Behavioural Diversity Study in Bottlenose Dolphin (Tursiops truncatus) Groups and Its Implications for Welfare Assessments
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites and Subjects
2.2. Assessment of Behavioural Diversity
2.3. Behavioural Sampling
2.4. Statistical Analysis
3. Results
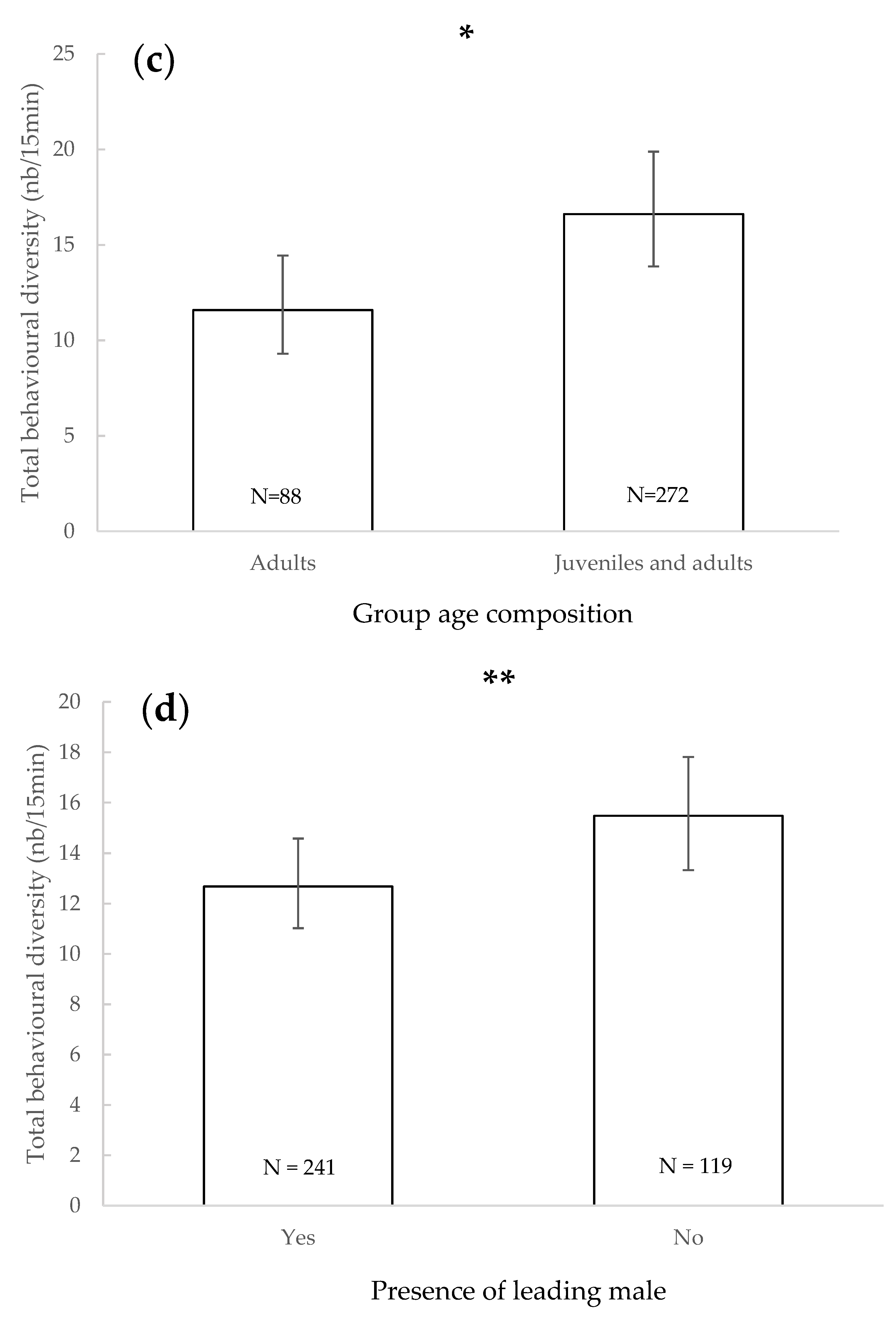
3.1. Total Behavioural Diversity
3.2. Affiliative Behavioural Diversity
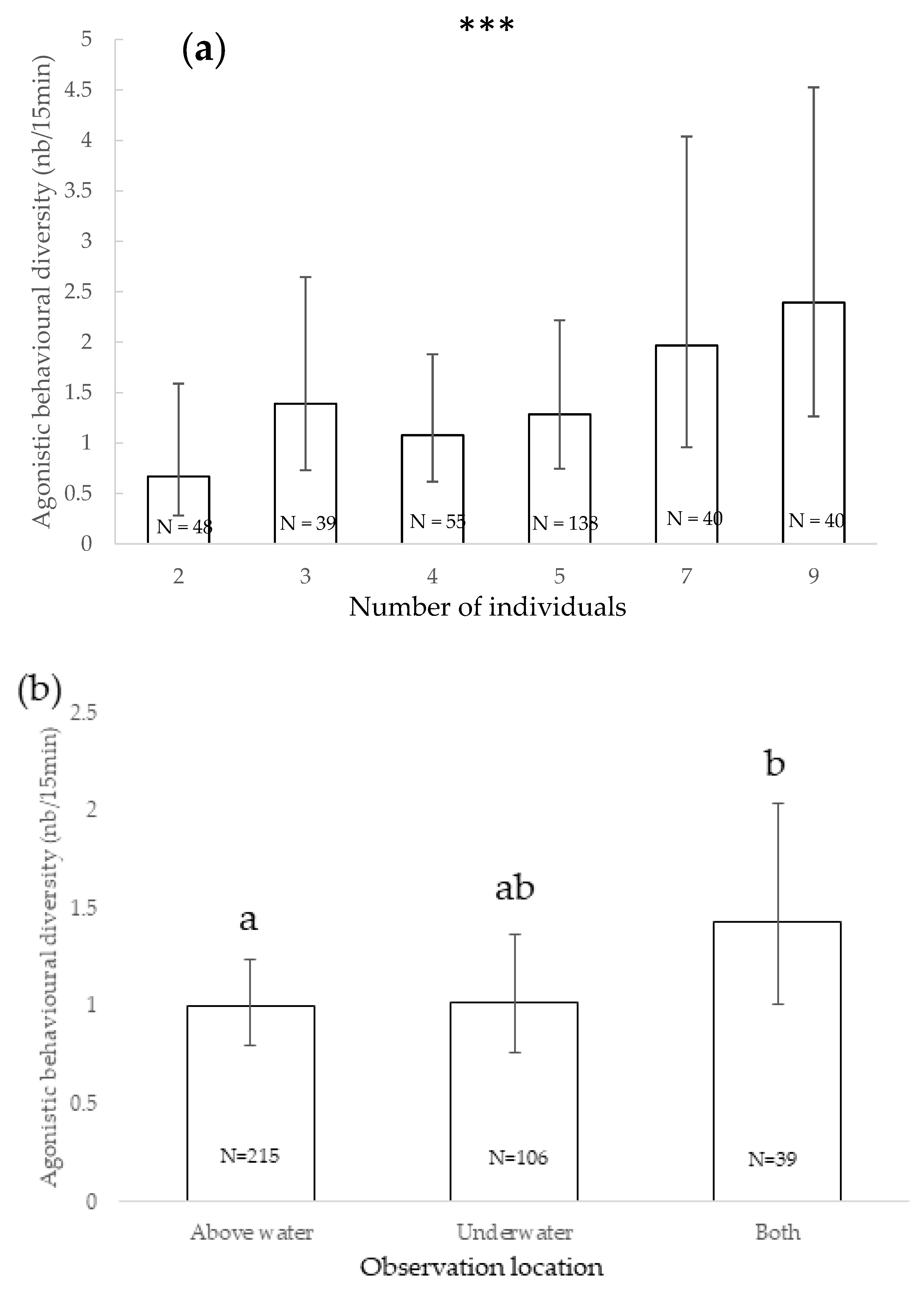
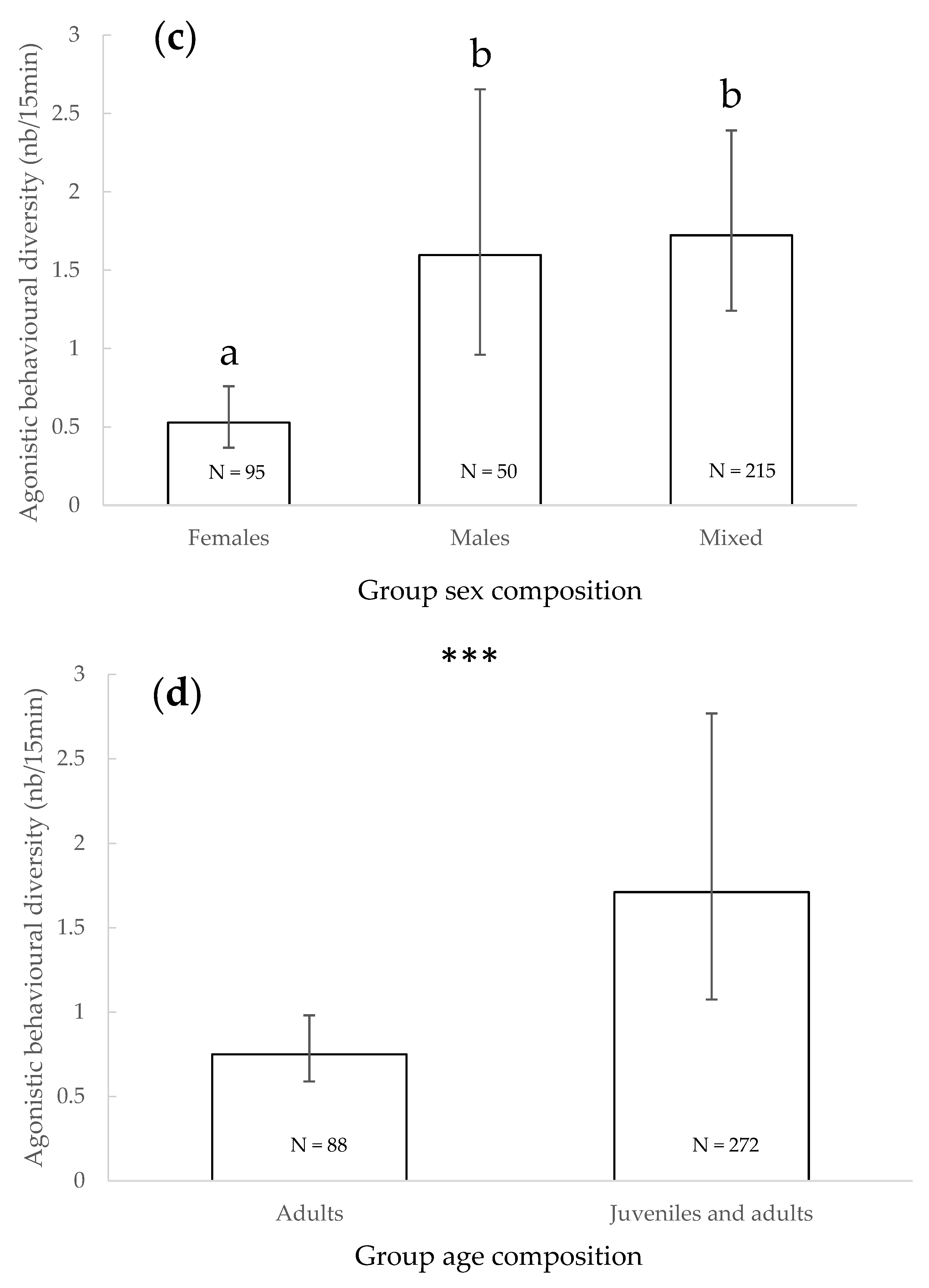
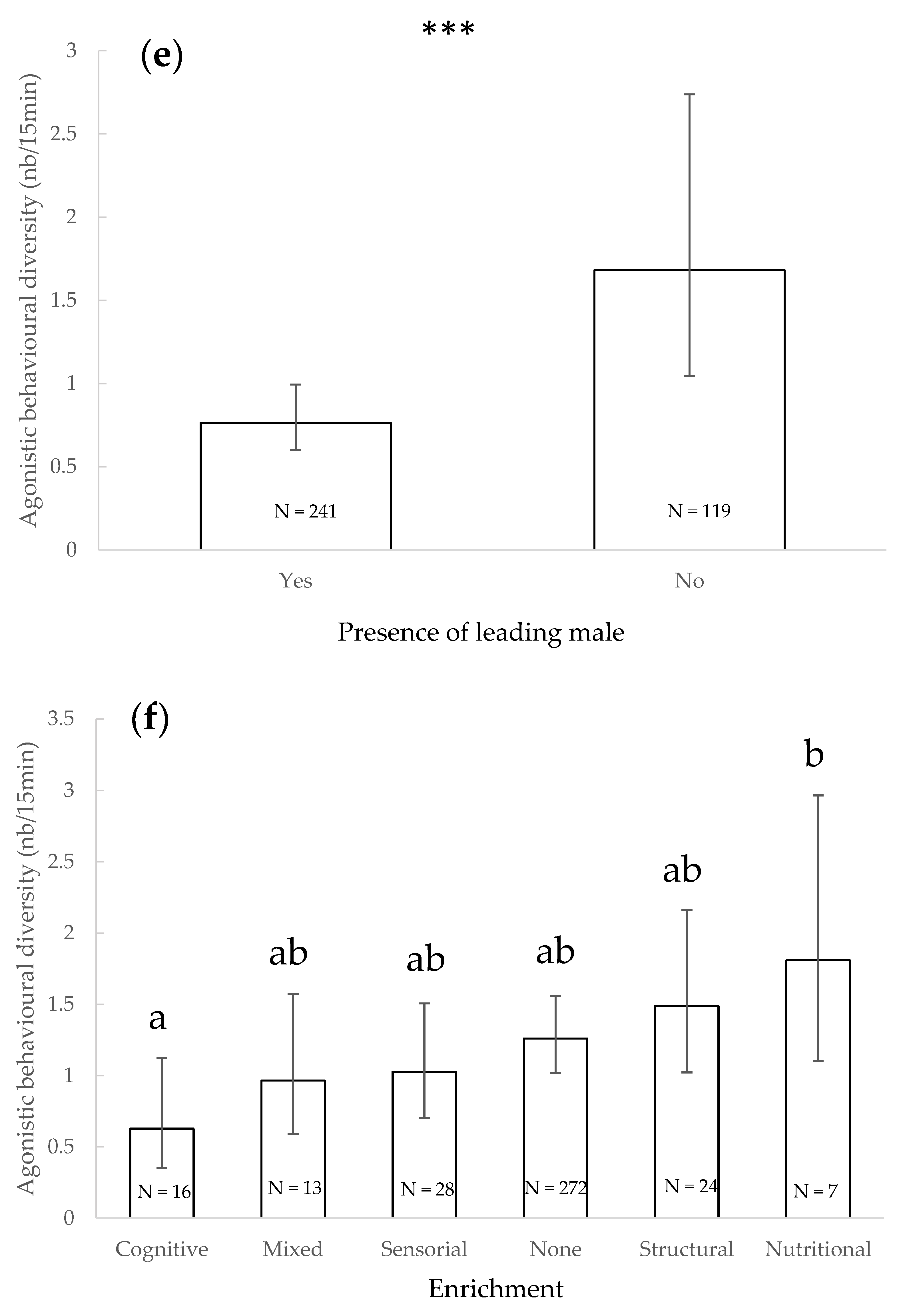
3.3. Agonistic Behavioural Diversity
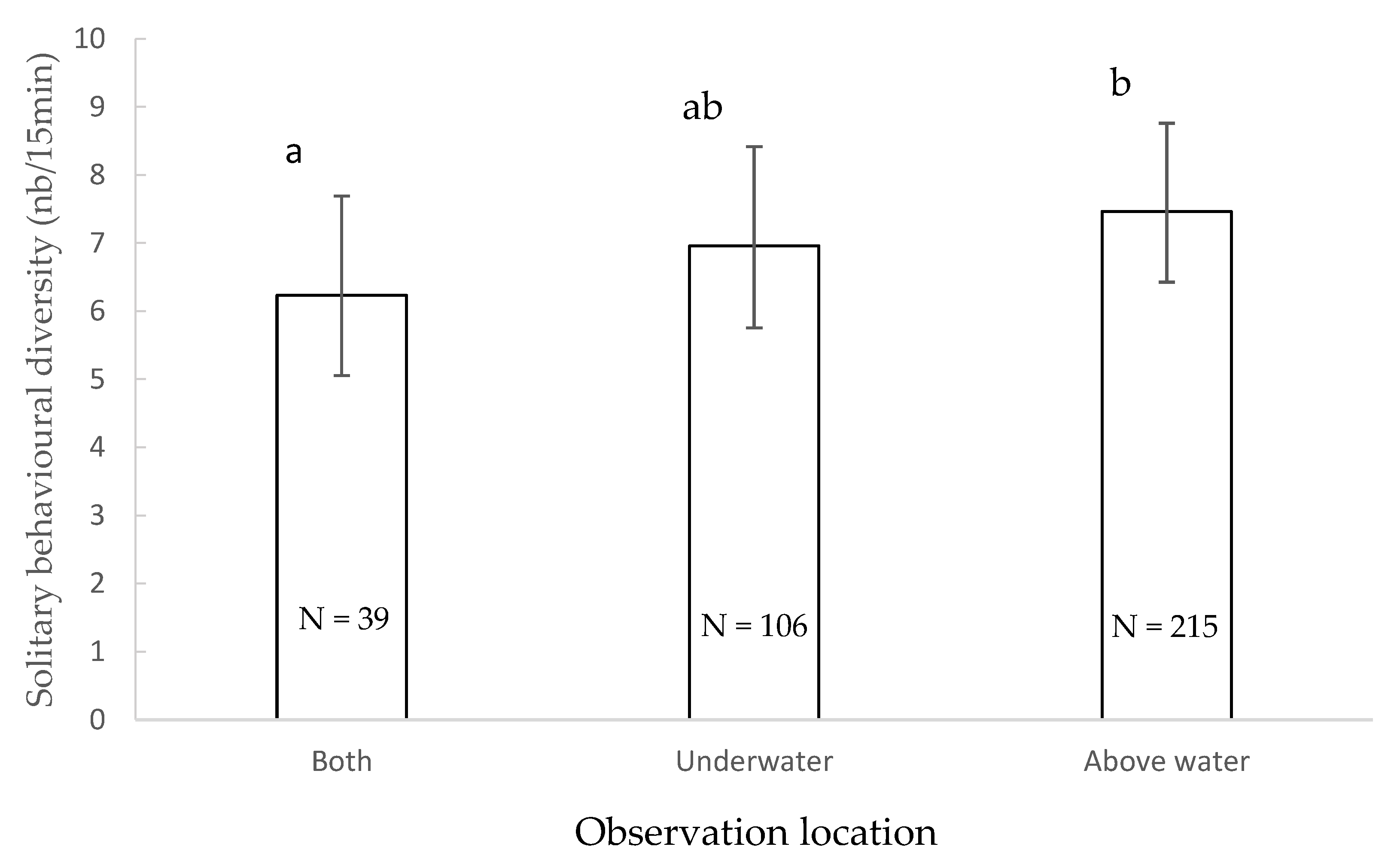
3.4. Solitary Behavioural Diversity
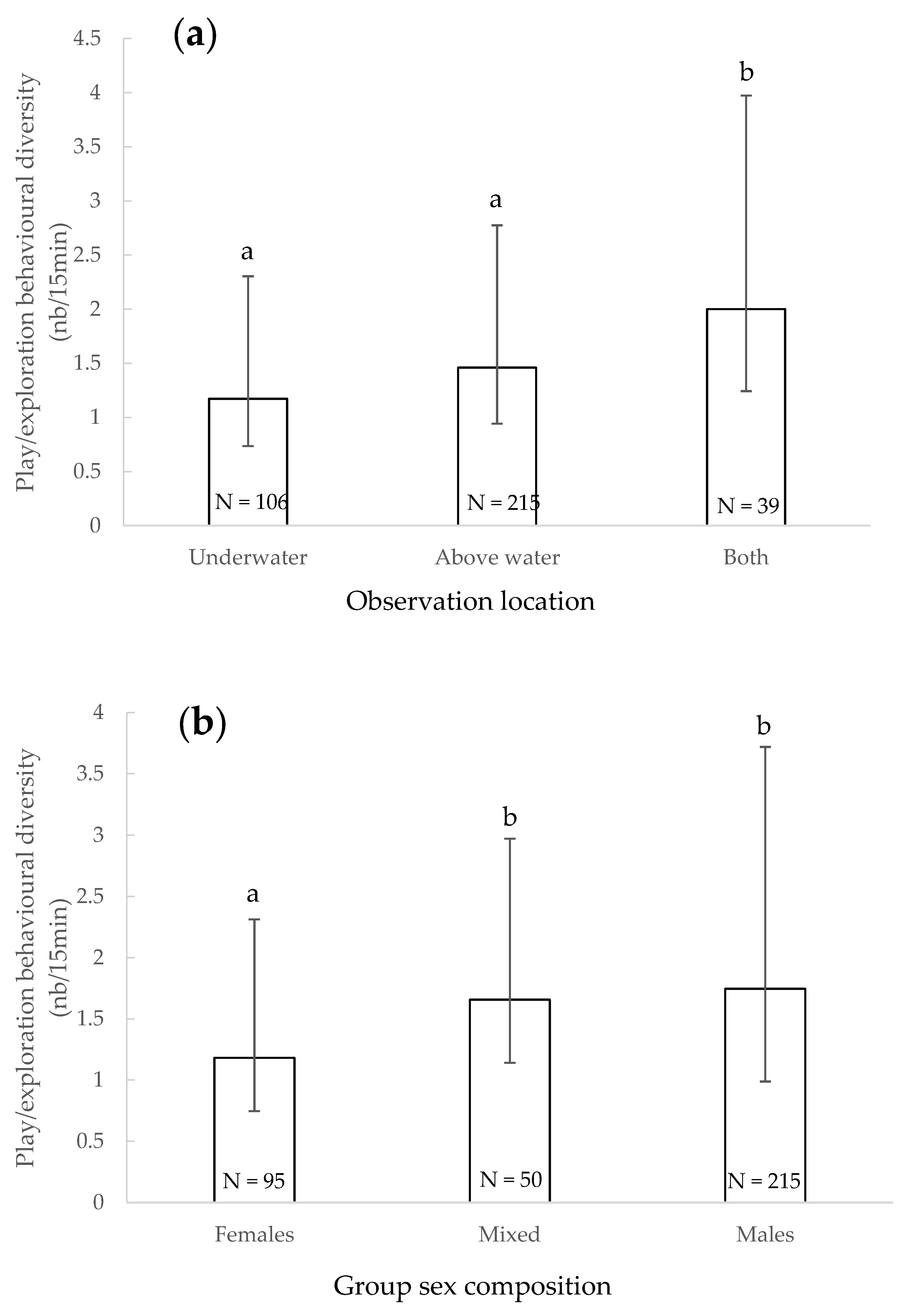
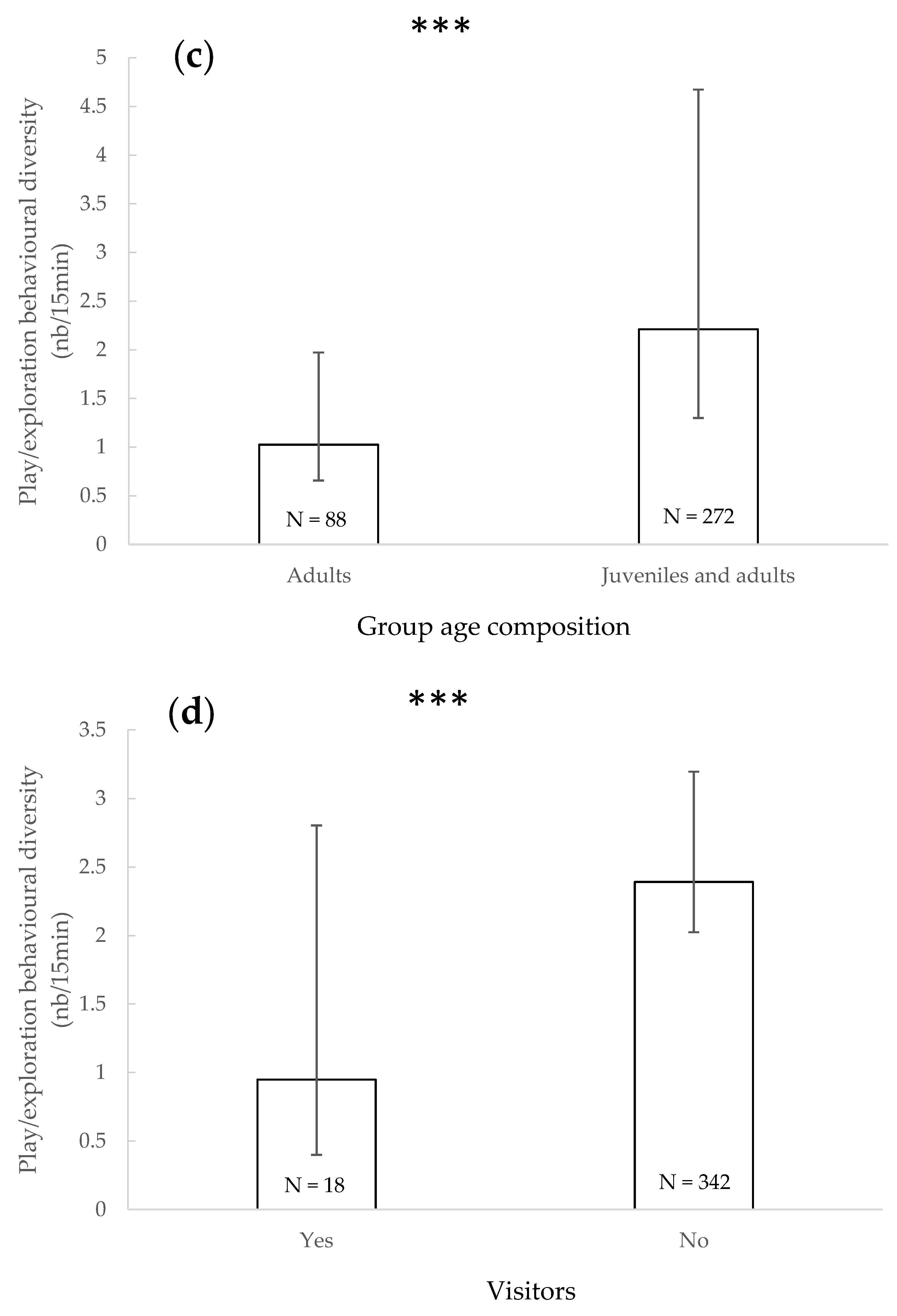
3.5. Play/Exploratory Behavioural Diversity
3.6. Sexual Behavioural Diversity
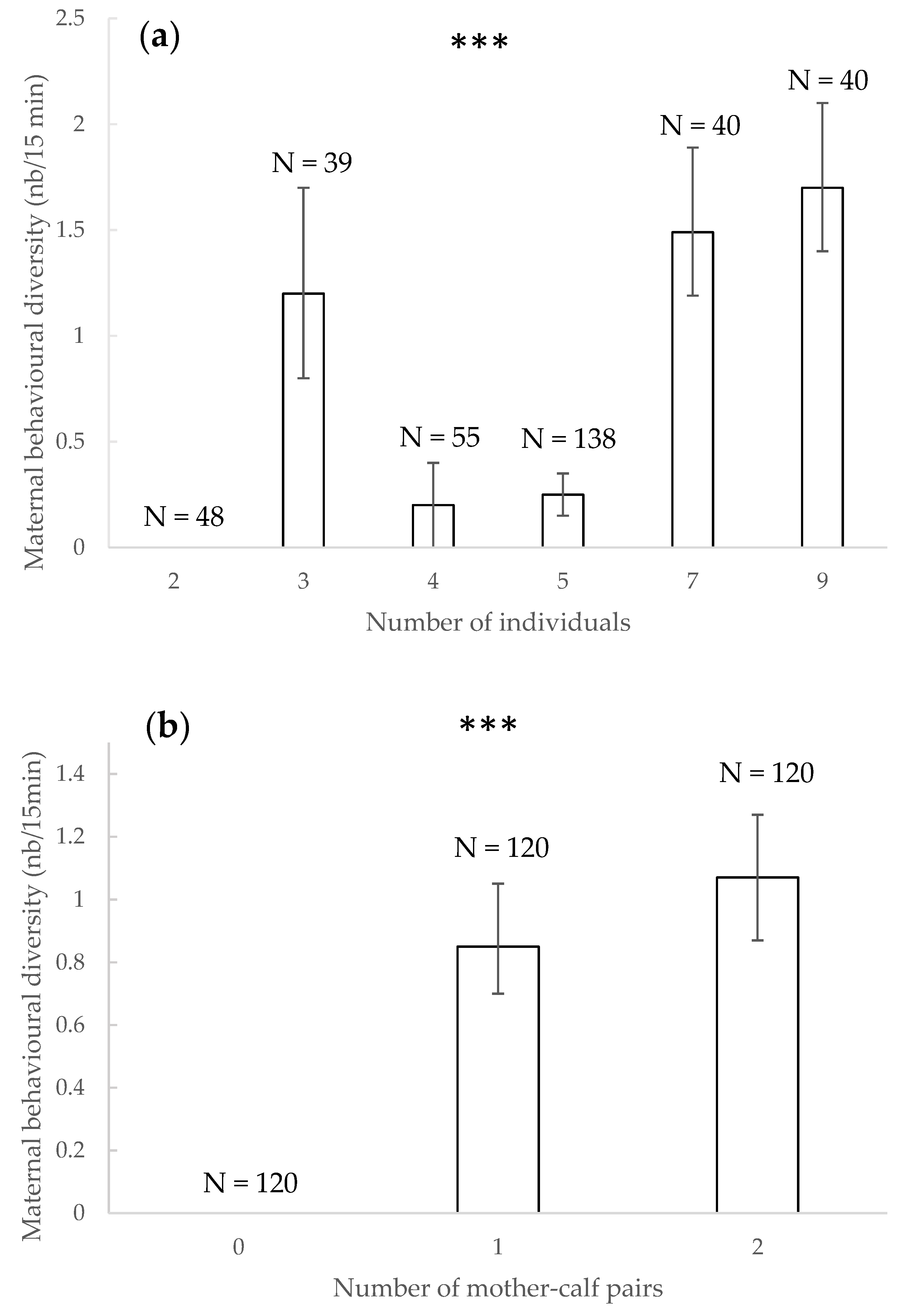
3.7. Maternal Behavioural Diversity
4. Discussion
4.1. Affiliative Behavioural Diversity
4.2. Agonistic Behavioural Diversity
4.3. Solitary Behavioural Diversity
4.4. Play/Exploratory Behavioural Diversity
4.5. Sexual Behavioural Diversity
4.6. Maternal Behavioural Diversity
4.7. Study Limitations and Further Developments for Dolphin Welfare Assessments
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Webster, J. Animal Welfare: Limping towards Eden: A Practical Approach to Redressing the Problem of Our Dominion Over the Animals; Blackwell Publishing Ltd.: Oxford, UK, 2005; ISBN 978-1-405-11877-4. [Google Scholar]
- Delfour, F.; Lassalle, J.M. Les Animaux de Laboratoire: Bien-Être et Conditions d’Hébergement; OPAL: Paris, France, 1996; 158p. [Google Scholar]
- Mason, G.J.; Cooper, J.; Clarebrough, C. Frustrations of fur-farmed mink. Nature 2001, 410, 35–36. [Google Scholar] [CrossRef]
- Wrangham, R.W.; de Waal, F.B.; McGrew, W.C. The challenge of behavioral diversity. In Chimpanzee Cultures; Wrangham, R.W., McGrew, W.C., de Waal, F.B.M., Heltne, P.G., Eds.; Harvard University Press: Cambridge, MA, USA, 1994; pp. 1–18. [Google Scholar]
- Cordero-Rivera, A. Behavioral diversity (ethodiversity): A neglected level in the study of biodiversity. Front. Ecol. Evol. 2017, 5, 7. [Google Scholar] [CrossRef]
- Spiezio, C.; Valsecchi, V.; Sandri, C.; Regaiolli, B. Investigating individual and social behaviour of the Northern bald ibis (Geronticus eremita): Behavioural variety and welfare. PeerJ 2018, 6, e5436. [Google Scholar] [CrossRef]
