Evaluation of Fecal Glucocorticoid Metabolite Levels in Response to a Change in Social and Handling Conditions in African Lions (Panthera leo bleyenberghi)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
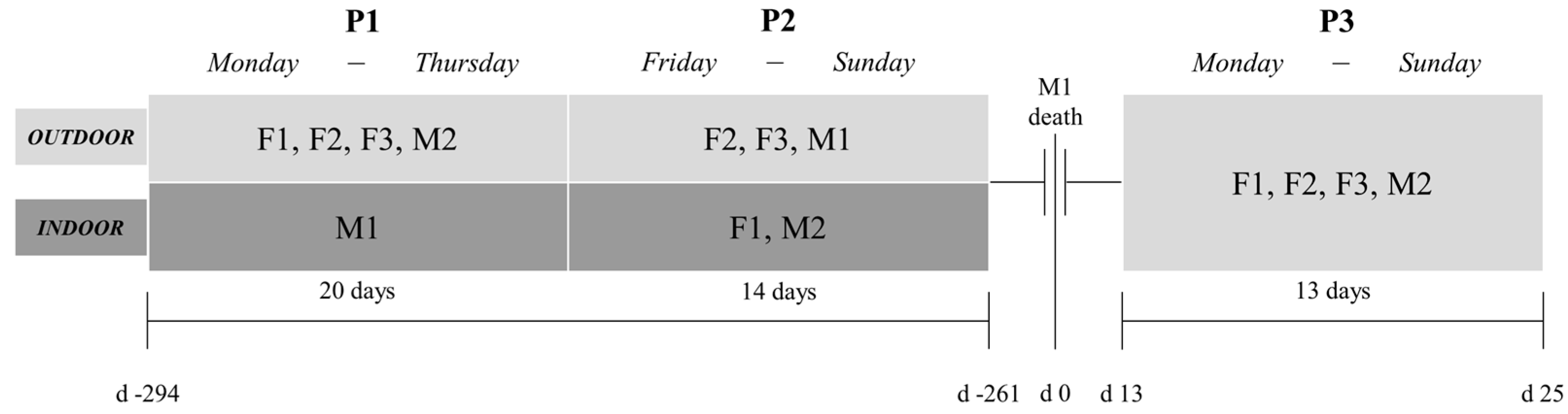
2.1. Experimental Design
2.2. Housing, Handling, and Environmental Conditions
2.3. Individual Identification and Sample Collection
2.4. Steroid Extraction
2.5. Steroid Analysis and Biochemical Validation
2.6. Data Analysis
3. Results
3.1. Biochemical Validation of the EIA
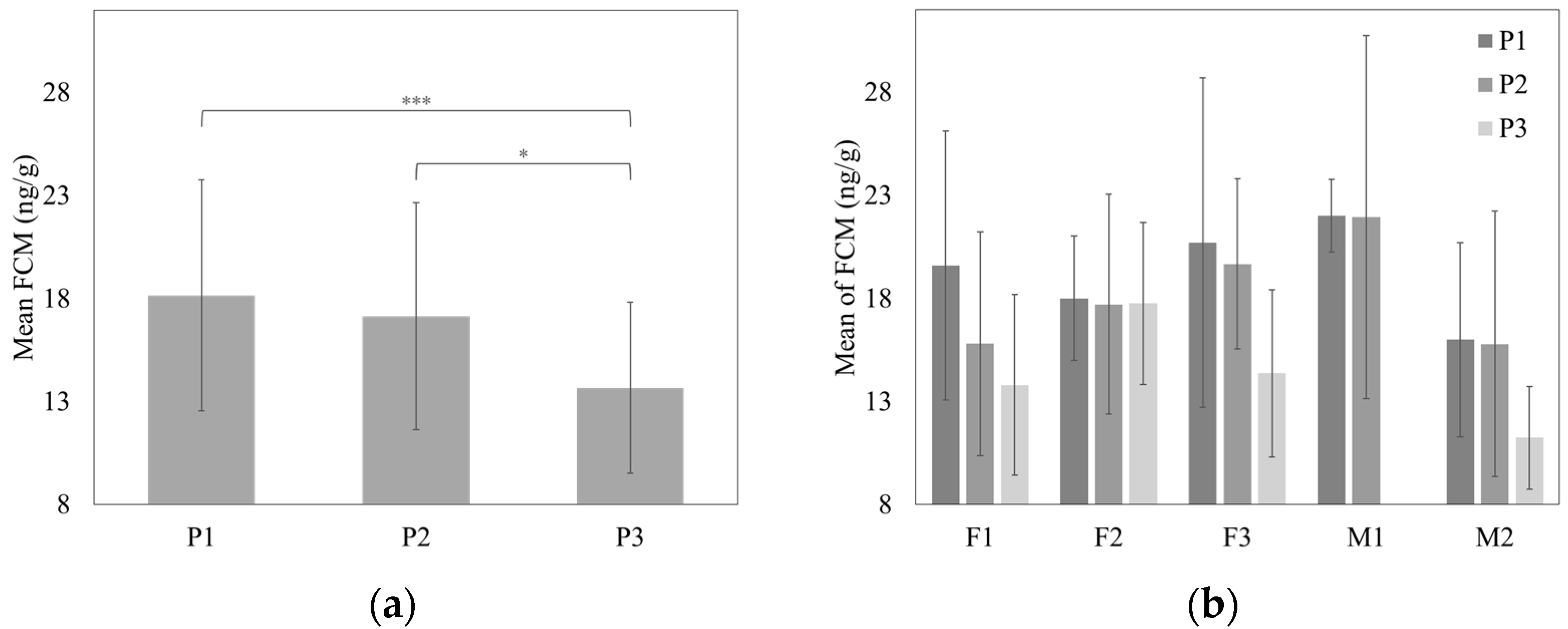
3.2. Mean FCM Concentrations
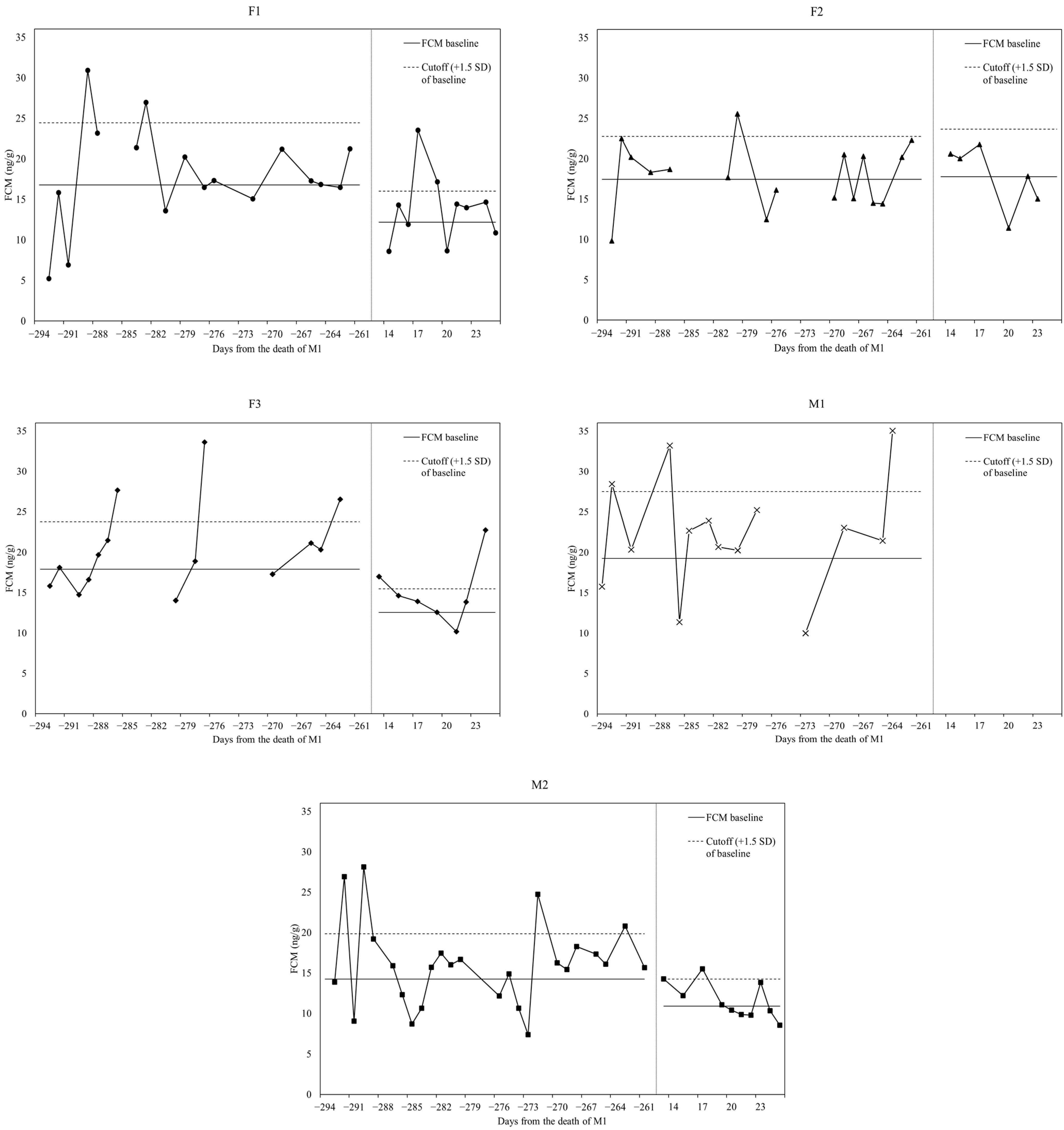
3.3. Longitudinal Assessment of Individual Hormonal Profiles
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mellen, J.D.; Shepherdson, D.J. Environmental enrichment for felids: An integrated approach. Int. Zoo Yearb. 1997, 35, 191–197. [Google Scholar] [CrossRef]
- Bellani, G.G. Subfamily Pantherinae. In Felines of the World; Academic Press Inc.: Cambridge, MA, USA, 2020; pp. 93–144. ISBN 9780128165034. [Google Scholar]
- Sogbohossou, E.A.; Bauer, H.; Loveridge, A.; Funston, P.J.; De Snoo, G.R.; Sinsin, B.; De Iongh, H.H. Social structure of lions (Panthera leo) is affected by management in Pendjari Biosphere Reserve, Benin. PLoS ONE 2014, 9, e84674. [Google Scholar] [CrossRef]
- Kirk, J.; Wascher, C.A.F. Temporal modification of social interactions in response to changing group demographics and offspring maturation in African lions (Panthera leo). Behav. Process. 2018, 157, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Mosser, A.; Packer, C. Group territoriality and the benefits of sociality in the African lion, Panthera leo. Anim. Behav. 2009, 78, 359–370. [Google Scholar] [CrossRef]
- Breed, M.D.; Moore, J. Comparative social behavior. In Animal Behavior; Academic Press Inc.: Cambridge, MA, USA, 2016; pp. 459–497. ISBN 9780128015322. [Google Scholar]
- Kohari, D.; Sunada, A.; Matsui, Y.; Ootaki, A.; Hori, H. Behavioral restriction effects on activity motivation of a captive lion (Panthera leo persica). J. Vet. Behav. Clin. Appl. Res. 2017, 17, 14–18. [Google Scholar] [CrossRef]
- Möstl, E.; Palme, R. Hormones as indicators of stress. Domest. Anim. Endocrinol. 2002, 23, 67–74. [Google Scholar] [CrossRef]
