucci, U.; Ronchi, B. Composition of colostrum from dairy heifers exposed to high air tem-peratures during late pregnancy and the early postpartum period. J. Dairy Sci. 1997, 80, 838–844. [Google Scholar] [CrossRef]
- Gross, J.J.; Kessler, E.C.; Bruckmaier, R.M. Quarter vs composite colostrum composition assesed by Brix Refractometry, Specific Gravity and visual Color Appearance in primiparous and multiparous dairy cows. Transl. Anim. Sci. 2017, 1, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Kume, S.-I.; Tanabe, S. Effect of Parity on Colostral Mineral Concentrations of Holstein Cows and Value of Colostrum as a Mineral Source for Newborn Calves. J. Dairy Sci. 1993, 76, 1654–1660. [Google Scholar] [CrossRef]
- Morin, D.; Constable, P.; Maunsell, F.; McCoy, G. Factors Associated with Colostral Specific Gravity in Dairy Cows. J. Dairy Sci. 2001, 84, 937–943. [Google Scholar] [CrossRef]
- Tyler, J.W.; Steevens, B.J.; Hostetler, D.E.; Holle, J.M.; Denbigh, J.L., Jr. Colostral IgG concentrations in Holstein and Guernsey cows. Am. J. Vet. Res. 1999, 60, 1136–1139. [Google Scholar]
- Moore, M.; Tyler, J.W.; Chigerwe, M.; Dawes, M.E.; Middleton, J.R. Effect of delayed colostrum collection on colostral IgG concentration in dairy cows. J. Am. Vet. Med. Assoc. 2005, 226, 1375–1377. [Google Scholar] [CrossRef] [PubMed]
- Donovan, G.A.; Badinga, L.; Collier, R.J.; Wilcox, C.J.; Braun, R.K. Factors influencing passive transfer in dairy calves. J. Dairy Sci. 1986, 69, 754–759. [Google Scholar] [CrossRef]
- Annen, E.L.; Collier, R.J.; McGuire, M.A.; Vicini, J.L.; Ballam, J.M.; Lormore, M.J. Effect of modified dry period lengths and bovine somatotropin on yield and composition of milk from dairy cows. J. Dairy Sci. 2004, 87, 3746–3761. [Google Scholar] [CrossRef] [Green Version]
- Mayasari, N.; de Vries Reilingh, G.; Nieuwland, M.G.B.; Remmelink, G.J.; Parmentier, H.K.; Kemp, B.; van Knegsel, A.T.M. Effect of maternal dry period length on colostrum immunoglobulin content and on natural and specific antibody titers in calves. J. Dairy Sci. 2015, 98, 3969–3979. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Factors | Observations | Mean | SD | Min | Max |
|---|---|---|---|---|---|
| Dry period length (days) | 687 | 67 | 27 | 1 | 213 |
| Milk yield of previous 305-d lactation (kg) | 640 | 9211 | 1913 | 3136 | 16,273 |
| Age at calving (months) | 1074 | 46.5 | 22.5 | 19.1 | 151.3 |
| Body Condition Score at calving | 1074 | 2.93 | 0.50 | 1.75 | 4.75 |
| Interval calving colostrum collection | 1074 | 232 | 195 | 10 | 960 |
| Trait | Observations | Mean | SD | CV (%) | Median | Lower Quartile | Upper Quartile |
|---|---|---|---|---|---|---|---|
| Fat (%) | 1074 | 6.37 | 3.33 | 52 | 5.96 | 2.54 | 10.92 |
| Protein (%) | 1074 | 17.83 | 3.97 | 22 | 17.88 | 12.67 | 22.70 |
| Lactose (%) | 1074 | 2.15 | 0.73 | 34 | 2.16 | 1.23 | 3.06 |
| Total solids (%Brix value) | 1074 | 25.80 | 4.68 | 18 | 25.90 | 19.76 | 31.55 |
| Energy (Mcal/kg) | 1074 | 1.35 | 0.29 | 21 | 1.33 | 0.99 | 1.74 |
| Colostrum yield (kg) | 1074 | 6.20 | 3.80 | 61 | 5.00 | 3.50 | 8.00 |
| Farm | Fat | Protein | Lactose | Total Solids | Energy | Yield |
|---|---|---|---|---|---|---|
| (%) | (%) | (%) | (%Brix Value) | (Mcal/kg) | (kg) | |
| Mean (SD) | ||||||
| 1 | 3.00 | 16.98 | 1.47 | 22.80 | 1.04 | 5.00 |
| (1.46) | (3.12) | (1.20) | (5.40) | (0.26) | (2.97) | |
| 2 | 5.33 | 16.72 | 2.39 | 24.55 | 1.23 | 7.70 |
| (3.33) | (3.83) | (0.67) | (4.29) | (0.27) | (3.89) | |
| 3 | 6.61 | 17.67 | 2.02 | 25.81 | 1.36 | 5.30 |
| (3.53) | (3.70) | (0.68) | (4.20) | (0.29) | (2.96) | |
| 4 | 7.50 | 18.04 | 2.09 | 26.50 | 1.44 | 6.40 |
| (3.35) | (3.94) | (0.71) | (4.84) | (0.29) | (4.17) | |
| 5 | 7.69 | 17.54 | 2.01 | 25.45 | 1.43 | 6.20 |
