Summer Is Coming! Tackling Ocean Warming in Atlantic Salmon Cage Farming
Abstract
:Simple Summary
Abstract
1. Introduction
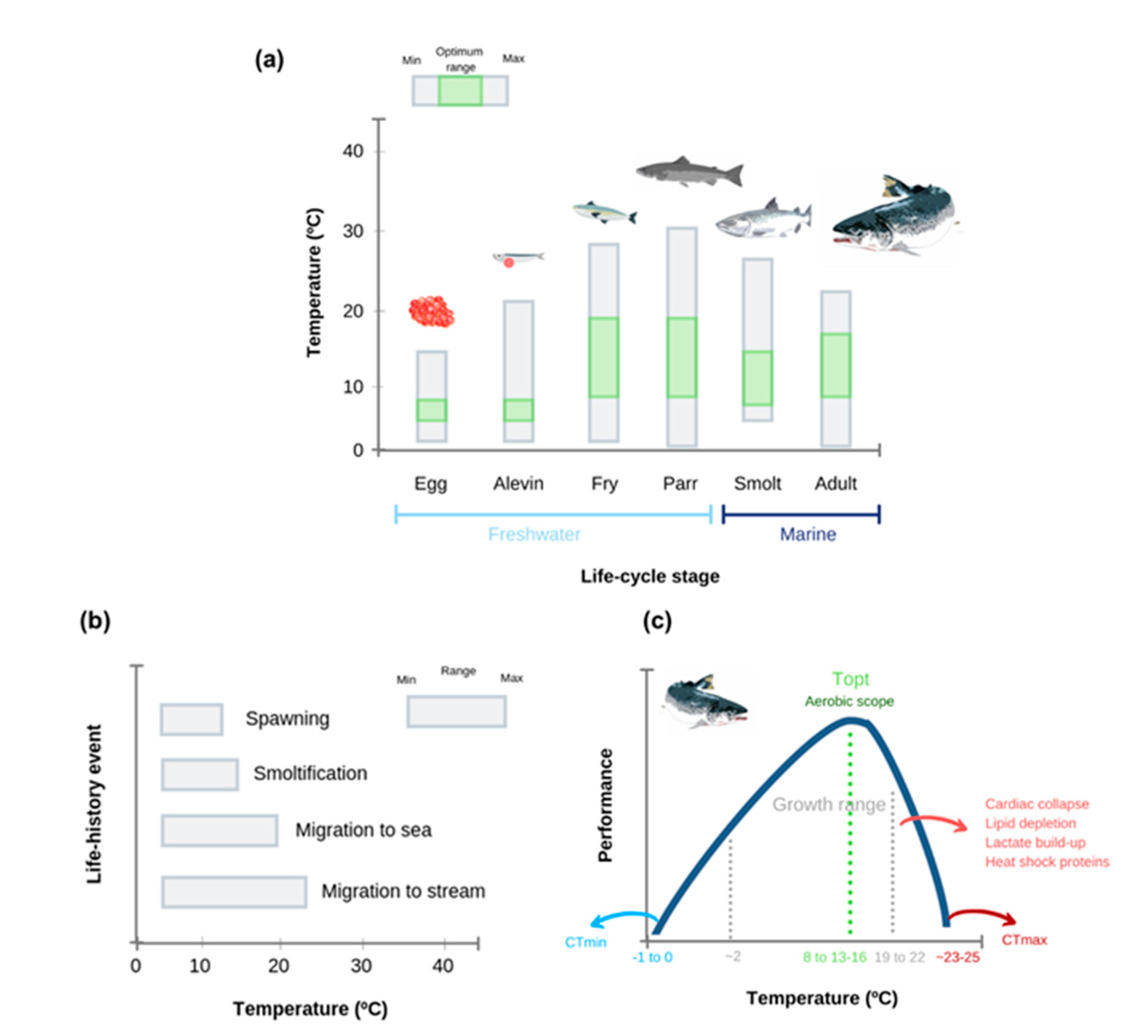
2. Adult Atlantic Salmon Thermal Tolerance and Physiological Responses to Heat Stress
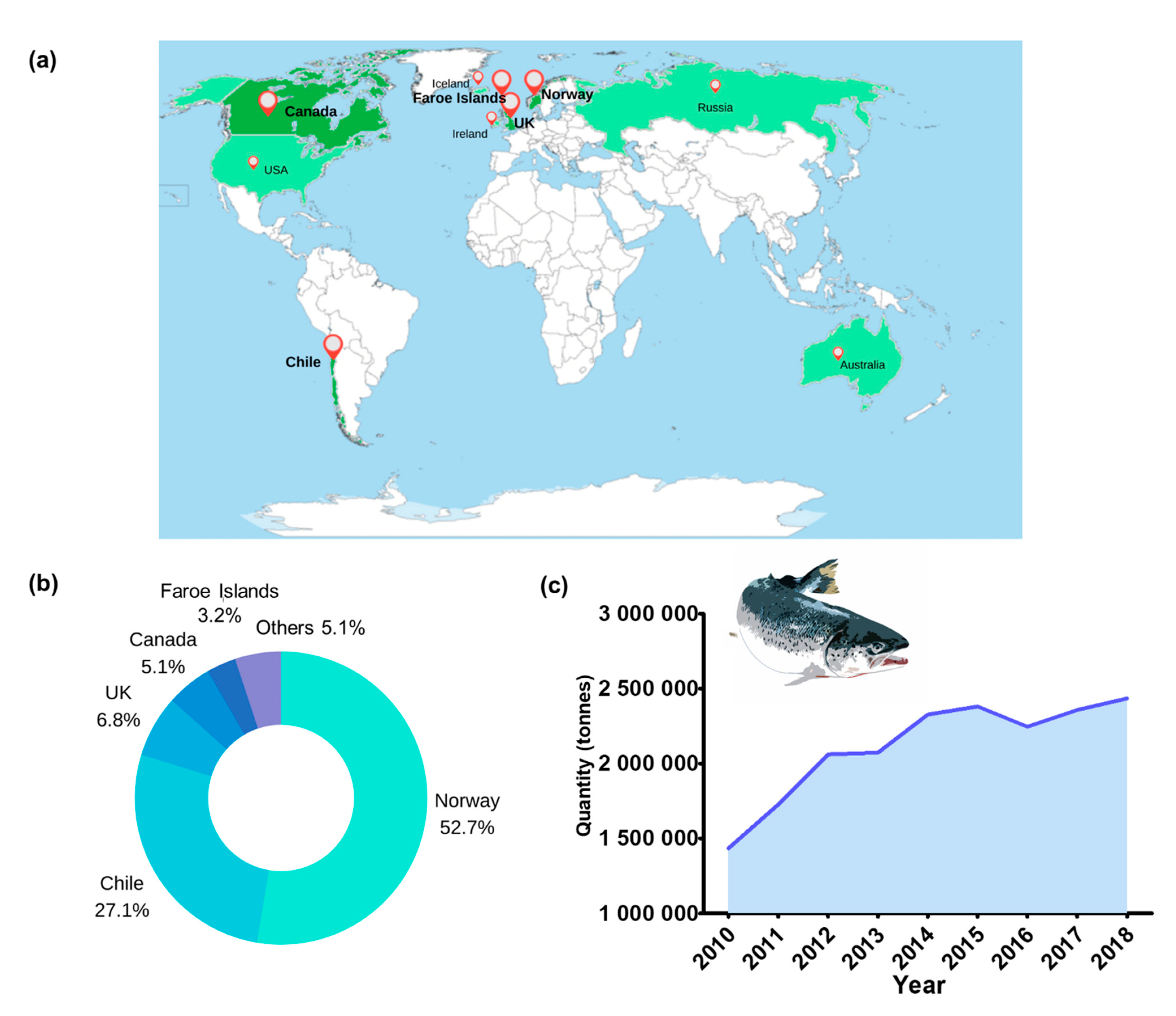
3. Impacts of Heat Stress on Atlantic Salmon Cage Farming
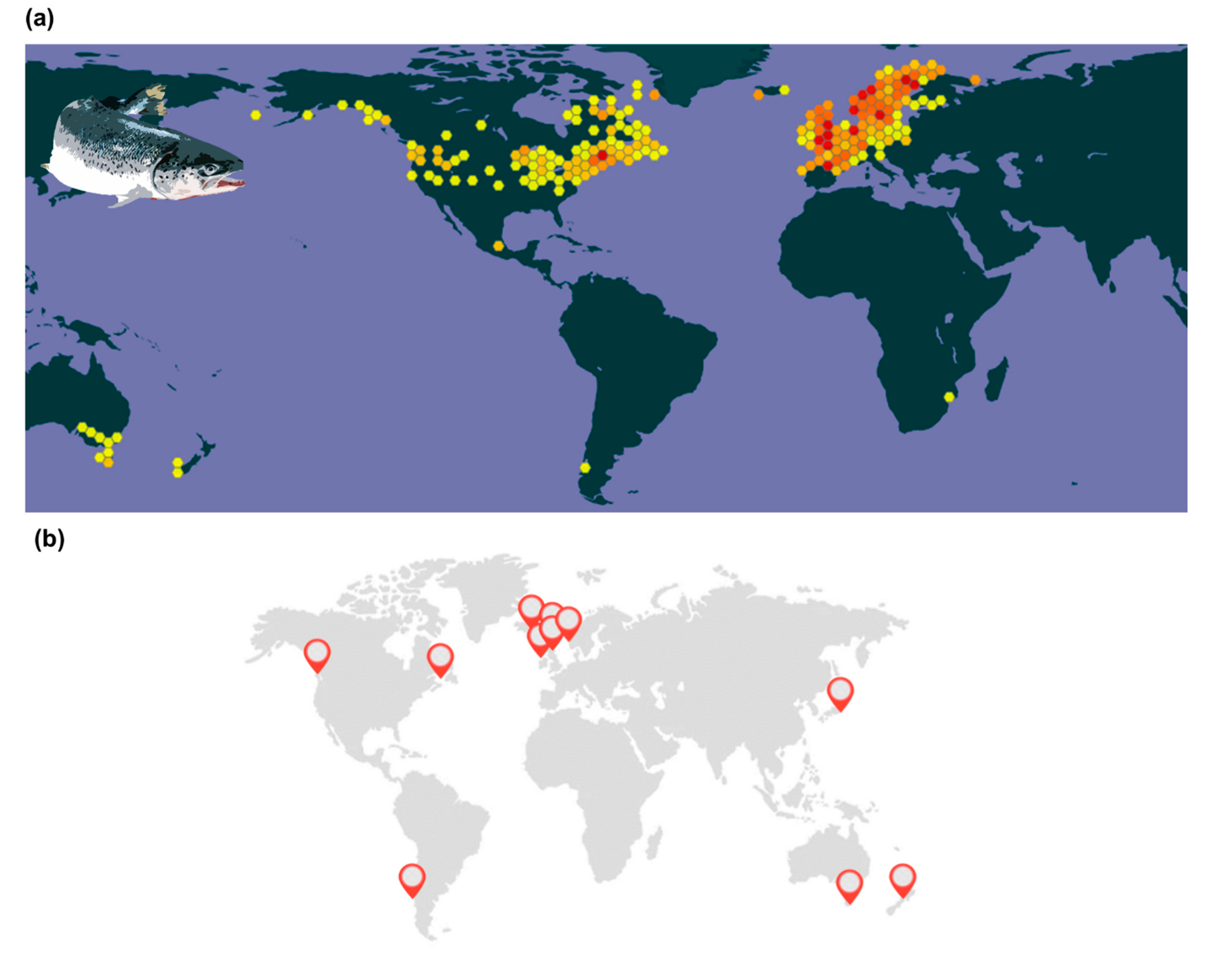
4. Accessing Local Adaptation and Phenotypic Plasticity to Foster Temperature-Dependent Selection of Atlantic Salmon
5. The Role of the Salmon Breeding Industry
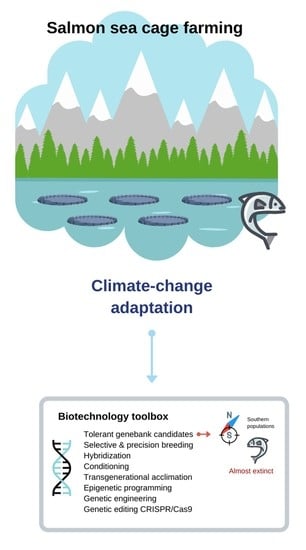
6. The Way Forward
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture 2020. Sustainability in Action; FAO: Rome, Italy, 2020. [Google Scholar]
- Asche, F.; Misund, B.; Oglend, A. The case and cause of salmon price volatility. Mar. Resour. Econ. 2019, 34, 23–38. [Google Scholar] [CrossRef]
- Overton, K.; Dempster, T.; Oppedal, F.; Kristiansen, T.S.; Gismervik, K.; Stien, L.H. Salmon lice treatments and salmon mortality in Norwegian aquaculture: A review. Rev. Aquac. 2019, 11, 1398–1417. [Google Scholar] [CrossRef] [Green Version]
- Pike, A. Sea lice—Major pathogens of farmed atlantic salmon. Parasitol. Today 1989, 5, 291–297. [Google Scholar] [CrossRef]
- Szekeres, P.; Eliason, E.; Lapointe, D.; Donaldson; Brownscombe, J.; Cooke, S. On the neglected cold side of climate change and what it means to fish. Clim. Res. 2016, 69, 239–245. [Google Scholar] [CrossRef]
- Callaway, R.; Shinn, A.P.; Grenfell, S.; Bron, J.E.; Burnell, G.; Cook, E.J.; Crumlish, M.; Culloty, S.; Davidson, K.; Ellis, R.; et al. Review of climate change impacts on marine aquaculture in the UK and Ireland. Aquat. Conserv. Mar. Freshw. Ecosyst. 2012, 22, 389–421. [Google Scholar] [CrossRef]