- Whitten, P.L.; Brockman, D.K.; Stavisky, R.C. Recent advances in noninvasive techniques to monitor hormone-behavior interactions. Am. J. Phys. Anthropol. 1998, 107, 1–23. [Google Scholar] [CrossRef]
- Fuller, G.; Margulis, S.W.; Santymire, R. The effectiveness of indigestible markers for identifying individual animal feces and their prevalence of use in North American zoos. Zoo Biol. 2011, 30, 379–398. [Google Scholar] [CrossRef] [PubMed]
- Mormède, P.; Andanson, S.; Aupérin, B.; Beerda, B.; Guémené, D.; Malmkvist, J.; Manteca, X.; Manteuffel, G.; Prunet, P.; van Reenen, C.G.; et al. Exploration of the hypothalamic-pituitary-adrenal function as a tool to evaluate animal welfare. Physiol. Behav. 2007, 92, 317–339. [Google Scholar] [CrossRef] [PubMed]
- Heimbürge, S.; Kanitz, E.; Otten, W. The use of hair cortisol for the assessment of stress in animals. Gen. Comp. Endocrinol. 2019, 270, 10–17. [Google Scholar] [CrossRef]
- Reeder, D.M.; Kramer, K.M. Stress in free-ranging mammals: Integrating physiology, ecology, and natural history. J. Mammal. 2005, 86, 225–235. [Google Scholar] [CrossRef]
- Palme, R. Non-invasive measurement of glucocorticoids: Advances and problems. Physiol. Behav. 2019, 199, 229–243. [Google Scholar] [CrossRef]
- Keay, J.M.; Singh, J.; Gaunt, M.C.; Kaur, T. Fecal glucocorticoids and their metabolites as indicators of stress in various mammalian species: A literature review. J. Zoo Wildl. Med. 2006, 37, 234–244. [Google Scholar] [CrossRef]
- Tsigos, C.; Chrousos, G.P. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J. Psychosom. Res. 2002, 53, 865–871. [Google Scholar] [CrossRef]
- Salas, M.; Temple, D.; Abáigar, T.; Cuadrado, M.; Delclaux, M.; Enseñat, C.; Almagro, V.; Martínez-Nevado, E.; Quevedo, M.Á.; Carbajal, A.; et al. Aggressive behavior and hair cortisol levels in captive Dorcas gazelles (Gazella dorcas) as animal-based welfare indicators. Zoo Biol. 2016, 35, 467–473. [Google Scholar] [CrossRef]
- Graham, L.H.; Brown, J.L. Cortisol metabolism in the domestic cat and implications for non-invasive monitoring of adrenocortical function in endangered felids. Zoo Biol. 1996, 15, 71–82. [Google Scholar] [CrossRef]
