Amino Acid Absorption Profiles in Growing Pigs Fed Different Protein Sources
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Diets
2.2. Protocol
2.3. Chemical Analysis
2.4. Data Analysis
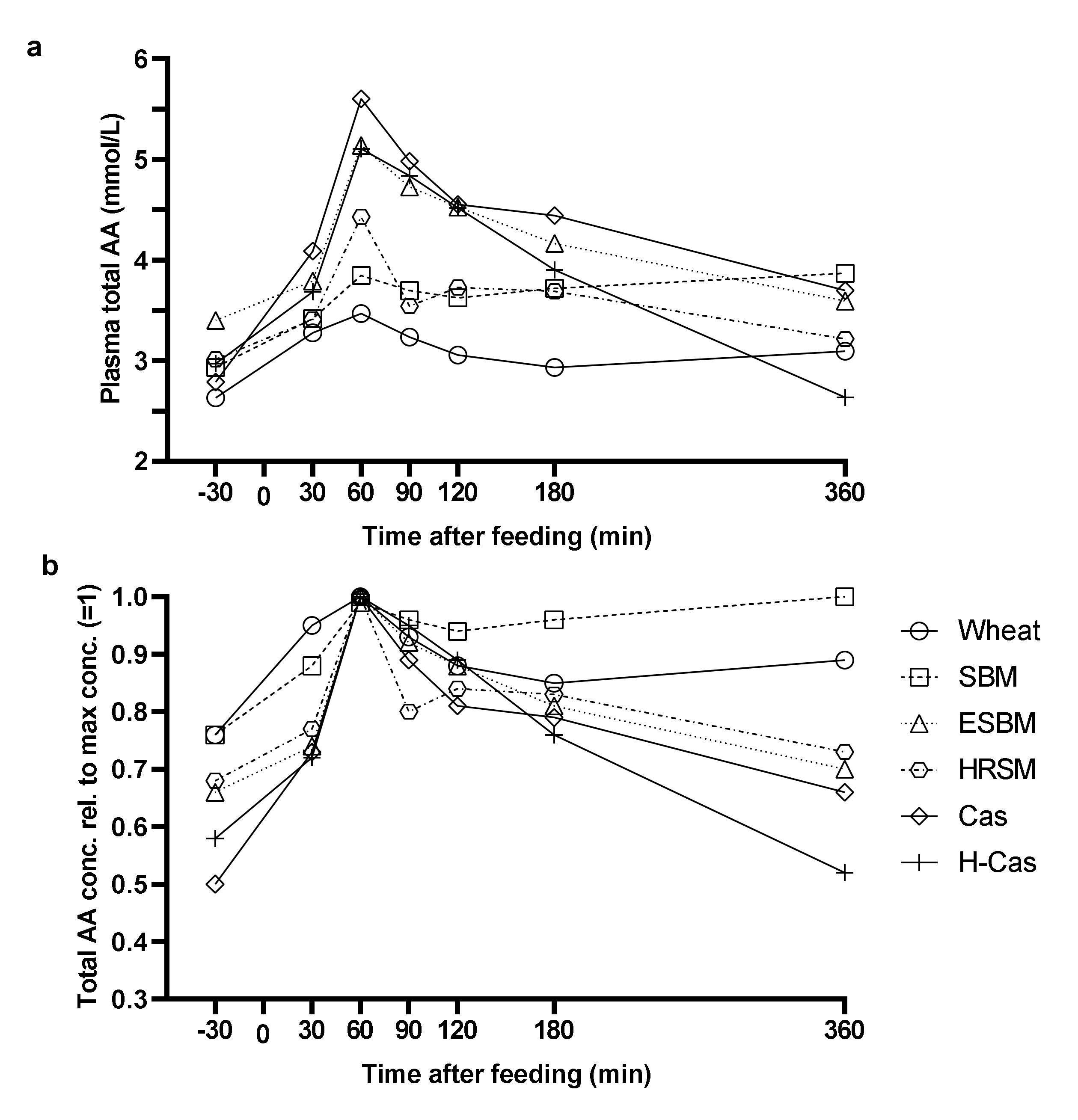
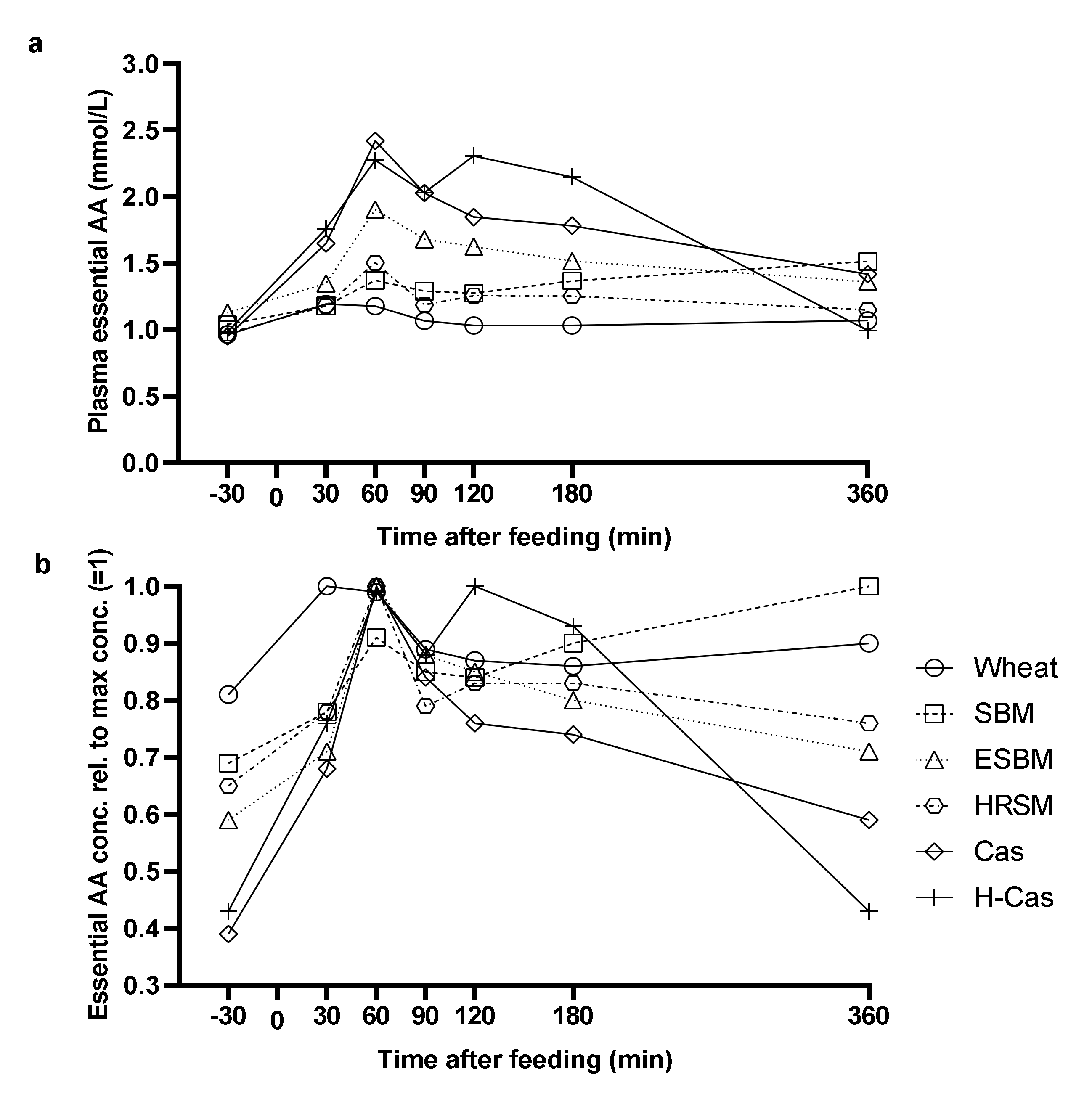
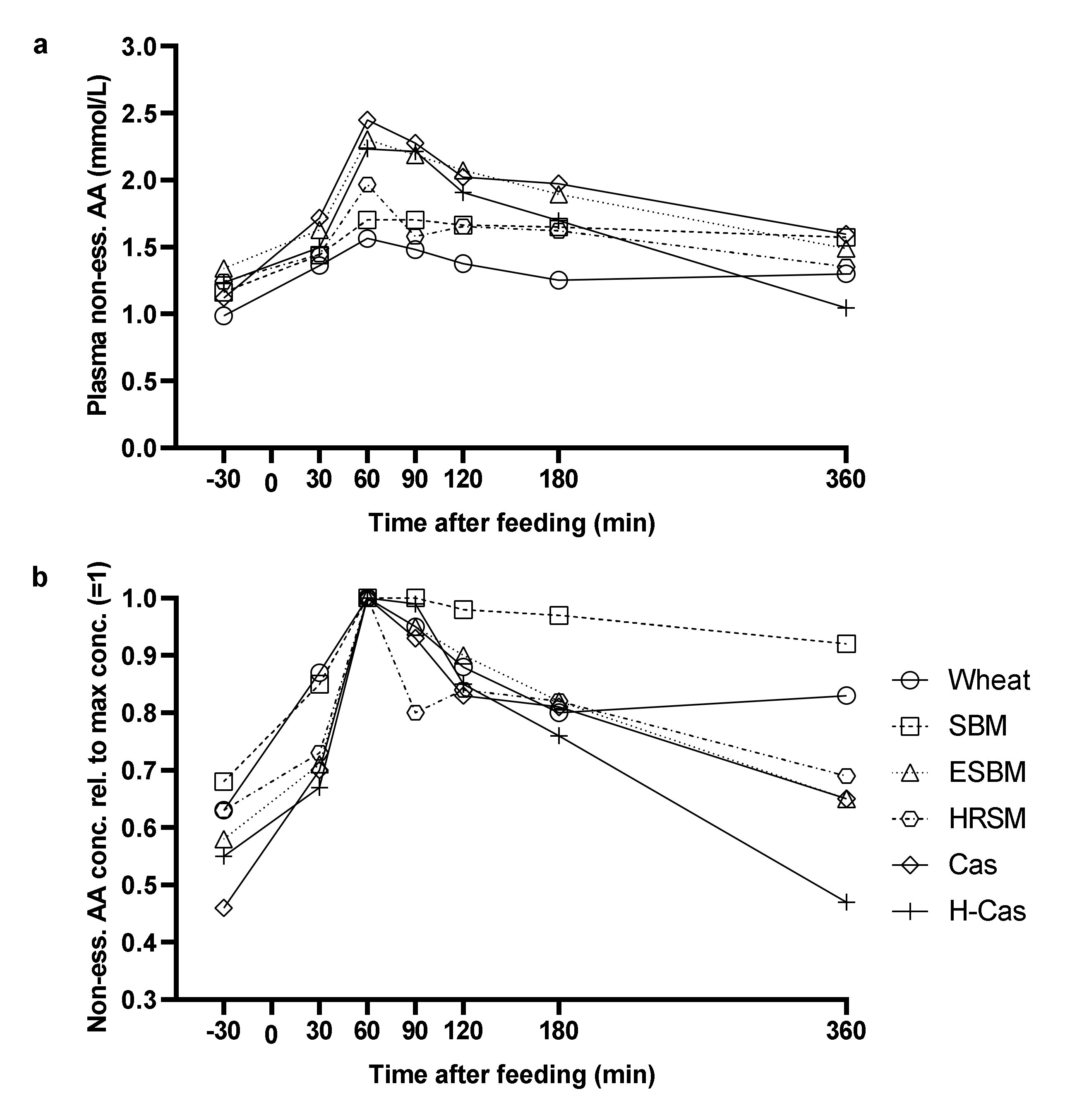
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nørgaard, J.V.; Hansen, M.J.; Soumeh, E.A.; Adamsen, A.P.S.; Poulsen, H.D. Effect of protein level on performance, nitrogen utilisation and carcass composition in finisher pigs. Acta Agri. Scand. A Anim. Sci. 2014, 64, 123–129. [Google Scholar] [CrossRef]
- Boirie, Y.; Dangin, M.; Gachon, P.; Vasson, M.P.; Maubois, J.L.; Beaufrère, B. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc. Natl. Acad. Sci. USA 1997, 94, 14930–14935. [Google Scholar] [CrossRef]
- Calbet, J.A.; Holst, J.J. Gastric emptying, gastric secretion and enterogastrone response after administration of milk proteins or their peptide hydrolysates in humans. Eur. J. Nutr. 2004, 43, 127–139. [Google Scholar] [CrossRef]
- Koopman, R.; Crombach, N.; Gijsen, A.P.; Walrand, S.; Fauquant, J.; Kies, A.K.; Lemosquet, S.; Saris, W.H.M.; Boirie, Y.; van Loon, L.J.C. Ingestion of a protein hydrolysate is accompanied by an accelerated in vivo digestion and absorption rate when compared with its intact protein. Am. J. Clin. Nutr. 2009, 90, 106–115. [Google Scholar] [CrossRef]
- Horner, K.; Drummond, E.; O’Sullivan, V.; Sri Harsha, P.S.C.; Brennan, L. Effects of a casein hydrolysate versus intact casein on gastric emptying and amino acid responses. Eur. J. Nutr. 2018, 58, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Hamuro, Y.; Coales, S.J.; Molnar, K.S.; Tuske, S.J.; Morrow, J.A. Specificity of immobilized porcine pepsin in h/d exchange compatible conditions. Rapid Commun. Mass Spectrom. 2008, 22, 1041–1046. [Google Scholar] [CrossRef] [PubMed]