- Jensen, L.B.; Boltana, S.; Obach, A.; McGurk, C.; Waagbo, R.; MacKenzie, S. Investigating the underlying mechanisms of temperature-related skin diseases in Atlantic salmon, Salmo salar L., as measured by quantitative histology, skin transcriptomics and composition. J. Fish Dis. 2015, 38, 977–992. [Google Scholar] [CrossRef] [Green Version]
- Benedicenti, O.; Pottinger, T.; Collins, C.; Secombes, C.J. Effects of temperature on amoebic gill disease development: Does it play a role? J. Fish Dis. 2019, 42, 1241–1258. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, N.J.; Reynolds, J.D.; Moore, J.W.; Neff, B.D. Salmon in clear and present danger. Science 2019, 366. [Google Scholar] [CrossRef]
- Wade, N.M.; Clark, T.; Maynard, B.T.; Atherton, S.; Wilkinson, R.J.; Smullen, R.P.; Taylor, R.S. Effects of an unprecedented summer heatwave on the growth performance, flesh colour and plasma biochemistry of marine cage-farmed Atlantic salmon (Salmo salar). J. Therm. Biol. 2019, 80, 64–74. [Google Scholar] [CrossRef]
- Oppedal, F.; Dempster, T.; Stien, L.H. Environmental drivers of Atlantic salmon behaviour in sea-cages: A review. Aquaculture 2011, 311, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Oliver, E.C.J.; Burrows, M.T.; Donat, M.; Gupta, A.S.; Alexander, L.V.; Perkins-Kirkpatrick, S.E.; Benthuysen, J.A.; Hobday, A.J.; Holbrook, N.; Moore, P.; et al. Projected Marine Heatwaves in the 21st Century and the Potential for Ecological Impact. Front. Mar. Sci. 2019, 6. [Google Scholar] [CrossRef] [Green Version]
- Sapin, R. Newfoundland Fisheries Minister Suspends Mowi’s Northern Harvest Salmon Farming Licenses over “Mass Mortality”. Available online: https://www.intrafish.com/news/newfoundland-fisheries-minister-suspends-mowis-northern-harvest-salmon-farming-licenses-over-mass-mortality/2-1-687631 (accessed on 30 January 2021).