- Coelho, C.M.; de Azevedo, C.S.; de Guimarães, M.A.B.V.; Young, R.J. Environmental enrichment effect on fecal glucocorticoid metabolites and captive maned wolf (Chrysocyon brachyurus) behavior. J. Appl. Anim. Welf. Sci. 2016, 19, 353–362. [Google Scholar] [CrossRef]
- Bayazit, V. Evaluation of cortisol and stress in captive animals. Aust. J. Basic Appl. Sci. 2009, 3, 1022–1031. [Google Scholar]
- Kersey, D.C.; Dehnhard, M. The use of noninvasive and minimally invasive methods in endocrinology for threatened mammalian species conservation. Gen. Comp. Endocrinol. 2014, 203, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Schwarzenberger, F. The many uses of non-invasive faecal steroid monitoring in zoo and wildlife species. Int. Zoo Yearb. 2007, 41, 52–74. [Google Scholar] [CrossRef]
- Santymire, R.M.; Freeman, E.W.; Lonsdorf, E.V.; Heintz, M.R.; Armstrong, D.M.; College, N.C. Using ACTH challenges to validate techniques for adrenocortical activity analysis in various African wildlife species. Int. J. Anim. Vet. Adv. 2012, 4, 99–108. [Google Scholar]
- Fanson, K.V.; Wielebnowski, N.C. Effect of housing and husbandry practices on adrenocortical activity in captive Canada lynx (Lynx canadensis). Anim. Welf. 2013, 22, 159–165. [Google Scholar] [CrossRef]
- Breton, G.; Barrot, S. Influence of enclosure size on the distances covered and paced by captive tigers (Panthera tigris). Appl. Anim. Behav. Sci. 2014, 154, 66–75. [Google Scholar] [CrossRef]
- Clubb, R.; Mason, G. Animal welfare: Captivity effects on wide-ranging carnivores. Nature 2003, 425, 473–474. [Google Scholar] [CrossRef] [PubMed]
- Chosy, J.; Wilson, M.; Santymire, R. Behavioral and physiological responses in felids to exhibit construction. Zoo Biol. 2014, 33, 267–274. [Google Scholar] [CrossRef]
- Wielebnowski, N.C.; Fletchall, N.; Carlstead, K.; Busso, J.M.; Brown, J.L. Noninvasive assessment of adrenal activity associated with husbandry and behavioral factors in the North American clouded leopard population. Zoo Biol. 2002, 21, 77–98. [Google Scholar] [CrossRef]
- Sapolsky, R.M. The influence of social hierarchy on primate health. Science 2005, 308, 648–652. [Google Scholar] [CrossRef]