- Miller, L.J.; Vicino, G.A.; Sheftel, J.; Lauderdale, L.K. Behavioral diversity as a potential indicator of positive animal welfare. Animals 2020, 10, 1211. [Google Scholar] [CrossRef]
- Whitehead, H.; Laland, K.N.; Rendell, L.; Thorogood, R.; Whiten, A. The reach of gene-culture coevolution in animals. Nat. Comm. 2019, 10, 2405. [Google Scholar] [CrossRef]
- Modlmeier, A.P.; Keiser, C.N.; Shearer, T.A.; Pruitt, J.N. Species-specific influence of group composition on collective behaviors in ants. Behav. Ecol Sociobiol. 2014, 68, 1929–1937. [Google Scholar] [CrossRef]
- Samuni, L.; Wegdell, F.; Surbeck, M. Behavioural diversity of bonobo prey preference as a potential cultural trait. eLife 2020, 9, 59191. [Google Scholar] [CrossRef]
- Kühl, H.S.; Boesch, C.; Kulik, L.; Haas, F.; Arandjelovic, M.; Dieguez, P.; Bocksberger, G.; Brooke McElreath, M.; Agbor, A.; Angedakin, A.; et al. Human impact erodes chimpanzee behavioral diversity. Science 2019, 363, 1453–1455. [Google Scholar] [CrossRef]
- Kalan, A.K.; Kulik, L.; Arandjelovic, M.; Boesch, C.; Haas, F.; Dieguez, P.; Barratt, C.D.; Abwe, E.E.; Agbor, A.; Angedakin, S.; et al. Environmental variability supports chimpanzee behavioural diversity. Nat. Comm. 2020, 11, 4451. [Google Scholar] [CrossRef]
- Boesch, C.; Hohmann, G.; Marchant, L. (Eds.) Behavioural Diversity in Chimpanzees and Bonobos; Cambridge University Press: Cambridge, UK, 2002. [Google Scholar]
- Frézard, A.; Pape, G.L. Contribution to the welfare of captive wolves (Canis lupus lupus): A behavioral comparison of six wolf packs. Zoo Biol. 2003, 22, 33–44. [Google Scholar] [CrossRef]
- Mellor, D.J.; Hunt, S.; Gusset, M. Caring for Wildlife: The World Zoo and Aquarium Animal Welfare Strategy; WAZA Executive Office: Gland, Switzerland, 2015. [Google Scholar]
- Meehan, C.L.; Mench, J.A.; Carlstead, K.; Hogan, J.N. Determining connections between the daily lives of zoo elephants and their welfare: An epidemiological approach. PLoS ONE 2016, 11, e0158124. [Google Scholar] [CrossRef]
- Bashaw, M.J.; Gibson, M.D.; Schowe, D.M.; Kucher, A.S. Does enrichment improve reptile welfare? Leopard geckos (Eublepharis macularius) respond to five types of environmental enrichment. Appl. Anim. Behav. Sci. 2016, 184, 150–160. [Google Scholar] [CrossRef]