| (3.41) | (3.43) | (0.66) | (4.11) | (0.28) | (2.61) | |
| 6 | 5.33 | 16.12 | 2.24 | 23.27 | 1.20 | 12.60 |
| (3.33) | (4.82) | (0.90) | (5.23) | (0.26) | (4.14) | |
| 7 | 6.06 | 18.14 | 2.19 | 26.03 | 1.34 | 5.60 |
| (3.09) | (3.90) | (0.74) | (4.64) | (0.29) | (3.64) | |
| 8 | 5.52 | 18.46 | 2.06 | 26.85 | 1.31 | 5.70 |
| (2.61) | (4.42) | (0.75) | (5.06) | (0.27) | (4.03) | |
| 9 | 5.99 | 19.42 | 2.20 | 26.59 | 1.40 | 5.40 |
| (3.23) | (3.85) | (0.73) | (4.87) | (0.31) | (1.36) | |
| 10 | 5.86 | 19.84 | 2.24 | 23.36 | 1.27 | 5.30 |
| (2.60) | (5.72) | (0.98) | (6.41) | (0.31) | (3.90) | |
| Factor | Size of Effect | |||||
|---|---|---|---|---|---|---|
| Fat (%) | Protein (%) | Lactose (%) | Energy (Mcal/kg) | Total Solids (%Brix) | Yield (kg) | |
| Parity | ** | * | * | * | * | * |
| 1 | 7.77 b | 17.15 a | 2.24 b | 1.36 b | 25.38 b | 4.95 a |
| 2 | 5.50 a | 16.93 a | 2.36 b | 1.35 a | 24.40 a | 6.75 b |
| 3 | 5.32 a | 17.98 a | 2.20 b | 1.32 a | 25.81 b | 7.69 b |
| 4+ | 5.92 a | 18.96 b | 1.97 a | 1.36 b | 26.77 c | 6.99 b |
| Season | * | ** | ** | * | ** | *** |
| Winter | 6.11 a,b | 18.01 b | 2.30 b | 1.35 a,b | 25.62 a,b | 6.01 a |
| Spring | 6.58 b | 17.64 a | 2.24 b | 1.37 b | 25.50 a | 7.52 b |
| Summer | 6.06 a | 17.33 a | 2.19 b | 1.32 a | 25.01 a | 6.99 b |
| Autumn | 5.77 a | 18.03 b | 2.04 a | 1.34 a | 26.23 b | 5.77 a |
| Milk yield of previous 305-day lactation (kg) | NS | NS | NS | NS | NS | *** |
| ≤7000 | 5.96 | 17.64 | 2.05 | 1.32 | 24.97 | 5.61 a |
| 7000–9000 | 5.35 | 17.69 | 2.11 | 1.37 | 25.11 | 6.42 a,b |
| 9000–11,000 | 5.10 | 18.26 | 2.00 | 1.37 | 25.81 | 7.39 b,c |
| ≥11,000 | 5.61 | 18.01 | 2.14 | 1.39 | 25.61 | 8.53 c |
| Dry period length (days) | * | NS | * | * | NS | * |
| ≤45 | 5.07 a | 18.33 | 2.02 a,b | 1.34 a | 25.68 | 6.10 a |
| 46–64 | 5.12 a | 17.41 | 2.22 b | 1.35 a | 24.66 | 6.66 a,b |
| 65–84 | 5.45 a,b | 17.70 | 2.06 a,b | 1.39 a,b | 25.07 | 7.45 a,b |
| ≥85 | 6.37 b | 18.14 | 1.99 a | 1.38 b | 26.09 | 7.74 b |
| Colostrum Yield (kg) | NS | *** | *** | NS | ** | |
| ≤4.0 | 5.83 | 18.46 b | 2.03 a | 1.37 | 26.33 b | |
| 4.1–8.5 | 6.25 | 18.06 b | 2.19 b | 1.33 | 25.94 b | |
| >8.5 | 6.30 | 16.75 a | 2.35 c | 1.34 | 24.51 a | |
| Time interval between calving and colostrum collection (h) | * | ** | *** | * | *** | * |
| ≤2 | 6.55 b | 18.24 b | 2.07 a | 1.38 b | 26.18 b | 6.14 a,b |
| 2–6 | 6.09 a,b | 17.91 b | 2.17 a | 1.37 b | 25.83 b | 6.26 a |
| ≥6 | 5.74 a | 17.11 a | 2.34 b | 1.34 a | 24.76 a | 7.39 b |
| Age at calving | −0.19 * | 0.24 * | −0.05 * | −0.02 * | 0.20 * | 0.47 * |
| Body Condition Score | 0.93 NS | −0.25 NS | 0.04 NS | −0.01 NS | −0.17 NS | 0.43 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soufleri, A.; Banos, G.; Panousis, N.; Fletouris, D.; Arsenos, G.; Kougioumtzis, A.; Valergakis, G.E. Evaluation of Factors Affecting Colostrum Quality and Quantity in Holstein Dairy Cattle. Animals 2021, 11, 2005. https://doi.org/10.3390/ani11072005
Soufleri A, Banos G, Panousis N, Fletouris D, Arsenos G, Kougioumtzis A, Valergakis GE. Evaluation of Factors Affecting Colostrum Quality and Quantity in Holstein Dairy Cattle. Animals. 2021; 11(7):2005. https://doi.org/10.3390/ani11072005
Chicago/Turabian StyleSoufleri, Aikaterini, Georgios Banos, Nikolaos Panousis, Dimitrios Fletouris, Georgios Arsenos, Alexandros Kougioumtzis, and Georgios E. Valergakis. 2021. "Evaluation of Factors Affecting Colostrum Quality and Quantity in Holstein Dairy Cattle" Animals 11, no. 7: 2005. https://doi.org/10.3390/ani11072005
APA StyleSoufleri, A., Banos, G., Panousis, N., Fletouris, D., Arsenos, G., Kougioumtzis, A., & Valergakis, G. E. (2021). Evaluation of Factors Affecting Colostrum Quality and Quantity in Holstein Dairy Cattle. Animals, 11(7), 2005. https://doi.org/10.3390/ani11072005