- Wooddell, L.J.; Kaburu, S.S.K.; Rosenberg, K.L.; Meyer, J.S.; Suomi, S.J.; Dettmer, A.M. Matrilineal behavioral and physiological changes following the death of a non-alpha matriarch in rhesus macaques (Macaca mulatta). PLoS ONE 2016, 11, e0157108. [Google Scholar] [CrossRef][Green Version]
- Engh, A.L.; Beehner, J.C.; Bergman, T.J.; Whitten, P.L.; Hoffmeier, R.R.; Seyfarth, R.M.; Cheney, D.L. Behavioural and hormonal responses to predation in female chacma baboons (Papio hamadryas ursinus). Proc. R. Soc. B Biol. Sci. 2006, 273, 707–712. [Google Scholar] [CrossRef]
- Soriano, A.; Vinyoles, D.; Maté, C. A short-term macro and microevaluation of an environmental enrichment program in a captive pride of Soutwest African lions (Panthera leo bleyenberghi): Age class differences. Res. J. Zool. 2018, 1, 1–9. [Google Scholar] [CrossRef]
- Tallo-Parra, O.; Manteca, X.; Sabes-Alsina, M.; Carbajal, A.; Lopez-Bejar, M. Hair cortisol detection in dairy cattle by using EIA: Protocol validation and correlation with faecal cortisol metabolites. Animal 2014, 9, 1059–1064. [Google Scholar] [CrossRef]
- Sabés-Alsina, M.; Planell, N.; Torres-Mejia, E.; Taberner, E.; Maya-Soriano, M.J.; Tusell, L.; Ramon, J.; Dalmau, A.; Piles, M.; Lopez-Bejar, M. Daily exposure to summer circadian cycles affects spermatogenesis, but not fertility in an invivo rabbit model. Theriogenology 2015, 83, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.L.; Citino, S.B.; Shaw, J.; Miller, C. Endocrine profiles during the estrous cycle and pregnancy in the Baird’s tapir (Tapirus bairdii). Zoo Biol. 1994, 13, 107–117. [Google Scholar] [CrossRef]
- Palme, R. Measuring fecal steroids: Guidelines for practical application. Ann. N. Y. Acad. Sci. 2005, 1046, 75–80. [Google Scholar] [CrossRef]
- Touma, C.; Palme, R. Measuring fecal glucocorticoid metabolites in mammals and birds: The importance of validation. Ann. N. Y. Acad. Sci. 2005, 1046, 54–74. [Google Scholar] [CrossRef] [PubMed]
- Morato, R.G.; Bueno, M.G.; Malmheister, P.; Verreschi, I.T.N.; Barnabe, R.C. Changes in the fecal concentrations of cortisol and androgen metabolites in captive male jaguars (Panthera onca) in response to stress. Braz. J. Med. Biol. Res. 2004, 37, 1903–1907. [Google Scholar] [CrossRef]
- Whitham, J.C.; Wielebnowski, N. New directions for zoo animal welfare science. Appl. Anim. Behav. Sci. 2013, 147, 247–260. [Google Scholar] [CrossRef]