- Sapin, R. Mowi Calls for Climate Change Action after Massive Farmed Salmon Die-Off in Newfoundland. Available online: https://www.intrafish.com/aquaculture/mowi-calls-for-climate-change-action-after-massive-farmed-salmon-die-off-in-newfoundland/2-1-679108 (accessed on 30 January 2021).
- Burke, H.; Gardner, I.; Farrell, A.P. A Review of the 2019 Newfoundland and Labrador South Coast Cultured Atlantic Salmon Mortality Event. Department of Fisheries and Land Resources, Government of Newfoundland and Labrador, Special Studies and Reports. 2020. Available online: https://www.gov.nl.ca/ffa/files/publications-pdf-2019-salmon-review-final-report.pdf; (accessed on 30 January 2021).
- Crozier, L.G.; Hutchings, J. Plastic and evolutionary responses to climate change in fish. Evol. Appl. 2014, 7, 68–87. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, N.J.; Farrell, A.P.; Heath, J.W.; Neff, B.D. Adaptive potential of a Pacific salmon challenged by climate change. Nat. Clim. Chang. 2014, 5, 163–166. [Google Scholar] [CrossRef]
- Schulte, P.M. The effects of temperature on aerobic metabolism: Towards a mechanistic understanding of the responses of ectotherms to a changing environment. J. Exp. Biol. 2015, 218, 1856–1866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delong, J.P.; Gibert, J.P.; Luhring, T.M.; Bachman, G.; Reed, B.; Neyer, A.; Montooth, K.L. The combined effects of reactant kinetics and enzyme stability explain the temperature dependence of metabolic rates. Ecol. Evol. 2017, 7, 3940–3950. [Google Scholar] [CrossRef]
- Morley, J.W.; Selden, R.L.; Latour, R.J.; Frölicher, T.L.; Seagraves, R.J.; Pinsky, M.L. Projecting shifts in thermal habitat for 686 species on the North American continental shelf. PLoS ONE 2018, 13, e0196127. [Google Scholar] [CrossRef] [Green Version]
- IPCC. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Pachauri, R.K., Meyer, L.A., Eds.; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- Tokarska, K.B.; Gillett, N.P. Cumulative carbon emissions budgets consistent with 1.5 °C global warming. Nat. Clim. Chang. 2018, 8, 296–299. [Google Scholar] [CrossRef] [Green Version]
- IPCC. Summary for Policymakers—IPCC Special Report on the Ocean and Cryosphere in a Changing Climate; Pörtner, H.O., Roberts, D.C., Masson-Delmotte, V., Zhai, P., Tignor, M., Poloczanska, E., Mintenbeck, K., Nicolai, M., Okem, A., Petzold, J., et al., Eds.; IPCC: Geneva, Switzerland, 2019. [Google Scholar]
- Jones, P.D.; Parker, D.E.; Osborn, T.J.; Briffa, K.R. Global and Hemispheric Temperature Anomalies: Land and Marine Instrumental Records (1850–2015); Carbon Dioxide Information Analysis Center (CDIAC), Oak Ridge National Laboratory (ORNL): Oak Ridge, TN, USA, 2016. [Google Scholar] [CrossRef]
- Hobday, A.J.; Alexander, L.V.; Perkins, S.E.; Smale, D.; Straub, S.C.; Oliver, E.C.; Benthuysen, J.A.; Burrows, M.T.; Donat, M.; Feng, M.; et al. A hierarchical approach to defining marine heatwaves. Prog. Oceanogr. 2016, 141, 227–238. [Google Scholar] [CrossRef] [Green Version]
- Smale, D.A.; Wernberg, T.; Oliver, E.C.J.; Thomsen, M.; Harvey, B.P.; Straub, S.C.; Burrows, M.T.; Alexander, L.V.; Benthuysen, J.A.; Donat, M.G.; et al. Marine heatwaves threaten global biodiversity and the provision of ecosystem services. Nat. Clim. Chang. 2019, 9, 306–312. [Google Scholar] [CrossRef] [Green Version]
- Bricknell, I.R.; Birkel, S.D.; Brawley, S.H.; Van Kirk, T.; Hamlin, H.J.; Capistrant-Fossa, K.; Huguenard, K.; Van Walsum, G.P.; Liu, Z.L.; Zhu, L.; et al. Resilience of cold water aquaculture: A review of likely scenarios as climate changes in the Gulf of Maine. Rev. Aquac. 2021, 13, 460–503. [Google Scholar] [CrossRef]
- Viglione, G. Fevers are plaguing the oceans—and climate change is making them worse. Nat. Cell Biol. 2021, 593, 26–28. [Google Scholar] [CrossRef]
- Laufkötter, C.; Zscheischler, J.; Frölicher, T.L. High-impact marine heatwaves attributable to human-induced global warming. Science 2020, 369, 1621–1625. [Google Scholar] [CrossRef]
- Filbee-Dexter, K.; Wernberg, T.; Grace, S.P.; Thormar, J.; Fredriksen, S.; Narvaez, C.N.; Feehan, C.J.; Norderhaug, K.M. Marine heatwaves and the collapse of marginal North Atlantic kelp forests. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Jensen, A.J.; Karlsson, S.; Fiske, P.; Hansen, L.P.; Østborg, G.M.; Hindar, K. Origin and life history of Atlantic salmon (Salmo salar) near their northernmost oceanic limit. Can. J. Fish. Aquat. Sci. 2014, 71, 1740–1746. [Google Scholar] [CrossRef] [Green Version]
- Mowi. Salmon Farming. Industry Handbook; Mowi: Bergen, Norway, 2019. [Google Scholar]
- Nicola, G.G.; Elvira, B.; Jonsson, B.; Ayllón, D.; Almodóvar, A. Local and global climatic drivers of Atlantic salmon decline in southern Europe. Fish. Res. 2018, 198, 78–85. [Google Scholar] [CrossRef]
- Webb, B.W.; Walsh, A.J. Changing UK river temperatures and their impact on fish populations. In Hydrology: Science & Practice for the 21st Century, Proceedings of the British Hydrological Society International Conference, London, UK, 01 January 2004; British Hydrological Society: London, UK, 2004; Volume 2, pp. 117–191. [Google Scholar]
- Williams, J.; Isaak, D.J.; Imhof, J.; Hendrickson, A.D.; McMillan, J. Cold-Water Fishes and Climate Change in North America. In Reference Module in Earth Systems and Environmental Sciences; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Bradbury, C.; Roberge, M.H.M.; Minns, C.K. Life History Characteristics of Freshwater Fishes Occurring in Newfoundland and Labrador, with Major Emphasis on Lake Habitat Requirements. In Canadian Manuscript Report of Fisheries and Aquatic Sciences; Fisheries and Oceans Canada 1979: Ottawa, ON, Canada, 1999. [Google Scholar]
- Elliott, S.R.; Coe, T.A.; Helfield, J.M.; Naiman, R.J. Spatial variation in environmental characteristics of Atlantic salmon (Salmo salar) rivers. Can. J. Fish. Aquat. Sci. 1998, 55, 267–280. [Google Scholar] [CrossRef]