- Spain, M.S.; Fuller, G.; Allard, S.M. Effects of habitat modifications on behavioral indicators of welfare for Madagascar giant hognose snakes (Leioheterodon madagascariensis). Anim. Behav. Cogn. 2020, 7, 70–81. [Google Scholar] [CrossRef]
- Miller, L.J.; Pisacane, C.B.; Vicino, G.A. Relationship between behavioural diversity and faecal glucocorticoid metabolites: A case study with cheetahs (Acinonyx jubatus). Anim. Welf. 2016, 25, 325–329. [Google Scholar] [CrossRef]
- Collins, C.K.; Quirke, T.; Overy, L.; Flannery, K.; O’Riordan, R. The effect of the zoo setting on the behavioural diversity of captive gentoo penguins and the implications for their educational potential. J. Zoo Aquar. Res. 2016, 4, 85–90. [Google Scholar]
- Rose, P.E.; Brereton, J.E.; Croft, D.P. Measuring welfare in captive flamingos: Activity patterns and exhibit usage in zoo-housed birds. Appl. Anim. Behav. Sci. 2018, 205, 115–125. [Google Scholar] [CrossRef]
- Goswami, S.; Patel, S.K.; Kadivar, R.; Tyagi, P.C.; Malik, P.K.; Mondol, S. Effects of a combined enrichment intervention on the behavioural and physiological welfare of captive Asiatic lions (Panthera leo persica). Appl. Anim. Behav. Sci. 2021, 236, 105222. [Google Scholar] [CrossRef]
- Hamilton, J.; Fuller, G.; Allard, S. Evaluation of the Impact of Behavioral Opportunities on Four Zoo-Housed Aardvarks (Orycteropus afer). Animals 2020, 10, 1433. [Google Scholar] [CrossRef]
- Kistler, C.; Hegglin, D.; Würbel, H.; König, B. Feeding enrichment in an opportunistic carnivore: The red fox. Appl. Anim. Behav. Sci. 2009, 116, 260–265. [Google Scholar] [CrossRef]
- Carlstead, K.; Shepherdson, D. Alleviating stress in zoo animals with environmental enrichment. In The Biology of Animal Stress: Basic Principles and Implications for Animal Welfare; CABI: Wallingford, UK, 2000; pp. 337–354. [Google Scholar]
- Powell, D.M. Preliminary evaluation of environmental enrichment techniques for African lions. Anim. Welf. 1995, 4, 361–370. [Google Scholar]
- Hocking, D.P.; Salverson, M.; Evans, A.R. Foraging-based enrichment promotes more varied behaviour in captive Australian fur seals (Arctocephalus pusillus doriferus). PLoS ONE 2015, 10, e0124615. [Google Scholar] [CrossRef]