- Zayan, R. The specificity of social stress. Behav. Process. 1991, 25, 81–93. [Google Scholar] [CrossRef]
- Sands, J.; Creel, S. Social dominance, aggression and faecal glucocorticoid levels in a wild population of wolves, Canis lupus. Anim. Behav. 2004, 67, 387–396. [Google Scholar] [CrossRef]
- Goymann, W.; East, M.L.; Wachter, B.; Höner, O.P.; Möstl, E.; Van’t Hof, T.J.; Hofer, H. Social, state-dependent and environmental modulation of faecal corticosteroid levels in free-ranging female spotted hyenas. Proc. R. Soc. B Biol. Sci. 2001, 268, 2453–2459. [Google Scholar] [CrossRef]
- Cockrem, J.F. Individual variation in glucocorticoid stress responses in animals. Gen. Comp. Endocrinol. 2013, 181, 45–58. [Google Scholar] [CrossRef]
- Putman, S.B.; Brown, J.L.; Franklin, A.D.; Schneider, E.C.; Boisseau, N.P.; Asa, C.S.; Pukazhenthi, B.S. Characterization of ovarian steroid patterns in female African lions (Panthera leo), and the effects of contraception on reproductive function. PLoS ONE 2015, 10, e0140373. [Google Scholar] [CrossRef]
- Putman, S.B.; Brown, J.L.; Saffoe, C.; Franklin, A.D.; Pukazhenthi, B.S. Linkage between fecal androgen and glucocorticoid metabolites, spermaturia, body weight and onset of puberty in male African lions (Panthera leo). PLoS ONE 2019, 14, e0217986. [Google Scholar] [CrossRef] [PubMed]
- Creel, S.; Christianson, D.; Schuette, P. Glucocorticoid stress responses of lions in relationship to group composition, human land use, and proximity to people. Conserv. Physiol. 2013, 1, cot021. [Google Scholar] [CrossRef] [PubMed]
| Model Selection | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Model | K | AICc | ΔAICc | Weight | |||||
| FCM~Phase + Lion | 8 | 700.00 | 0.00 | 0.63 | |||||
| FCM~Phase | 5 | 701.09 | 1.09 | 0.36 | |||||
| FCM~Phase ∗ Lion | 4 | 709.14 | 9.14 | 0.01 | |||||
| Final Model Output | |||||||||
| Factor | Estimate | Standard Error | t-Value (df) | p-Value | 95% CI | ||||
| Intercept | 18.47 | 1.87 | 9.88 (108) | <0.001 | [14.80, 22.13] | ||||
| Phase (P2) | −1.13 | 1.15 | −0.99 (108) | 0.33 | [−3.38, 1.12] | ||||
| Phase (P3) | −4.48 | 1.11 | −4.03 (108) | <0.001 | [−6.66, −2.30] | ||||