- Anttila, K.; Couturier, C.S.; Øverli, Ø.; Johnsen, A.; Marthinsen, G.; Nilsson, G.E.; Farrell, A.P. Atlantic salmon show capability for cardiac acclimation to warm temperatures. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jensen, A.J.; Zubchenko, A.; Hvidsten, N.A.; Johnsen, B.O.; Kashin, E.; Næsje, T.F. A Five Year Study of Atlantic Salmon in Two Russian and Two Norwegian Rivers. NINA•NIKU Project Report 008; Foundation for Nature Research and Cultural Heritage Research: Trondheim, Norway, 1998; pp. 1–38. [Google Scholar]
- DFO. Temperature threshold to define management strategies for Atlantic salmon (Salmo salar) fisheries under environmen-tally stressful conditions. DFO Can. Sci. Advis. Sec. Sci. Advis. Rep. 2012, 19, 1–17. [Google Scholar]
- Stehfest, K.M.; Carter, C.G.; McAllister, J.D.; Ross, J.D.; Semmens, J.M. Response of Atlantic salmon Salmo salar to temperature and dissolved oxygen extremes established using animal-borne environmental sensors. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gollock, M.J.; Currie, S.; Petersen, L.H.; Gamperl, A.K. Cardiovascular and haematological responses of Atlantic cod (Gadus morhua) to acute temperature increase. J. Exp. Biol. 2006, 209, 2961–2970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olusanya, H.O.; Jong, M.V.Z.D. Assessing the vulnerability of freshwater fishes to climate change in Newfoundland and Labrador. PLoS ONE 2018, 13, e0208182. [Google Scholar] [CrossRef]
- Mueter, F.J.; Peterman, R.M.; Pyper, B.J. Opposite effects of ocean temperature on survival rates of 120 stocks of Pacific salmon (Oncorhynchus spp.) in northern and southern areas. Can. J. Fish. Aquat. Sci. 2002, 59, 456–463. [Google Scholar] [CrossRef]
- Swansburg, E.; Chaput, G.; Moore, D.; Caissie, D.; El-Jabi, N. Size variability of juvenile Atlantic salmon: Links to environmental conditions. J. Fish Biol. 2002, 61, 661–683. [Google Scholar] [CrossRef]
- Quinn, T.P. The Behavior and Ecology of Pacific Salmon and Trout; University of Washington Press: Seattle, WA, USA, 2018. [Google Scholar]
- Breau, C.; Cunjak, R.A.; Peake, S.J. Behaviour during elevated water temperatures: Can physiology explain movement of ju-venile Atlantic salmon to cool water? J. Anim. Ecol. 2011, 80, 844–853. [Google Scholar] [CrossRef] [PubMed]
- Wedemeyer, G.A.; Saunders, R.L.; Clarke, W.C. Environmental factors affecting smoltification and early marine survival of anadromous salmonids. Mar. Fish. Rev. 1980, 42, 1–14. [Google Scholar]
- McCormick, S.D.; Hansen, L.P.; Quinn, T.P.; Saunders, R.L. Movement, migration, and smolting of Atlantic salmon (Salmo salar). Can. J. Fish. Aquat. Sci. 1998, 55, 77–92. [Google Scholar] [CrossRef]
- Crossin, G.T.; Hinch, S.G.; Cooke, S.J.; Welch, D.W.; Patterson, D.A.; Jones, S.R.; Lotto, A.G.; Leggatt, R.A.; Mathes, M.T.; Shrimpton, J.M.; et al. Exposure to high temperature influences the behaviour, physiology, and survival of sockeye salmon during spawning migration. Can. J. Zool. 2008, 86, 127–140. [Google Scholar] [CrossRef] [Green Version]
- Farrell, A.P.; Hinch, S.G.; Cooke, S.J.; Patterson, D.A.; Crossin, G.T.; Lapointe, M.; Mathes, M.T. Pacific salmon in hot water: Applying aerobic scope models and biotelemetry to predict the success of spawning migrations. Physiol. Biochem. Zool. 2008, 81, 697–709. [Google Scholar] [CrossRef] [Green Version]
- Gamperl, A.K.; Ajiboye, O.O.; Zanuzzo, F.S.; Sandrelli, R.M.; Peroni, E.D.F.C.; Beemelmanns, A. The impacts of increasing temperature and moderate hypoxia on the production characteristics, cardiac morphology and haematology of Atlantic Salmon (Salmo salar). Aquaculture 2020, 519. [Google Scholar] [CrossRef]
- Leeuwis, R.H.; Nash, G.W.; Sandrelli, R.M.; Zanuzzo, F.S.; Gamperl, A.K. The environmental tolerances and metabolic physiology of sablefish (Anoplopoma fimbria). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2019, 231, 140–148. [Google Scholar] [CrossRef]
- Penney, C.M.; Nash, G.W.; Gamperl, A.K. Cardiorespiratory responses of seawater-acclimated adult Arctic char (Salvelinus alpinus) and Atlantic salmon (Salmo salar) to an acute temperature increase. Can. J. Fish. Aquat. Sci. 2014, 71, 1096–1105. [Google Scholar] [CrossRef]
- Zanuzzo, F.S.; Bailey, J.A.; Garber, A.F.; Gamperl, A.K. The acute and incremental thermal tolerance of Atlantic cod (Gadus morhua) families under normoxia and mild hypoxia. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2019, 233, 30–38. [Google Scholar] [CrossRef]
- Alabaster, J.S.; Calamari, D.; Dethlefsen, V.; Konemann, H.; Lloyd, R.; Solbé, J.F.; Alabaster, J.S.; Calamari, D.; Dethlefsen, V.; Konemann, H.; et al. Water quality criteria for European freshwater fish. Chem. Ecol. 1988, 3, 165–253. [Google Scholar] [CrossRef]
- Dwyer, W.P.; Piper, R.G. Atlantic salmon growth efficiency as affected by temperature. Progress. Fish Cult. 1987, 49, 57–59. [Google Scholar] [CrossRef]
- Elliott, J.M.; Hurley, M. A functional model for maximum growth of Atlantic Salmon parr, Salmo salar, from two populations in northwest England. Funct. Ecol. 1997, 11, 592–603. [Google Scholar] [CrossRef]
- Hevrøy, E.M.; Hunskår, C.; De Gelder, S.; Shimizu, M.; Waagbo, R.; Breck, O.; Takle, H.; Sussort, S.; Hansen, T. GH–IGF system regulation of attenuated muscle growth and lipolysis in Atlantic salmon reared at elevated sea temperatures. J. Comp. Physiol. B 2012, 183, 243–259. [Google Scholar] [CrossRef] [PubMed]
- Vikeså, V. Regulation of Appetite and Growth of Atlantic Salmon (Salmo salar L.) and Effect of Water Oxygen, Temperature and Dietary Energy. Ph.D. Thesis, The University of Bergen, Bergen, Norway, 27 June 2017. [Google Scholar]
- Hvas, M.; Folkedal, O.; Imsland, A.; Oppedal, F. The effect of thermal acclimation on aerobic scope and critical swimming speed in Atlantic salmon Salmo salar. J. Exp. Biol. 2017, 220, 2757–2764. [Google Scholar] [CrossRef] [Green Version]
- Anttila, K.; Jã¸rgensen, S.M.; Casselman, M.T.; Timmerhaus, G.; Farrell, A.P.; Takle, H.; Jørgensen, S.M. Association between swimming performance, cardiorespiratory morphometry, and thermal tolerance in Atlantic salmon (Salmo salar L.). Front. Mar. Sci. 2014, 1. [Google Scholar] [CrossRef] [Green Version]