- Riggio, G.; Mariti, C.; Boncompagni, C.; Corosaniti, S.; Di Giovanni, M.; Ogi, A.; Gazzano, A.; Thomas, R. Feeding Enrichment in a Captive Pack of European Wolves (Canis lupus lupus): Assessing the Effects on Welfare and on a Zoo’s Recreational, Educational and Conservational Role. Animals 2019, 9, 331. [Google Scholar] [CrossRef]
- Melfi, V.A.; Ward, S.J. Welfare Implications of Zoo Animal Training. In Zoo Animal Learning and Training; Melfi, V.A., Dorey, N.R., Ward, S.J., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2020; pp. 271–288. [Google Scholar]
- Brando, S.; Broom, D.M.; Acasuso-Rivero, C.; Clark, F. Optimal marine mammal welfare under human care: Current efforts and future directions. Behav. Proc. 2018, 156, 16–36. [Google Scholar] [CrossRef]
- Vaicekauskaite, R.; Schneider, J.N.; Delfour, F. Does enrichment improve wellbeing in animals under human care? A case study of two harbor seals (Phoca vitulina). J. Appl. Anim. Welf. Sci. 2019, 22, 255–266. [Google Scholar] [CrossRef]
- Samuelson, M.M.; Lauderdale, L.K.; Pulis, K.; Solangi, M.; Hoffland, T.; Lyn, H. Olfactory enrichment in California sea lions (Zalophus californianus): An effective tool for captive welfare? J. Appl. Anim. Welf. Sci. 2017, 20, 75–85. [Google Scholar] [CrossRef]
- Ross, S.R. Issues of choice and control in the behaviour of a pair of captive polar bears (Ursus maritimus). Behav. Proc. 2006, 73, 117–120. [Google Scholar] [CrossRef]
- Skovlund, C.R.; Kirchner, M.K.; Moos, L.W.; Alsted, N.; Manteca, X.; Tallo-Parra, O.; Stelvig, M.; Forkman, B. A critical review of animal-based welfare indicators for polar bears (Ursus maritimus) in zoos: Identification and evidence of validity. Anim. Welf. 2021, 30, 1–18. [Google Scholar] [CrossRef]
- Castellote, M.; Fossa, F. Measuring acoustic activity as a method to evaluate welfare in captive beluga whales (Delphinapterus leucas). Aqua. Mamm. 2006, 32, 325–333. [Google Scholar] [CrossRef]
- Clegg, I.L.K.; Van Elk, C.E.; Delfour, F. Applying welfare science to bottlenose dolphins (Tursiops truncatus). Anim. Welf. 2017, 26, 165–176. [Google Scholar] [CrossRef]
- Clegg, I.L.K.; Rödel, H.G.; Cellier, M.; Vink, D.; Michaud, I.; Mercera, B.; Böye, M.; Hausberger, M.; Lemasson, A.; Delfour, F. Schedule of human-controlled periods structures bottlenose dolphin (Tursiops truncatus) behavior in their free-time. J. Comp. Psychol. 2017, 131, 214–224. [Google Scholar] [CrossRef]