- Nørgaard, J.V.; Buxant, L.; Clausen, D.; Sharma, N.; Sorensen, P. Utilization of crystalline and protein-bound amino acids by growing-finishing pigs. J. Anim. Sci. 2016, 94, 246–248. [Google Scholar] [CrossRef]
- Rérat, A. Nutritional value of protein hydrolysis products (oligopeptides and free amino acids) as a consequence of absorption and metabolism kinetics. Arch. Tierernaehr. 1995, 48, 23–36. [Google Scholar] [CrossRef]
- Munck, B.G. Transport of sugars and amino acids across guinea pig small intestine. Biochim. Biophys. Acta Biomembr. 1980, 597, 411–417. [Google Scholar] [CrossRef]
- Stoll, B.; Burrin, D.G.; Henry, J.; Yu, H.; Jahoor, F.; Reeds, P.J. Dietary amino acids are the preferential source of hepatic protein synthesis in piglets. J. Nutr. 1998, 128, 1517–1524. [Google Scholar] [CrossRef]
- Dai, Z.-L.; Li, X.-L.; Xi, P.-B.; Zhang, J.; Wu, G.; Zhu, W.-Y. Metabolism of select amino acids in bacteria from the pig small intestine. Amino Acids 2012, 42, 1597–1608. [Google Scholar] [CrossRef] [PubMed]
- Rérat, A.A. Nutritional supply of proteins and absorption of their hydrolysis products: Consequences on metabolism. Proc. Nutr. Soc. 1993, 52, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Van Der Meulen, J.; Bakker, J.G.M.; Smits, B.; De Visser, H. Effect of source of starch on net portal flux of glucose, lactate, volatile fatty acids and amino acids in the pig. Br. J. Nutr. 1997, 78, 533–544. [Google Scholar] [CrossRef]
- Deglaire, A.; Fromentin, C.; Fouillet, H.; Airinei, G.; Gaudichon, C.; Boutry, C.; Benamouzig, R.; Moughan, P.J.; Tomé, D.; Bos, C. Hydrolyzed dietary casein as compared with the intact protein reduces postprandial peripheral, but not whole-body, uptake of nitrogen in humans. Am. J. Clin. Nutr. 2009, 90, 1011–1022. [Google Scholar] [CrossRef] [PubMed]