- Pörtner, H.-O.; Bock, C.; Mark, F.C. Oxygen- and capacity-limited thermal tolerance: Bridging ecology and physiology. J. Exp. Biol. 2017, 220, 2685–2696. [Google Scholar] [CrossRef] [Green Version]
- Pörtner, H.-O.; Farrell, A.P. Ecology: Physiology and Climate Change. Science 2008, 322, 690–692. [Google Scholar] [CrossRef]
- Jutfelt, F.; Norin, T.; Ern, R.; Overgaard, J.; Wang, T.; McKenzie, D.; Lefevre, S.; Nilsson, G.E.; Metcalfe, N.; Hickey, A.J.R.; et al. Oxygen- and capacity-limited thermal tolerance: Blurring ecology and physiology. J. Exp. Biol. 2018, 221. [Google Scholar] [CrossRef] [Green Version]
- Clark, T.D.; Sandblom, E.; Jutfelt, F. Aerobic scope measurements of fishes in an era of climate change: Respirometry, relevance and recommendations. J. Exp. Biol. 2013, 216, 2771–2782. [Google Scholar] [CrossRef] [Green Version]
- Ern, R. A mechanistic oxygen- and temperature-limited metabolic niche framework. Philos. Trans. R. Soc. B Biol. Sci. 2019, 374. [Google Scholar] [CrossRef]
- Madeira, D.; Vinagre, C.; Costa, P.; Diniz, M. Histopathological alterations, physiological limits, and molecular changes of juvenile Sparus aurata in response to thermal stress. Mar. Ecol. Prog. Ser. 2014, 505, 253–266. [Google Scholar] [CrossRef]
- Haverinen, J.; Vornanen, M. Reduced ventricular excitability causes atrioventricular block and depression of fish heart rate in fish at critically high temperatures. J. Exp. Biol. 2020, 223. [Google Scholar] [CrossRef]
- Christen, F.; Dufresne, F.; LeDuc, G.A.; Dupont-Cyr, B.; Vandenberg, G.W.; Le François, N.R.; Tardif, J.-C.; Lamarre, S.G.; Blier, P.U. Thermal tolerance and fish heart integrity: Fatty acids profiles as predictors of species resilience. Conserv. Physiol. 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Gerber, L.; Clow, K.A.; Mark, F.C.; Gamperl, A.K. Improved mitochondrial function in salmon (Salmo salar) following high temperature acclimation suggests that there are cracks in the proverbial ‘ceiling’. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Andreassen, A.H.; Hall, P.; Khatibzadeh, P.; Jutfelt, F.; Kermen, F. Neural dysfunction at the upper thermal limit in the zebrafish. bioRxiv 2020. [Google Scholar] [CrossRef]
- Gerber, L.; Clow, K.A.; Gamperl, A.K. Acclimation to warm temperatures has important implications for mitochondrial function in Atlantic salmon (Salmo salar). J. Exp. Biol. 2021, 224. [Google Scholar] [CrossRef]
- Beemelmanns, A.; Ribas, L.; Anastasiadi, D.; Moraleda-Prados, J.; Zanuzzo, F.S.; Rise, M.L.; Gamperl, A.K. DNA Methylation dynamics in Atlantic salmon (Salmo salar) challenged with high temperature and moderate hypoxia. Front. Mar. Sci. 2021, 7. [Google Scholar] [CrossRef]
- Beemelmanns, A.; Zanuzzo, F.S.; Xue, X.; Sandrelli, R.M.; Rise, M.L.; Gamperl, A.K. The transcriptomic responses of Atlantic salmon (Salmo salar) to high temperature stress alone, and in combination with moderate hypoxia. BMC Genom. 2021, 22, 1–33. [Google Scholar] [CrossRef]
- Nuez-Ortín, W.G.; Carter, C.G.; Nichols, P.D.; Cooke, I.R.; Wilson, R. Liver proteome response of pre-harvest Atlantic salmon following exposure to elevated temperature. BMC Genom. 2018, 19. [Google Scholar] [CrossRef] [Green Version]
- Solomon, D.J.; Lightfoot, G.W. Using Science to Create a Better Place. The Thermal Biology of Brown Trout and Atlantic Salmon. Science Report; Environment Agency: Bristol, UK, 2008. [Google Scholar]
- Elliott, J.M. Temperature requirements of Atlantic salmon Salmo salar, brown trout Salmo trutta and Arctic charr Salvelinus alpinus: Predicting the effects of climate change. J. Fish Biol. 2010, 77, 1793–1817. [Google Scholar] [CrossRef]
- Lund, S.G.; Caissie, D.; Cunjak, A.R.; Vijayan, M.M.; Tufts, B.L. The effects of environmental heat stress on heat-shock mRNA and protein expression in Miramichi Atlantic salmon (Salmo salar) parr. Can. J. Fish. Aquat. Sci. 2002, 59, 1553–1562. [Google Scholar] [CrossRef]
- Handeland, S.O.; Imsland, A.K.; Stefansson, S.O. The effect of temperature and fish size on growth, feed intake, food conversion efficiency and stomach evacuation rate of Atlantic salmon post-smolts. Aquaculture 2008, 283, 36–42. [Google Scholar] [CrossRef]
- Kullgren, A.; Jutfelt, F.; Fontanillas, R.; Sundell, K.; Samuelsson, L.; Wiklander, K.; Kling, P.; Koppe, W.; Larsson, D.J.; Björnsson, B.T.; et al. The impact of temperature on the metabolome and endocrine metabolic signals in Atlantic salmon (Salmo salar). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2013, 164, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Hevrøy, E.; Waagbo, R.; Torstensen, B.; Takle, H.; Stubhaug, I.; Jørgensen, S.; Torgersen, T.; Tvenning, L.; Susort, S.; Breck, O.; et al. Ghrelin is involved in voluntary anorexia in Atlantic salmon raised at elevated sea temperatures. Gen. Comp. Endocrinol. 2012, 175, 118–134. [Google Scholar] [CrossRef]
- Burt, K.; Hamoutene, D.; Mabrouk, G.; Lang, C.; Puestow, T.; Drover, D.; Losier, R.; Page, F.H. Environmental conditions and occurrence of hypoxia within production cages of Atlantic salmon on the south coast of Newfoundland. Aquac. Res. 2011, 43, 607–620. [Google Scholar] [CrossRef]
- Solstorm, D.; Oldham, T.M.W.; Solstorm, F.; Klebert, P.; Stien, L.H.; Vågseth, T.; Oppedal, F. Dissolved oxygen variability in a commercial sea-cage exposes farmed Atlantic salmon to growth limiting conditions. Aquaculture 2018, 486, 122–129. [Google Scholar] [CrossRef]
- Vikeså, V.; Nankervis, L.; Hevrøy, E.M. Appetite, metabolism and growth regulation in Atlantic salmon (Salmo salar L.) exposed to hypoxia at elevated seawater temperature. Aquac. Res. 2017, 48, 4086–4101. [Google Scholar] [CrossRef]
- Johansson, D.; Ruohonen, K.; Kiessling, A.; Oppedal, F.; Stiansen, J.-E.; Kelly, M.; Juell, J.-E. Effect of environmental factors on swimming depth preferences of Atlantic salmon (Salmo salar L.) and temporal and spatial variations in oxygen levels in sea cages at a fjord site. Aquaculture 2006, 254, 594–605. [Google Scholar] [CrossRef]
- Dempson, J.B.; O’Connell, M.F.; Cochrane, N.M. Potential impact of climate warming on recreational fishing opportunities for Atlantic salmon, Salmo salar L., in Newfoundland, Canada. Fish. Manag. Ecol. 2001, 8, 69–82. [Google Scholar] [CrossRef]