- Trone, M.; Kuczaj, S.; Solangi, M. Does participation in Dolphin–Human Interaction Programs affect bottlenose dolphin behaviour? Appl. Anim. Behav. Sci. 2005, 93, 363–374. [Google Scholar] [CrossRef]
- Miller, L.J.; Mellen, J.; Greer, T.; Kuczaj, S.A. The effects of education programmes on Atlantic bottlenose dolphin (Tursiops truncatus) behaviour. Anim. Welf. 2011, 20, 159–172. [Google Scholar]
- Serres, A.; Delfour, F. Environmental changes and anthropogenic factors modulate social play in captive bottlenose dolphins (Tursiops truncatus). Zoo Biol. 2017, 36, 99–111. [Google Scholar] [CrossRef]
- Serres, A.; Hao, Y.; Wang, D. Body Contacts and Social Interactions in Captive Odontocetes Are Influenced by the Context: An Implication for Welfare Assessment. Animals 2020, 10, 924. [Google Scholar] [CrossRef] [PubMed]
- Monreal-Pawlowsky, T.; Carbajal, A.; Tallo-Parra, O.; Sabés-Alsina, M.; Monclús, L.; Almunia, J.; Fernández-Bellon, H.; Lopez-Bejar, M. Daily salivary cortisol levels in response to stress factors in captive common bottlenose dolphins (Tursiops truncatus): A potential welfare indicator. Vet. Rec. 2017, 180, 593. [Google Scholar] [CrossRef]
- Pedernera-Romano, C.; Valdez, R.A.; Singh, S.; Chiappa, X.; Romano, M.C.; Galindo, F. Salivary cortisol in captive dolphins (Tursiops truncatus): A non-invasive technique. Anim. Welf. 2006, 15, 359–362. [Google Scholar]
- Mercera, K.; Pilot-Storck, F.; Mercera, B.; Gilbert, C.; Delfour, F. Exploration of fecal glucocorticoid metabolites in the bottlenose dolphin (Tursiops truncatus) under human care by enzyme Immunoassay. Aquat. Mamm. 2021, 47, 227–238. [Google Scholar] [CrossRef]
- Clegg, I.L.K.; Rödel, H.G.; Mercera, B.; van der Heul, S.; Schrijvers, T.; de Laender, P.; Gojceta, R.; Zimmitti, M.; Verhoeven, E.; Burger, J.; et al. Dolphins’ willingness to participate (WtP) in positive reinforcement training as a potential welfare indicator, where WtP predicts early changes in health status. Front. Psychol. 2019, 10, 2112. [Google Scholar] [CrossRef]
- Delfour, F.; Monreal-Pawlowsky, T.; Vaicekauskaite, R.; Pilenga, C.; Garcia-Parraga, D.; Rödel, H.G.; García Caro, N.; Perlado Campos, E.; Mercera, B. Dolphin Welfare Assessment under Professional Care: “Willingness to Participate”, an Indicator Significantly Associated with Six Potential “Alerting Factors”. J. Zool. Bot. Gard. 2020, 1, 4. [Google Scholar] [CrossRef]