- Bindari, Y.R.; Laerke, H.N.; Nørgaard, J.V. Standardized ileal digestibility and digestible indispensable amino acid score of porcine and bovine hydrolyzates in pigs. J. Sci. Food Agric. 2018, 98, 2131–2137. [Google Scholar] [CrossRef]
- Nørgaard, J.V.; Soumeh, E.A.; Curtasu, M.; Corrent, E.; van Milgen, J.; Hedemann, M.S. Use of metabolic profile in short-term studies for estimating optimum dietary isoleucine, leucine, and valine for pigs. Anim. Feed Sci. Technol. 2017, 228, 39–47. [Google Scholar] [CrossRef]
- Larsen, M.; Lapierre, H.; Kristensen, N.B. Abomasal protein infusion in postpartum transition dairy cows: Effect on performance and mammary metabolism. J. Dairy Sci. 2014, 97, 5608–5622. [Google Scholar] [CrossRef]
- Stoll, B.; Henry, J.; Reeds, P.J.; Yu, H.; Jahoor, F.; Burrin, D.G. Catabolism dominates the first-pass intestinal metabolism of dietary essential amino acids in milk protein-fed piglets. J. Nutr. 1998, 128, 606–614. [Google Scholar] [CrossRef]
- Soumeh, E.A.; van Milgen, J.; Sloth, N.M.; Corrent, E.; Poulsen, H.D.; Nørgaard, J.V. The optimum ratio of standardized ileal digestible leucine to lysine for 8 to 12 kg female pigs. J. Anim. Sci. 2015, 93, 2218–2224. [Google Scholar] [CrossRef]
- Yen, J.T.; Kerr, B.J.; Easter, R.A.; Parkhurst, A.M. Difference in rates of net portal absorption between crystalline and protein-bound lysine and threonine in growing pigs fed once daily. J. Anim. Sci. 2004, 82, 1079–1090. [Google Scholar] [CrossRef]