- Richter, A.; Kolmes, S.A. Maximum temperature limits for Chinook, Coho, and Chum salmon, and steelhead trout in the Pacific Northwest. Rev. Fish. Sci. 2005, 13, 23–49. [Google Scholar] [CrossRef]
- Valiente, A.G.; Juanes, F.; Garcia-Vazquez, E. Increasing regional temperatures associated with delays in Atlantic salmon sea-run timing at the southern edge of the European distribution. Trans. Am. Fish. Soc. 2011, 140, 367–373. [Google Scholar] [CrossRef]
- Otero, J.; Jensen, A.J.; L’Abée-Lund, J.H.; Stenseth, N.C.; Storvik, G.O.; Vøllestad, L.A. Contemporary ocean warming and freshwater conditions are related to later sea age at maturity in Atlantic salmon spawning in Norwegian rivers. Ecol. Evol. 2012, 2, 2192–2203. [Google Scholar] [CrossRef] [PubMed]
- Crozier, L. Impacts of Climate Change on Salmon of the Pacific Northwest. A Review of the Scientific Literature Published in 2015; National Marine Fisheries Service NOAA: Seattle, WA, USA, 2016. [Google Scholar]
- Jacox, M.G.; Alexander, M.A.; Mantua, N.; Scott, J.D.; Hervieux, G.; Webb, R.S.; Werner, F.E. Forcing of multiyear extreme Ocean temperatures that impacted California Current living marine resources in 2016. Bull. Am. Meteorol. Soc. 2018, 99, 27–33. [Google Scholar] [CrossRef]
- King, J.; Boldt, J.; Burke, B.; Greene, C.; Moss, J.; Neville, C. Northeast Pacific Juvenile Salmon Summer Surveys in 2018; PICES Press: Sidney, BC, Canada, 2019; Volume 27, pp. 19–26. [Google Scholar]
- Alfonso, S.; Gesto, M.; Sadoul, B. Temperature increase and its effects on fish stress physiology in the context of global warming. J. Fish Biol. 2020. [Google Scholar] [CrossRef] [PubMed]
- KGO-TV. abc News: Scientists say heat wave is killing large numbers of salmon in Alaska. 2019. Available online: https://abc7news.com/salmon-alaska-die-off-whats-killing-the/5475563/ (accessed on 30 January 2021).
- White, C. Mowi Canada seeks to repair damage done by Newfoundland salmon die-off. SeafoodSource. 2019. Available online: https://www.seafoodsource.com/news/aquaculture/mowi-canada-seeks-to-repair-damage-done-by-newfoundland-salmon-die-off (accessed on 18 October 2019).
- Magra, I. Millions of salmon in Norway killed by algae bloom. The New York Times. 2019. Available online: https://www.savingseafood.org/news/conservation-environment/millions-of-salmon-in-norway-killed-by-algae-bloom/ (accessed on 24 May 2019).
- Soto, D.; León-Muñoz, J.; Dresdner, J.; Luengo, C.; Tapia, F.J.; Garreaud, R. Salmon farming vulnerability to climate change in southern Chile: Understanding the biophysical, socioeconomic and governance links. Rev. Aquac. 2019, 11, 354–374. [Google Scholar] [CrossRef] [Green Version]
- León-Muñoz, J.; Urbina, M.A.; Garreaud, R.; Iriarte, J.L. Hydroclimatic conditions trigger record harmful algal bloom in western Patagonia (summer 2016). Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Oliver, E.C.J.; Donat, M.G.; Burrows, M.T.; Moore, P.; Smale, D.A.; Alexander, L.V.; Benthuysen, J.A.; Feng, M.; Gupta, A.S.; Hobday, A.J.; et al. Longer and more frequent marine heatwaves over the past century. Nat. Commun. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Lorentzen, T. Modeling climate change and the effect on the norwegian salmon farming industry. Nat. Resour. Model. 2008, 21, 416–435. [Google Scholar] [CrossRef]
- Hermansen, Ø.; Heen, K. Norwegian salmonid farming and global warming: Socioeconomic impacts. Aquac. Econ. Manag. 2012, 16, 202–221. [Google Scholar] [CrossRef]
- Thyholdt, S.B. The importance of temperature in farmed salmon growth: Regional growth functions for norwegian farmed salmon. Aquac. Econ. Manag. 2014, 18, 189–204. [Google Scholar] [CrossRef] [Green Version]
- Zanuzzo, F.S.; Beemelmanns, A.; Hall, J.R.; Rise, M.L.; Gamperl, A.K. The innate immune response of Atlantic salmon (Salmo salar) is not negatively affected by high temperature and moderate hypoxia. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef]
- Falconer, L.; Hjøllo, S.S.; Telfer, T.C.; McAdam, B.J.; Hermansen, Ø.; Ytteborg, E. The importance of calibrating climate change projections to local conditions at aquaculture sites. Aquaculture 2020, 514. [Google Scholar] [CrossRef]
- Huntsman, A.G. Death of salmon and trout with high temperature. J. Fish. Res. Board Can. 1942, 5c, 485–501. [Google Scholar] [CrossRef]
- Hjeltnes, B.; Bang-Jensen, B.; Bornø, G.; Haukaas, A.; Walde, C. The Health Situation in Norwegian Aquaculture 2016; Norwegian Veterinary Institute: As, Norway, 2017. [Google Scholar]
- Pigliucci, M. Phenotypic Plasticity: Beyond Nature and Nurture; John Hopkins Press: Baltimore, MD, USA, 2001. [Google Scholar]
- Olson-Manning, C.F.; Wagner, R.M.; Mitchell-Olds, T. Adaptive evolution: Evaluating empirical support for theoretical pre-dictions. Nat. Rev. Genet. 2013, 13, 867–877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charlesworth, D.; Barton, N.H.; Charlesworth, B. The sources of adaptive variation. Proc. R. Soc. B 2017, 284. [Google Scholar] [CrossRef] [Green Version]
- Elliott, J.M. Tolerance and resistance to thermal stress in juvenile Atlantic salmon, Salmo salar. Freshw. Biol. 1991, 25, 61–70. [Google Scholar] [CrossRef]
- Finstad, A.G.; Naesje, T.F.; Forseth, T.; Næsje, T.F. Seasonal variation in the thermal performance of juvenile Atlantic salmon (Salmo salar). Freshw. Biol. 2004, 49, 1459–1467. [Google Scholar] [CrossRef]
- Corey, E.; Linnansaari, T.; Cunjak, R.A.; Currie, S. Physiological effects of environmentally relevant, multi-day thermal stress on wild juvenile Atlantic salmon (Salmo salar). Conserv. Physiol. 2017, 5. [Google Scholar] [CrossRef] [PubMed]
- De Leaniz, C.G.; Fleming, I.A.; Einum, S.; Verspoor, E.; Jordan, W.C.; Consuegra, S.; Aubin-Horth, N.; Lajus, D.; Letcher, B.H.; Youngson, A.F.; et al. A critical review of adaptive genetic variation in Atlantic salmon: Implications for conservation. Biol. Rev. 2007, 82, 173–211. [Google Scholar] [CrossRef] [PubMed]