- Clegg, I.L.; Rödel, H.G.; Delfour, F. Bottlenose dolphins engaging in more social affiliative behaviour judge ambiguous cues more optimistically. Behav. Brain Res. 2017, 322, 115–122. [Google Scholar] [CrossRef]
- Serres, A.; Hao, Y.; Wang, D. Swimming features in captive odontocetes: Indicative of animals’ emotional state? Behav. Proc. 2020, 170, 103998. [Google Scholar] [CrossRef]
- Jensen, A.L.M.; Delfour, F.; Carter, T. Anticipatory behavior in captive bottlenose dolphins (Tursiops truncatus): A preliminary study. Zoo Biol. 2013, 32, 436–444. [Google Scholar] [CrossRef]
- Clegg, I.L.; Rödel, H.G.; Boivin, X.; Delfour, F. Looking forward to interacting with their caretakers: Dolphins’ anticipatory behaviour indicates motivation to participate in specific events. Appl. Anim. Behav. Sci. 2018, 202, 85–93. [Google Scholar] [CrossRef]
- Council Directive 1999/22/EC of 29 March 1999 Relating to the Keeping of Wild Animals in Zoos. Available online: https://www.eumonitor.eu/9353000/1/j9vvik7m1c3gyxp/vjccgy56xozw (accessed on 1 November 2020).
- Carlstead, K.; Shepherdson, D.J. Effects of environmental enrichment on reproduction. Zoo Biol. 1994, 13, 447–458. [Google Scholar] [CrossRef]
- Makecha, R.N.; Highfill, L.E. Environmental Enrichment, Marine Mammals, and Animal Welfare: A Brief Review. Aquat. Mamm. 2018, 44, 221–230. [Google Scholar] [CrossRef]
- Altmann, J. Observational study of behavior: Sampling methods. Behaviour 1974, 49, 227–266. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 5 June 2021).
- Brooks, M.E.; Kristensen, K.; van Benthem, K.J.; Magnusson, A.; Berg, C.W.; Nielsen, A.; Skaug, H.J.; Maechler, M.; Bolker, B.M. glmmTMB Balances Speed and Flexibility Among Packages for Zero-inflated Generalized Linear Mixed Modeling. R J. 2017, 9, 378–400. [Google Scholar] [CrossRef]
- Hartig, F. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models. R Package; Version 0.1.1. Available online: https://github.com/florianhartig/DHARMa (accessed on 5 June 2021).
- Barton, K. Mu-MIn: Multi-Model Inference. R Package; Version 0.12.2/r18. Available online: http://R-Forge.R-project.org/projects/mumin/ (accessed on 5 June 2021).