- van den Borne, J.J.G.C.; Alferink, S.J.J.; Heetkamp, M.J.W.; Jacobs, A.A.A.; Verstegen, M.W.A.; Gerrits, W.J.J. Asynchronous supply of indispensable amino acids reduces protein deposition in milk-fed calves. J. Nutr. 2012, 142, 2075–2082. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bos, C.; Metges, C.C.; Gaudichon, C.; Petzke, K.J.; Pueyo, M.E.; Morens, C.; Everwand, J.; Benamouzig, R.; Tomé, D. Postprandial kinetics of dietary amino acids are the main determinant of their metabolism after soy or milk protein ingestion in humans. J. Nutr. 2003, 133, 1308–1315. [Google Scholar] [CrossRef] [PubMed]
- Deutz, N.E.; Bruins, M.J.; Soeters, P.B. Infusion of soy and casein protein meals affects interorgan amino acid metabolism and urea kinetics differently in pigs. J. Nutr. 1998, 128, 2435–2445. [Google Scholar] [CrossRef] [PubMed]
- Löhrke, B.; Saggau, E.; Schadereit, R.; Beyer, M.; Bellmann, O.; Kuhla, S.; Hagemeister, H. Activation of skeletal muscle protein breakdown following consumption of soyabean protein in pigs. Br. J. Nutr. 2001, 85, 447–457. [Google Scholar]
- Moran, K.; Boyd, R.D.; Zier-Rush, C.; Wilcock, P.; Bajjalieh, N.; van Heugten, E. Effects of high inclusion of soybean meal and a phytase superdose on growth performance of weaned pigs housed under the rigors of commercial conditions. J. Anim. Sci. 2017, 95, 5455–5465. [Google Scholar] [CrossRef][Green Version]
- Shi, C.; Zhang, Y.; Yin, Y.; Wang, C.; Lu, Z.; Wang, F.; Feng, J.; Wang, Y. Amino acid and phosphorus digestibility of fermented corn-soybean meal mixed feed with bacillus subtilis and enterococcus faecium fed to pigs. J. Anim. Sci. 2017, 95, 3996–4004. [Google Scholar] [CrossRef]
- Shoveller, A.K.; Brunton, J.A.; Pencharz, P.B.; Ball, R.O. The methionine requirement is lower in neonatal piglets fed parenterally than in those fed enterally. J. Nutr. 2003, 133, 1390–1397. [Google Scholar] [CrossRef]