- Anttila, K.; Dhillon, R.S.; Boulding, E.G.; Farrell, A.P.; Glebe, B.D.; Elliott, J.A.; Wolters, W.R.; Schulte, P.M. Variation in tem-perature tolerance among families of Atlantic salmon (Salmo salar) is associated with hypoxia tolerance, ventricle size and myoglobin level. J. Exp. Biol. 2013, 216, 1183–1190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlson, S.M.; Seamons, T.R. A review of quantitative genetic components of fitness in salmonids: Implications for adaptation to future change. Evol. Appl. 2008, 1, 222–238. [Google Scholar] [CrossRef] [PubMed]
- Solberg, M.F.; Dyrhovden, L.; Matre, I.H.; Glover, K.A. Thermal plasticity in farmed, wild and hybrid Atlantic salmon during early development: Has domestication caused divergence in low temperature tolerance? BMC Evol. Biol. 2016, 16, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Jonsson, B.; Forseth, T.; Jensen, A.J.; Naesje, T.F. Thermal performance of juvenile Atlantic Salmon, Salmo salar L. Funct. Ecol. 2001, 15, 701–711. [Google Scholar] [CrossRef]
- Taylor, E.B. A review of local adaptation in Salmonidac, with particular reference to Pacific and Atlantic salmon. Aquaculture 1991, 98, 185–207. [Google Scholar] [CrossRef]
- Crozier, L.G.; Hendry, A.P.; Lawson, P.W.; Quinn, T.P.; Mantua, N.J.; Battin, J.; Shaw, R.G.; Huey, R.B. Potential responses to climate change in organisms with complex life histories: Evolution and plasticity in Pacific salmon. Evol. Appl. 2008, 1, 252–270. [Google Scholar] [CrossRef]
- Eliason, E.J.; Clark, T.; Hague, M.J.; Hanson, L.M.; Gallagher, Z.S.; Jeffries, K.M.; Gale, M.K.; Patterson, D.A.; Hinch, S.G.; Farrell, A.P. Differences in thermal tolerance among sockeye salmon populations. Science 2011, 332, 109–112. [Google Scholar] [CrossRef] [Green Version]
- Hutchings, A.J. Old wine in new bottles: Reaction norms in salmonid fishes. Heredity 2011, 106, 421–437. [Google Scholar] [CrossRef] [Green Version]
- Nicieza, A.G.; Reyes-Gavilán, F.G.; Braña, F. Differentiation in juvenile growth and bimodality patterns between northern and southern populations of Atlantic salmon (Salmo salar L.). Can. J. Zool. 1994, 72, 1603–1610. [Google Scholar] [CrossRef]
- Nicieza, A.G.; Reiniz, L.; Braña, F. Variation in digestive performance between geographically disjunct populations of Atlantic salmon: Countergradient in passage time and digestion rate. Oecologia 1994, 99, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Dionne, M.; Miller, K.M.; Dodson, J.J.; Caron, F.; Bernatchez, L. Clinal variation in mhc diversity with temperature: Evidence for the role of host? Pathogen interaction on local adaptation in atlantic salmon. Evolution 2007, 61, 2154–2164. [Google Scholar] [CrossRef]
- Verspoor, E.; Jordan, W.C. Genetic variation at the Me-2 locus in the Atlantic salmon within and between rivers: Evidence for its selective maintenance. J. Fish Biol. 2006, 35, 205–213. [Google Scholar] [CrossRef]
- Jordan, W.C.; Cross, T.F.; Crozier, W.W.; Ferguson, A.; Galvin, P.; Hurrell, R.H.; McGinnity, P.; Martin, S.; Moffett, I.J.J.; Price, D.J.; et al. Allozyme variation in Atlantic salmon from the British Isles: Associations with geography and the environment. J. Fish Biol. 2005, 67, 146–168. [Google Scholar] [CrossRef]
- OECD. Part II Biology of Animals. Chapter 3. Atlantic salmon (Salmo salar). In Harmonisation of Regulatory Oversight in Biotechnology. Safety Assessment of Transgenic Organisms in the Environment. OECD Consensus Documents; OECD Publishing: Paris, France, 2017. [Google Scholar]
- Skaala, O.; Taggart, J.B.; Gunnes, K. Genetic differences between five major domesticated strains of Atlantic salmon and wild salmon. J. Fish Biol. 2005, 67, 118–128. [Google Scholar] [CrossRef]
- Van Oppen, M.J.H.; Gates, R.D.; Blackall, L.L.; Cantin, N.; Chakravarti, L.J.; Chan, W.Y.; Cormick, C.; Crean, A.; Damjanovic, K.; Epstein, H.; et al. Shifting paradigms in restoration of the world’s coral reefs. Glob. Chang. Biol. 2017, 23, 3437–3448. [Google Scholar] [CrossRef] [PubMed]
- van Oppen, M.J.H.; Oliver, J.K.; Putnam, H.; Gates, R.D. Building coral reef resilience through assisted evolution. Proc. Natl. Acad. Sci. USA 2015, 112, 2307–2313. [Google Scholar] [CrossRef] [Green Version]
- Schaefer, J.; Ryan, A. Developmental plasticity in the thermal tolerance of zebrafish Danio rerio. J. Fish Biol. 2006, 69, 722–734. [Google Scholar] [CrossRef]
- Beaman, J.E.; White, C.; Seebacher, F. Evolution of plasticity: Mechanistic link between development and reversible acclimation. Trends Ecol. Evol. 2016, 31, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Refsnider, J.M.; Clifton, I.T.; Vazquez, T.K. Developmental plasticity of thermal ecology traits in reptiles: Trends, potential benefits, and research needs. J. Therm. Biol. 2019, 84, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Gavery, M.R.; Roberts, S.B. Epigenetic considerations in aquaculture. PeerJ 2017, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, N.A.; Johnsen, H.; Moghadam, H.; Andersen, Ø.; Tveiten, H. Early developmental stress affects subsequent gene expression response to an acute stress in Atlantic salmon: An approach for creating robust fish for aquaculture? G3: Genes|Genomes|Genetics 2019, 9, 1597–1611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saito, T.; Whatmore, P.; Taylor, J.F.; Fernandes, J.M.; Adam, A.-C.; Tocher, D.R.; Espe, M.; Skjærven, K.H. Micronutrient supplementation affects transcriptional and epigenetic regulation of lipid metabolism in a dose-dependent manner. Epigenetics 2020, 1–18. [Google Scholar] [CrossRef]
- Froese, R.; Pauly, D. FishBase. World Wide Web Electronic Publication. www.fishbase.org, Version (08/2019). 2019. [Google Scholar]
- Mota, M.; Rochard, E.; Antunes, C. Status of the diadromous fish of the Iberian Peninsula: Past, present and trends. Limnetica 2016, 35, 1–18. [Google Scholar]
- IUCN. Atlantic Salmon Salmo Salar. Available online: https://www.iucnredlist.org/search?query=atlantic%20salmon%20&searchType=species (accessed on 30 January 2021).
- ICNF. Salmo Salar Salmão do Atlântico. Available online: http://www2.icnf.pt/portal/pn/biodiversidade/patrinatur/lvv/resource/doc/peix/salm-sal (accessed on 30 January 2021).