| Lion (F2) | 0.86 | 2.54 | 0.34 (108) | 0.74 | [−4.13, 5.84] | ||||
| Lion (F3) | 1.64 | 2.55 | 0.64 (108) | 0.52 | [−3.37, 6.64] | ||||
| Lion (M2) | −2.29 | 2.46 | −0.93 (108) | 0.36 | [−7.11, 2.54] | ||||
| Individual | Time | FCM (ng/g) | Proportion of Peaks | ||
|---|---|---|---|---|---|
| Baseline | Baseline Cut-Off | Peak Mean | |||
| Female 1 (F1) | Before | 16.78 ± 5.12 | 24.45 | 28.93 ± 2.81 | 11% |
| After | 12.17 ± 2.55 | 16.00 | 20.35 ± 4.51 | 20% | |
| Female 2 (F2) | Before | 17.46 ± 3.53 | 22.75 | 25.53 1 | 6% |
| After | 17.76 ± 3.93 | 23.66 | - | 0% | |
| Female 3 (F3) | Before | 17.89 ± 3.90 | 23.74 | 29.25 ± 3.80 | 20% |
| After | 12.55 ± 1.95 | 15.48 | 19.85 ± 4.08 | 25% | |
| Male 1 (M1) | Before | 19.28 ± 5.49 | 27.51 | 33.55 ± 1.33 | 19% |
| After | - | - | - | - | |
| Male 2 (M2) | Before | 14.25 ± 3.73 | 19.84 | 25.15 ± 3.22 | 15% |
| After | 10.90 ± 2.24 | 14.25 | 15.50 1 | 8% | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serres-Corral, P.; Fernández-Bellon, H.; Padilla-Solé, P.; Carbajal, A.; López-Béjar, M. Evaluation of Fecal Glucocorticoid Metabolite Levels in Response to a Change in Social and Handling Conditions in African Lions (Panthera leo bleyenberghi). Animals 2021, 11, 1877. https://doi.org/10.3390/ani11071877
Serres-Corral P, Fernández-Bellon H, Padilla-Solé P, Carbajal A, López-Béjar M. Evaluation of Fecal Glucocorticoid Metabolite Levels in Response to a Change in Social and Handling Conditions in African Lions (Panthera leo bleyenberghi). Animals. 2021; 11(7):1877. https://doi.org/10.3390/ani11071877
Chicago/Turabian StyleSerres-Corral, Paula, Hugo Fernández-Bellon, Pilar Padilla-Solé, Annaïs Carbajal, and Manel López-Béjar. 2021. "Evaluation of Fecal Glucocorticoid Metabolite Levels in Response to a Change in Social and Handling Conditions in African Lions (Panthera leo bleyenberghi)" Animals 11, no. 7: 1877. https://doi.org/10.3390/ani11071877
APA StyleSerres-Corral, P., Fernández-Bellon, H., Padilla-Solé, P., Carbajal, A., & López-Béjar, M. (2021). Evaluation of Fecal Glucocorticoid Metabolite Levels in Response to a Change in Social and Handling Conditions in African Lions (Panthera leo bleyenberghi). Animals, 11(7), 1877. https://doi.org/10.3390/ani11071877