- Bauchart-Thevret, C.; Stoll, B.; Burrin, D.G. Intestinal metabolism of sulfur amino acids. Nutr. Res. Rev. 2009, 22, 175–187. [Google Scholar] [CrossRef]
| Ingredient | Wheat | SBM | ESBM | HRSM | Casein | H-Cas | C-AA |
|---|---|---|---|---|---|---|---|
| Wheat | 100.00 | - | - | - | - | - | - |
| SBM | - | 40.70 | - | - | - | - | - |
| ESBM | - | - | 34.80 | - | - | - | - |
| HRSM | - | - | - | 61.50 | - | - | - |
| Casein | - | - | - | - | 24.30 | - | - |
| H-Cas | - | - | - | - | - | 24.30 | - |
| C-AA 1 | - | - | - | - | - | - | 20.20 |
| Cellulose fibre | - | 2.74 | 2.74 | 2.74 | 2.74 | 2.74 | 2.74 |
| Corn oil | - | 4.56 | 4.56 | 4.56 | 4.56 | 4.56 | 4.56 |
| Corn starch | - | 38.20 | 44.10 | 17.40 | 54.60 | 54.60 | 58.70 |
| Limestone | - | 1.74 | 1.74 | 1.74 | 1.74 | 1.74 | 1.74 |
| Min. vit. Premix 2 | - | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 |
| MCP 3 | - | 2.47 | 2.47 | 2.47 | 2.47 | 2.47 | 2.47 |
| Salt | - | 0.26 | 0.26 | 0.26 | 0.26 | 0.26 | 0.26 |
| Sugar | - | 9.13 | 9.13 | 9.13 | 9.13 | 9.13 | 9.13 |
| Wheat | SBM | ESBM | HRSM | Casein | H-Cas | C-AA | |
|---|---|---|---|---|---|---|---|
| Crude protein | 9.4 | 49.1 | 57.5 | 32.5 | 82.3 | 82.3 | 99.0 |
| Essential AA | |||||||
| Cys | 2.4 | 8.5 | 8.9 | 8.4 | 3.8 | 3.8 | - |
| His | 2.4 | 14.3 | 15.8 | 9.7 | 28.6 | 28.6 | 28.6 |
| Ile | 3.7 | 27.8 | 30.6 | 15.5 | 49.4 | 49.4 | 49.4 |
| Leu | 7.0 | 42.4 | 48.4 | 25.7 | 93.5 | 93.5 | 93.5 |
| Lys | 3.1 | 33.2 | 34.1 | 20.3 | 77.5 | 77.5 | 77.5 |
| Met | 1.7 | 7.5 | 8.1 | 7.3 | 28.0 | 28.0 | 28.0 |
| Phe | 4.7 | 28.1 | 31.8 | 14.5 | 50.6 | 50.6 | 50.6 |
| Thr | 3.1 | 21.6 | 24.3 | 16.4 | 35.5 | 35.5 | 35.5 |
| Trp | 1.4 | 7.4 | 8.0 | 5.0 | 11.9 | 11.9 | 11.9 |
| Val | 4.8 | 27.5 | 30.9 | 19.9 | 65.1 | 65.1 | 65.1 |
| Sum essential AA | 34 | 218 | 241 | 143 | 444 | 444 | 440 |