- Horreo, J.L.; Griffiths, A.M.; Machado-Schiaffino, G.; Stevens, J.R.; Garcia-Vazquez, E. Northern areas as refugia for temperate species under current climate warming: Atlantic salmon (Salmo salar L.) as a model in northern Europe. J. Fish Biol. 2018, 95, 304–310. [Google Scholar] [CrossRef] [Green Version]
- Lassalle, G.; Rochard, E. Impact of twenty-first century climate change on diadromous fish spread over Europe, North Africa and the Middle East. Glob. Chang. Biol. 2009, 15, 1072–1089. [Google Scholar] [CrossRef]
- Lehnert, S.J.; Kess, T.; Bentzen, P.; Kent, M.P.; Lien, S.; Gilbey, J.; Clément, M.; Jeffery, N.W.; Waples, R.S.; Bradbury, I.R. Genomic signatures and correlates of widespread population declines in salmon. Nat. Commun. 2019, 10. [Google Scholar] [CrossRef]
- Valiente, A.G.; Beall, E.; Garcia-Vazquez, E. Population genetics of south European Atlantic salmon under global change. Glob. Chang. Biol. 2009, 16, 36–47. [Google Scholar] [CrossRef]
- Jonsson, B.; Jonsson, N. Cultured Atlantic salmon in nature: A review of their ecology and interaction with wild fish. ICES J. Mar. Sci. 2006, 63, 1162–1181. [Google Scholar] [CrossRef] [Green Version]
- Glover, A.K.; Solberg, M.F.; McGinnity, P.; Hindar, K.; Verspoor, E.; Coulson, M.W.; Hansen, M.M.; Araki, H.; Skaala, Ø.; Svåsand, T. Half a century of genetic interaction between farmed and wild Atlantic salmon: Status of knowledge and unanswered questions. Fish Fish. 2017, 18, 890–927. [Google Scholar] [CrossRef]
- Almodóvar, A.; Leal, S.; Nicola, G.; Hórreo, J.; Garcia-Vazquez, E.; Elvira, B. Long-term stocking practices threaten the original genetic diversity of the southernmost European populations of Atlantic salmon Salmo salar. Endanger. Species Res. 2020, 41, 303–317. [Google Scholar] [CrossRef]
- Neiva, J.; Assis, J.; Coelho, N.C.; Fernandes, F.; Pearson, G.A.; Serrão, E.A. Genes left behind: Climate change threatens cryptic genetic diversity in the canopy-forming seaweed Bifurcaria bifurcata. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Pearson, G.A.; Lago-Leston, A.; Mota, C. Frayed at the edges: Selective pressure and adaptive response to abiotic stressors are mismatched in low diversity edge populations. J. Ecol. 2009, 97, 450–462. [Google Scholar] [CrossRef]
- Gjedrem, T.; Robinson, N.; Rye, M. The importance of selective breeding in aquaculture to meet future demands for animal protein: A review. Aquaculture 2012, 350-353, 117–129. [Google Scholar] [CrossRef]
- Chavanne, H.; Janssen, K.; Hofherr, J.; Contini, F.; Haffray, P.; Komen, H.; Nielsen, E.E.; Bargelloni, L. A comprehensive survey on selective breeding programs and seed market in the European aquaculture fish industry. Aquac. Int. 2016, 24, 1287–1307. [Google Scholar] [CrossRef]
- Janssen, K.; Chavanne, H.; Berentsen, P.; Komen, H. Impact of selective breeding on European aquaculture. Aquaculture 2017, 472, 8–16. [Google Scholar] [CrossRef]
- Glover, K.A.; Otterå, H.; Olsen, R.; Slinde, E.; Taranger, G.L.; Skaala, Ø. A comparison of farmed, wild and hybrid Atlantic salmon (Salmo salar L.) reared under farming conditions. Aquaculture 2009, 286, 203–210. [Google Scholar] [CrossRef]
- Burgess, M. A New Breed of Warm-Water Salmon Being Farmed Down Under. Available online: https://www.businesslive.co.za/bd/world/2019-09-29-a-new-breed-of-warm-water-salmon-being-farmed-down-under (accessed on 30 January 2021).
- Sae-Lim, P.; Kause, A.; Mulder, H.A.; Olesen, I. Breeding and Genetics Symposium: Climate change and selective breeding in aquaculture. J. Anim. Sci. 2017, 95. [Google Scholar] [CrossRef]
- Cole, J.; Bormann, J.; Gill, C.; Khatib, H.; Koltes, J.; Maltecca, C.; Miglior, F. Breeding and Genetics Symposium: Resilience of livestock to changing environments. J. Anim. Sci. 2017, 95. [Google Scholar] [CrossRef]
- Jablonka, E.; Raz, G. Transgenerational epigenetic inheritance: Prevalence, mechanisms, and implications for the study of heredity and evolution. Q. Rev. Biol. 2009, 84, 131–176. [Google Scholar] [CrossRef] [Green Version]
- Veilleux, H.D.; Ryu, T.W.; Donelson, J.; Van Herwerden, L.; Seridi, L.; Ghosheh, Y.; Berumen, M.L.; Leggat, W.; Ravasi, T.; Munday, P.L. Molecular processes of transgenerational acclimation to a warming ocean. Nat. Clim. Chang. 2015, 5, 1074–1078. [Google Scholar] [CrossRef] [Green Version]
- McCaw, B.A.; Stevenson, T.J.; Lancaster, L.T. Epigenetic responses to temperature and climate. Integr. Comp. Biol. 2020, 60, 1469–1480. [Google Scholar] [CrossRef] [PubMed]
- O’Dea, R.; Noble, D.W.A.; Johnson, S.L.; Hesselson, D.; Nakagawa, S. The role of non-genetic inheritance in evolutionary rescue: Epigenetic buffering, heritable bet hedging and epigenetic traps. Environ. Epigenetics 2016, 2, dvv014. [Google Scholar] [CrossRef] [Green Version]
- Myhr, A.I.; Dalmo, R.A. Introduction of genetic engineering in aquaculture: Ecological and ethical implications for science and governance. Aquaculture 2005, 250, 542–554. [Google Scholar] [CrossRef]
- Cleveland, B.M. A perspective of the future value and challenges of genetic engineering in aquaculture. J. World Aquac. Soc. 2018, 50, 890–893. [Google Scholar] [CrossRef]
- Yang, Y.-L.; Zhou, R.; Li, K. Future livestock breeding: Precision breeding based on multi-omics information and population personalization. J. Integr. Agric. 2017, 16, 2784–2791. [Google Scholar] [CrossRef]
- Yang, H.; Hu, E.; Buchanan, J.T.; Tiersch, T.R. A Strategy for Sperm Cryopreservation of Atlantic Salmon, Salmo salar, for Remote Commercial-scale High-throughput Processing. J. World Aquac. Soc. 2018, 49, 96–112. [Google Scholar] [CrossRef]
- Diwan, A.D.; Harke, S.N.; Gopalkrishna; Panche, A.N. Cryobanking of fish and shellfish egg, embryos and larvae: An overview. Front. Mar. Sci. 2020, 7. [Google Scholar] [CrossRef]
- Lien, S.; Koop, B.F.; Sandve, S.R.; Miller, J.R.; Kent, M.P.; Nome, T.; Hvidsten, T.R.; Leong, J.S.; Minkley, D.R.; Zimin, A.; et al. The Atlantic salmon genome provides insights into rediploidization. Nature 2016, 533, 200–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calado, R.; Mota, V.C.; Madeira, D.; Leal, M.C. Summer Is Coming! Tackling Ocean Warming in Atlantic Salmon Cage Farming. Animals 2021, 11, 1800. https://doi.org/10.3390/ani11061800
Calado R, Mota VC, Madeira D, Leal MC. Summer Is Coming! Tackling Ocean Warming in Atlantic Salmon Cage Farming. Animals. 2021; 11(6):1800. https://doi.org/10.3390/ani11061800
Chicago/Turabian StyleCalado, Ricardo, Vasco C. Mota, Diana Madeira, and Miguel C. Leal. 2021. "Summer Is Coming! Tackling Ocean Warming in Atlantic Salmon Cage Farming" Animals 11, no. 6: 1800. https://doi.org/10.3390/ani11061800
APA StyleCalado, R., Mota, V. C., Madeira, D., & Leal, M. C. (2021). Summer Is Coming! Tackling Ocean Warming in Atlantic Salmon Cage Farming. Animals, 11(6), 1800. https://doi.org/10.3390/ani11061800