- Akaile, H. Information theory and an extension of the maximum likelihood principle. In Proceedings of the Second International Symposium on Information Theory, Tsahkadsor, Armenia, 2–8 September 1971; Petrov, B.N., Caski, F., Eds.; Akademiai Kiado: Budapest, Hungary, 1973; pp. 267–281. [Google Scholar]
- Birgersson, S.; de la Pommeraye, S.B.; Delfour, F. Dolphin Personality Study Based on Ethology and Social Network Theory; LAP Lambert, Academic Publishing: Düsseldorf, Germany, 2014. [Google Scholar]
- Gosling, S.D. Personality in non-human animals. Soc. Pers. Psychol. Compass. 2008, 2, 985–1001. [Google Scholar] [CrossRef]
- Sih, A.; Bell, A.M.; Johnson, J.C.; Ziemba, R.E. Behavioural syndromes: An integrative overview. Quart. Rev. Biol. 2004, 79, 241–277. [Google Scholar] [CrossRef]
- Campbell, G.S.; Bilgre, B.A.; Defran, R.H. Bottlenose dolphins (Tursiops truncatus) in Tuneffe Atoll, Belize: Occurrence, site fidelity, group size, and abundance. Aquat. Mamm. 2002, 28, 170–180. [Google Scholar]
- Möller, L.M.; Allen, S.J.; Harcourt, R.G. Group characteristics, site fidelity and seasonal abundance of bottlenosed dolphins (Tursiops aduncus) in Jervis Bay and Port Stephens, South-Eastern Australia. Austral. Mammal. 2002, 24, 11–22. [Google Scholar] [CrossRef]
- Connor, R.C.; Watson-Capps, J.J.; Sargeant, B.L.; Scott, E.M.; Mann, J. Aggression in bottlenose dolphins: Evidence for sexual coercion, male-male competition, and female tolerance through analysis of tooth-rake marks and behaviour. Behaviour 2005, 142, 21–44. [Google Scholar] [CrossRef]
- Weinrich, M.T.; Mann, J.; Connor, R.C.; Tyack, P.L.; Whitehead, H. (Eds.) Cetacean Societies: Field Studies of Dolphins and Whales; University of Chicago Press: Chicago, IL, USA, 2000. [Google Scholar]
- Harvey, B.S.; Dudzinski, K.M.; Kuczaj, S.A. Associations and the role of affiliative, agonistic, and socio-sexual behaviors among common bottlenose dolphins (Tursiops truncatus). Behav. Proc. 2017, 135, 145–156. [Google Scholar] [CrossRef]
- Hill, H.M.; Greer, T.; Solangi, M.; Kuczaj, I.I.; Stan, A. All mothers are not the same: Maternal styles in bottlenose dolphins (Tursiops truncatus). Int. J. Comp. Psychol. 2007, 20, 35–54. [Google Scholar]
- Clark, F.E. Marine mammal cognition and captive care: A proposal for cognitive enrichment in zoos and aquariums. J. Zoo Aquar. Res. 2013, 1, 1–6. [Google Scholar]
- Matrai, E.; Ng, A.K.; Chan, M.M.; Gendron, S.M.; Dudzinski, K.M. Testing use of a potential cognitive enrichment device by an Indo-Pacific bottlenose dolphin (Tursiops aduncus). Zoo Biol. 2020, 39, 156–167. [Google Scholar] [CrossRef]
- Greene, W.E.; Melillo-Sweeting, K.; Dudzinski, K.M. Comparing object play in captive and wild dolphins. Int. J. Comp. Psychol. 2011, 24, 292–306. [Google Scholar]
- Eskelinen, H.C.; Winship, K.A.; Borger-Turner, J.L. Sex, age, and individual differences in bottlenose dolphins (Tursiops truncatus) in response to environmental enrichment. Anim. Behav. Cogn. 2015, 2, 241–253. [Google Scholar] [CrossRef]
- Kuczaj, S.A.; Eskelinen, H.C. Why do dolphins play. Anim. Behav. Cogn. 2014, 1, 113–127. [Google Scholar] [CrossRef]
- Delfour, F. Object manipulation and play behavior in bottlenose dolphins (Tursiops truncatus) under human care. Int. J. Comp. Psychol. 2017, 30. [Google Scholar] [CrossRef]
- Kuczaj, S.A.; Makecha, R.; Trone, M.; Paulos, R.D.; Ramos, J.A. Role of peers in cultural innovation and cultural transmission: Evidence from the play of dolphin calves. Int. J. Comp. Psychol. 2006, 19, 223–240. [Google Scholar]
- Hill, H.; Ramirez, D. Adults play but not like their young: The frequency and types of play by belugas (Delphinapterus leucas) in human care. Anim. Behav. Cogn. 2014, 1, 166–185. [Google Scholar] [CrossRef]
- Antonenko, T.V.; Medvedeva, J.E.; Panchuk, K.A. The influence of olfactory stimulation on the welfare of big cats in captivity. Ukr. J. Ecol. 2017, 7, 134–138. [Google Scholar] [CrossRef]
- Van Metter, J.E.; Harriger, M.D.; Bolen, R.H. Environmental enrichment utilizing stimulus objects for African lions (Panthera leo leo) and Sumatran tigers (Panthera tigris sumatrae). BIOS 2008, 79, 7–16. [Google Scholar] [CrossRef]
- Haskell, M.; Wemelsfelder, F.; Mendl, M.T.; Calvert, S.; Lawrence, A.B. The effect of substrate-enriched and substrate-impoverished housing environments on the diversity of behaviour in pigs. Behaviour 1996, 133, 741–761. [Google Scholar]
- Hirt, H.; Wechsler, B. Behavioural diversity as a measure of welfare: A study in pigs. Appl. Anim. Behav. Sci. 1994, 40, 82–83. [Google Scholar] [CrossRef]
- Wemelsfelder, F.; Haskell, M.; Mendl, M.T.; Calvert, S.; Lawrence, A.B. Diversity of behaviour during novel object tests is reduced in pigs housed in substrate-impoverished conditions. Anim. Behav. 2000, 60, 385–394. [Google Scholar] [CrossRef]
- Renner, M.J.; Lussier, J.P. Environmental enrichment for the captive spectacled bear (Tremarctos ornatus). Pharm. Biochem. Behav. 2002, 73, 279–283. [Google Scholar] [CrossRef]
- Wagman, J.D. The Effects of Feeding Enrichment on Behavioral Measures of Animal Welfare in Four Bear Species. Ph.D. Thesis, Case Western Reserve University, Cleveland, OH, USA, 2015. [Google Scholar]
- Scott, N.L.; LaDue, C.A. The behavioral effects of exhibit size versus complexity in African elephants: A potential solution for smaller spaces. Zoo Biol. 2019, 38, 448–457. [Google Scholar] [CrossRef]
- Swaisgood, R.R.; White, A.M.; Zhou, X.; Zhang, G.; Lindburg, D.G. How do giant pandas (Ailuropoda melanoleuca) respond to varying properties of enrichments? A comparison of behavioral profiles among five enrichment items. J. Comp. Psychol. 2005, 119, 325–334. [Google Scholar] [CrossRef]
- Robinson, K.P.; Sim, T.M.; Culloch, R.M.; Bean, T.S.; Cordoba Aguilar, I.; Eisfeld, S.M.; Filan, M.; Haskins, G.N.; Williams, G.; Pierce, G.J. Female reproductive success and calf survival in a North Sea coastal bottlenose dolphin (Tursiops truncatus) population. PLoS ONE 2017, 12, e0185000. [Google Scholar] [CrossRef]
- Mann, J. Establishing trust: Socio-sexual behaviour and the development of male-male bonds among Indian Ocean bottlenose dolphins. In Homosexual Behaviour in Animals: An Evolutionary Perspective; Sommer, V., Vasey, P.L., Eds.; Cambridge University Press: Cambridge, UK, 2006; pp. 107–130. [Google Scholar]
- Connor, R.C.; Smolker, R.S. Habituated dolphins (Tursiops sp.) in western Australia. J. Mammal. 1985, 66, 398–400. [Google Scholar] [CrossRef]
- Welsh, T.; Ward, S.J. Implications of human-animal interactions on mother–calf interactions in a Bottlenose Dolphin (Tursiops truncatus) dyad. J. Zoo Aquar. Res. 2019, 7, 162–169. [Google Scholar]
- Gubbins, C.; McCowan, B.; Lynn, S.; Hooper, S.; Reiss, D. Mother–infant spatial relations in captive bottlenose dolphins, Tursiops truncatus. Mar. Mammal Sci. 1999, 15, 751–765. [Google Scholar] [CrossRef]
- Mann, J.; Smuts, B.B. Natal attraction: Allomaternal care and mothereinfant separations in wild bottlenose dolphins. Anim. Behav. 1998, 55, 1097–1113. [Google Scholar] [CrossRef]
- Miles, J.A.; Herzing, D.L. Underwater analysis of the behavioural development of free-ranging Atlantic spotted dolphin (Stenella frontalis) calves (birth to 4 years of age). Aquat. Mamm. 2003, 29, 363–377. [Google Scholar] [CrossRef]
- Bagley, K.C.; Winship, K.; Bolton, T.; Foerder, P. Personality and Affiliation in a Cooperative Task for Bottlenose Dolphin (Tursiops truncatus) Dyads. Int. J. Comp. Psychol. 2020, 33. [Google Scholar] [CrossRef]
- Connor, R.C.; Smolker, R.; Bejder, L. Synchrony, social behaviour and alliance affiliation in Indian Ocean bottlenose dolphins, Tursiops aduncus. Anim. Behav. 2006, 72, 1371–1378. [Google Scholar] [CrossRef]
- Hill, H.; Guarino, S.; Crandall, S.; Lenhart, E.; Dietrich, S. Young belugas diversify adult beluga (Delphinapterus leucas) behavior. Anim. Behav. Cogn. 2015, 2, 267–284. [Google Scholar] [CrossRef]




| Affiliative Behaviours (N = 9) | |
| Approaching | Dolphin swims towards a conspecific |
| Contact swimming | Two or more dolphins are swimming close to each other with a part of their body in contact |
| Petting/Rubbing | Dolphin rubs its pectoral fin or fluke through an active movement with a conspecific’s pectoral fin or fluke; or fluke to fluke rubbing |
| Slow group swimming | Several dolphin swim together; slow speed |
| Synchronous swimming | Two or more dolphins swimming more or less close to each other with synchronised swimming movements |