| Non-essential AA | |||||||
| Ala | 3.9 | 23.9 | 26.6 | 16.1 | 27.4 | 27.4 | 27.4 |
| Arg | 5.2 | 40.1 | 44.4 | 21.8 | 36.2 | 36.2 | - |
| Asp | 5.4 | 62.9 | 70.9 | 27.1 | 67.2 | 67.2 | - |
| Glu | 28.0 | 98.2 | 110.7 | 60.1 | 209.0 | 209.0 | 299.1 |
| Gly | 4.4 | 23.2 | 25.8 | 18.6 | 17.6 | 17.6 | 74.1 |
| Pro | 9.4 | 27.2 | 30.1 | 21.6 | 100.6 | 100.6 | 127.7 |
| Ser | 5.1 | 29.5 | 33.3 | 16.7 | 56.5 | 56.5 | - |
| Sum non-essential AA | 61 | 305 | 342 | 182 | 515 | 515 | 528 |
| Sum AA | 96 | 523 | 583 | 325 | 958 | 958 | 968 |
| N-Free 1 | Wheat | SBM | ESBM | HRSM | Casein | H-Cas | |
|---|---|---|---|---|---|---|---|
| Dry matter, g/100 g as-fed | 92.2 | 87.5 | 91.2 | 92.7 | 90.0 | 92.0 | 92.8 |
| Crude protein, g/100 g | 0.4 | 10.9 | 20.3 | 21.7 | 21.8 | 22.1 | 19.1 |
| Crude fat, g/100 g | 5.2 | 2.4 | 4.1 | 4.7 | 4.6 | 4.0 | 4.0 |
| Ash, g/100 g | 5.1 | 1.8 | 5.2 | 5.9 | 7.0 | 6.2 | 5.8 |
| Gross energy, MJ/kg | 17.1 | 18.0 | 18.0 | 18.3 | 18.3 | 18.2 | 18.0 |
| Essential AA, g/kg | |||||||
| Cys | - | 2.4 | 3.1 | 3.1 | 4.6 | 0.8 | 0.8 |
| His | - | 2.5 | 5.4 | 5.6 | 5.4 | 6.4 | 5.9 |
| Ile | - | 3.9 | 10.2 | 11.1 | 8.7 | 11.1 | 10.5 |
| Leu | - | 7.3 | 16.0 | 17.2 | 14.4 | 20.6 | 19.3 |
| Lys | - | 3.5 | 12.7 | 12.5 | 11.7 | 17.3 | 16.2 |
| Met | - | 1.7 | 2.8 | 2.9 | 4.1 | 6.2 | 5.8 |
| Phe | - | 4.8 | 10.5 | 11.3 | 8.1 | 11.2 | 10.5 |
| Thr | - | 3.2 | 8.1 | 8.6 | 9.2 | 7.9 | 7.4 |
| Trp | - | 1.5 | 2.7 | 2.8 | 2.9 | 2.8 | 2.4 |
| Val | - | 5.0 | 10.6 | 11.3 | 11.3 | 14.4 | 13.5 |
| Sum essential AA | - | 36 | 82 | 86 | 80 | 99 | 92 |
| Non-essential AA, g/kg | |||||||
| Ala | - | 4.0 | 9.1 | 9.6 | 9.2 | 6.2 | 5.8 |
| Arg | - | 5.5 | 15.1 | 15.8 | 12.2 | 8.0 | 7.6 |
| Asp | - | 5.9 | 23.8 | 25.4 | 15.3 | 15.0 | 14.1 |
| Glu | - | 27.6 | 36.7 | 39.4 | 33.7 | 46.3 | 43.2 |