| Synchronous breathing | Two or more dolphins breathe in unison |
| Nibbling | Dolphin nibbles conspecific’s body, usually the fluke |
| Follow | Dolphin follows another dolphin |
| Nudge | Dolphin pushes rostrum on another dolphin’s body part |
| Agonistic/aggressive behaviour (N = 9) | |
| Bite/Rake | Dolphin bites or rakes teeth on another dolphin(s) |
| Chase | Dolphin follows a conspecific rapidly and intensively |
| Fast group swimming | At least three dolphins swim in the same direction with a distance of less than one body length between them |
| Pivot dive | Dolphin briefly leaps out of the water re-entering face first, often during chasing between individuals |
| Side mounting | Dolphin side mounts or is side mounted by other dolphin/dolphins |
| Slapping behaviour | Dolphin strikes another dolphin with its head or fluke |
| Hit/Bump | Dolphin charges into another dolphin using its rostrum or flank in a quick manner |
| Jaw clapping | Dolphin opens and shuts its jaws rapidly once or consecutively; a loud clapping sound is made |
| Leaving | Dolphin swims rapidly away from a conspecific |
| Sexual behaviour (N = 5) | |
| Erection | Male dolphin shows penis out of the genital slit |
| Genital inspection | Dolphin inspects the genital region of a conspecific and emits a burst pulsed sound; no physical contact is observed |
| Genital rubbing | Dolphin rubs its genital area on conspecifics |
| Mating | Two dolphins in ventral contact with intromission observed |
| Penis insertion | Dolphin inserts its penis into blow hole/anus of a male or female conspecific, but not in its genital area |
| Maternal behaviour (N = 5) | |
| Nurturant behaviour | A mother carries its calf away from danger |
| Bumping/genitals | The calf swims underneath its mother and bumps several times into her ventral/genital areas |
| Nursing | Calf takes milk from its mother |
| Echelon swimming | Calf is in close proximity of its mother’s mid-lateral flank |
| Infant swimming | Calf swims underneath its mothers’ peduncle |
| Solitary behaviours (N = 19) | |
| Arching | Dolphin bends head and tail ventrally |
| Belly up swimming | Dolphin swims ventral side up for more than five seconds |
| Bubbles | Dolphin expels air from its blowhole, forming a line of tiny bubbles |
| Carrying objects | Dolphin carries objects with/on body parts |
| Circular swimming | Dolphin swims in clockwise or counterclockwise direction in large and regular circles |
| Fast solitary swimming | Dolphin swims fast, making waves on the surface, or making riddles on its skin |
| Fluke out of the water | Dolphin hangs vertically in the water, head downward, the tail and the peduncle protruding above water |
| Head-up swim | Dolphin has its eyes, or the entire head, above the water surface while swimming slowly forward, toward the point of interest |
| Jumping | Dolphin jumps with a high curvature between head and body; the dolphin jumps and lands in the same place and checks the surrounding environment; the jump is higher than longer |
| Logging | Dolphin floats at the surface, back and dorsal fin above water and may drift or remains motionless while logging |
| Looking/surface | Dolphin floats sideways at the surface with one eye above the water to observe; this behaviour may be brief or prolonged, and the dolphin may drift |
| Regurgitating | Dolphin brings swallowed food up again to the mouth |
| Resting | Dolphin rests motionless, breathing regularly or it swims slowly and steadily |
| Rolling | Dolphin’s body is rotated 360° on the longitudinal axis to either side of the dolphin |
| Rubbing/habitat | Dolphin rubs its body on pool walls, gate or other parts of the environment. |
| Side swimming | Forward progress in a 90-degree rotation from the dorsal position, orienting one pectoral fin upward and the other downward |
| Slow solitary swimming | Dolphin swims alone; slow speed |
| Spy-hopping | Dolphin is vertical in the water and propels itself out vertically usually as far as the pectoral fins, with the eyes directed to a point above the water surface, and then descends again vertically; often the movement can be repeated several times consecutively |
| Vertical standing | Dolphin hangs/suspends itself vertically with its head up or down in mid-water |
| Play/explorative behaviours (N = 8) | |
| Exploration | Dolphin investigates or explores habitat (gates, sides and bottom of the pool) or non-enrichment objects (such as tree leaves) by closely looking at them or touching them |
| Exploration/enrichment | Dolphin investigates or explores enrichment objects by closely looking or touching them |
| Social play | Dolphin plays with two or more dolphins (including social bubble play) |
| Solitary play | One dolphin plays (including solitary bubble play) |
| Object circle | Dolphin begins swimming around an object in wide circles, in other words the object is now included in the swimming circle |
| Play with object | Dolphin carries a human-made object by using its rostrum, the fins or the melon, the fluke, the mouth, passing and slightly touching an object, balancing/dribbling/catching/throwing and catching/pushing or pulling an object with its rostrum, pressing it under water/rolling it on the bottom of the pool by using the rostrum or the body and holding it in the rostrum while swimming |
| Play with environment | Dolphin touches, rubs, scratches on, pulls, pushes, splashes, etc., on environmental items |
| Rough play | Two or more dolphins display intense plays, might include biting, chasing, pivoting |
| Time of Day | Observer Location | Number of Individuals | Sex | Age | Social Grouping | Presence of Leading Male | Activity before or after Session | Presence of Visitors | Enrichment | |
|---|---|---|---|---|---|---|---|---|---|---|
| Total behavioural diversity | - | Increased with number of individuals | - | Juveniles + adults/adults | - | In absence/in presence | - | - | - | |
| Affiliative behaviours diversity | Morning Midday | - | - | - | - | - | - | - | ||
| Agonistic behaviours diversity | - | Under + above observations/above | Increased with number of individuals | Males/females or both sexes | Juveniles + adults/adults | - | In absence/in presence | - | - | Presence/absence Nutritional/cognitive |
| Solitary behaviours diversity | - | Above water > under or both | - | - | - | - | - | - | - | - |
| Play/exploratory behaviours diversity | - | Under + above water/under or above water | - | Males or both sexes/females | Juveniles + adults/adults | - | - | - | In absence/in presence | Presence/absence Sensorial, structural, cognitive, mixed. |
| Sexual behaviours diversity | - | - | - | Both sexes/females | - | Separation/together | - | - | - | - |
| Maternal behaviours diversity | - | - | Increased with number of individuals | Number of mother-calf pairs | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delfour, F.; Vaicekauskaite, R.; García-Párraga, D.; Pilenga, C.; Serres, A.; Brasseur, I.; Pascaud, A.; Perlado-Campos, E.; Sánchez-Contreras, G.J.; Baumgartner, K.; et al. Behavioural Diversity Study in Bottlenose Dolphin (Tursiops truncatus) Groups and Its Implications for Welfare Assessments. Animals 2021, 11, 1715. https://doi.org/10.3390/ani11061715
Delfour F, Vaicekauskaite R, García-Párraga D, Pilenga C, Serres A, Brasseur I, Pascaud A, Perlado-Campos E, Sánchez-Contreras GJ, Baumgartner K, et al. Behavioural Diversity Study in Bottlenose Dolphin (Tursiops truncatus) Groups and Its Implications for Welfare Assessments. Animals. 2021; 11(6):1715. https://doi.org/10.3390/ani11061715
Chicago/Turabian StyleDelfour, Fabienne, Ruta Vaicekauskaite, Daniel García-Párraga, Cristina Pilenga, Agathe Serres, Isabelle Brasseur, Ana Pascaud, Enrique Perlado-Campos, Guillermo J. Sánchez-Contreras, Katrin Baumgartner, and et al. 2021. "Behavioural Diversity Study in Bottlenose Dolphin (Tursiops truncatus) Groups and Its Implications for Welfare Assessments" Animals 11, no. 6: 1715. https://doi.org/10.3390/ani11061715
APA StyleDelfour, F., Vaicekauskaite, R., García-Párraga, D., Pilenga, C., Serres, A., Brasseur, I., Pascaud, A., Perlado-Campos, E., Sánchez-Contreras, G. J., Baumgartner, K., & Monreal-Pawlowsky, T. (2021). Behavioural Diversity Study in Bottlenose Dolphin (Tursiops truncatus) Groups and Its Implications for Welfare Assessments. Animals, 11(6), 1715. https://doi.org/10.3390/ani11061715