| Gly | - | 4.5 | 8.8 | 9.2 | 10.5 | 4.0 | 3.8 |
| Pro | - | 9.6 | 10.3 | 11.0 | 12.3 | 22.7 | 21.1 |
| Ser | - | 5.2 | 11.0 | 11.8 | 9.3 | 12.5 | 11.7 |
| Sum non-essential AA | - | 62 | 115 | 122 | 103 | 115 | 107 |
| Sum AA | 98 | 197 | 209 | 183 | 213 | 200 | |
| Period | Diets | |||||||
|---|---|---|---|---|---|---|---|---|
| Wheat | SBM | ESBM | HRSM | Casein | H-Cas | SEM | p-Value 1 | |
| 0–30 min | 95 b | 98 a,b | 101 a,b | 96 b | 115 a | 103 a,b | 5.6 | 0.04 |
| 0–60 min | 198 b | 210 a,b | 236 a,b | 205 b | 268 a | 239 a,b | 16.8 | 0.009 |
| 0–90 min | 301 b | 324 a,b | 385 a,b | 317 b | 425 a | 389 a,b | 27.4 | 0.003 |
| 0–120 min | 398 b | 434 a,b | 524 a,b | 426 b | 565 a | 532 a,b | 36.7 | 0.002 |
| 0–180 min | 582 b | 655 a | 786 a | 649 a,b | 830 a | 832 a | 55.4 | 0.002 |
| 0–360 min | 1137 b | 1340 a b | 1485 a,b | 1283 a,b | 1544 a | 1519 a,b | 106.4 | 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nørgaard, J.V.; Florescu, I.C.; Krogh, U.; Nielsen, T.S. Amino Acid Absorption Profiles in Growing Pigs Fed Different Protein Sources. Animals 2021, 11, 1740. https://doi.org/10.3390/ani11061740
Nørgaard JV, Florescu IC, Krogh U, Nielsen TS. Amino Acid Absorption Profiles in Growing Pigs Fed Different Protein Sources. Animals. 2021; 11(6):1740. https://doi.org/10.3390/ani11061740
Chicago/Turabian StyleNørgaard, Jan V., Iulia C. Florescu, Uffe Krogh, and Tina Skau Nielsen. 2021. "Amino Acid Absorption Profiles in Growing Pigs Fed Different Protein Sources" Animals 11, no. 6: 1740. https://doi.org/10.3390/ani11061740
APA StyleNørgaard, J. V., Florescu, I. C., Krogh, U., & Nielsen, T. S. (2021). Amino Acid Absorption Profiles in Growing Pigs Fed Different Protein Sources. Animals, 11(6), 1740. https://doi.org/10.3390/ani11061740






