Laying Performance, Egg Quality Characteristics, and Egg Yolk Fatty Acids Profile in Layer Hens Housed with Free Access to Chicory- and/or White Clover-Vegetated or Non-Vegetated Areas
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Hens, Housing, and Feeding
2.2. Data Collection, Measurements, and Analysis
2.3. Statistical Analysis
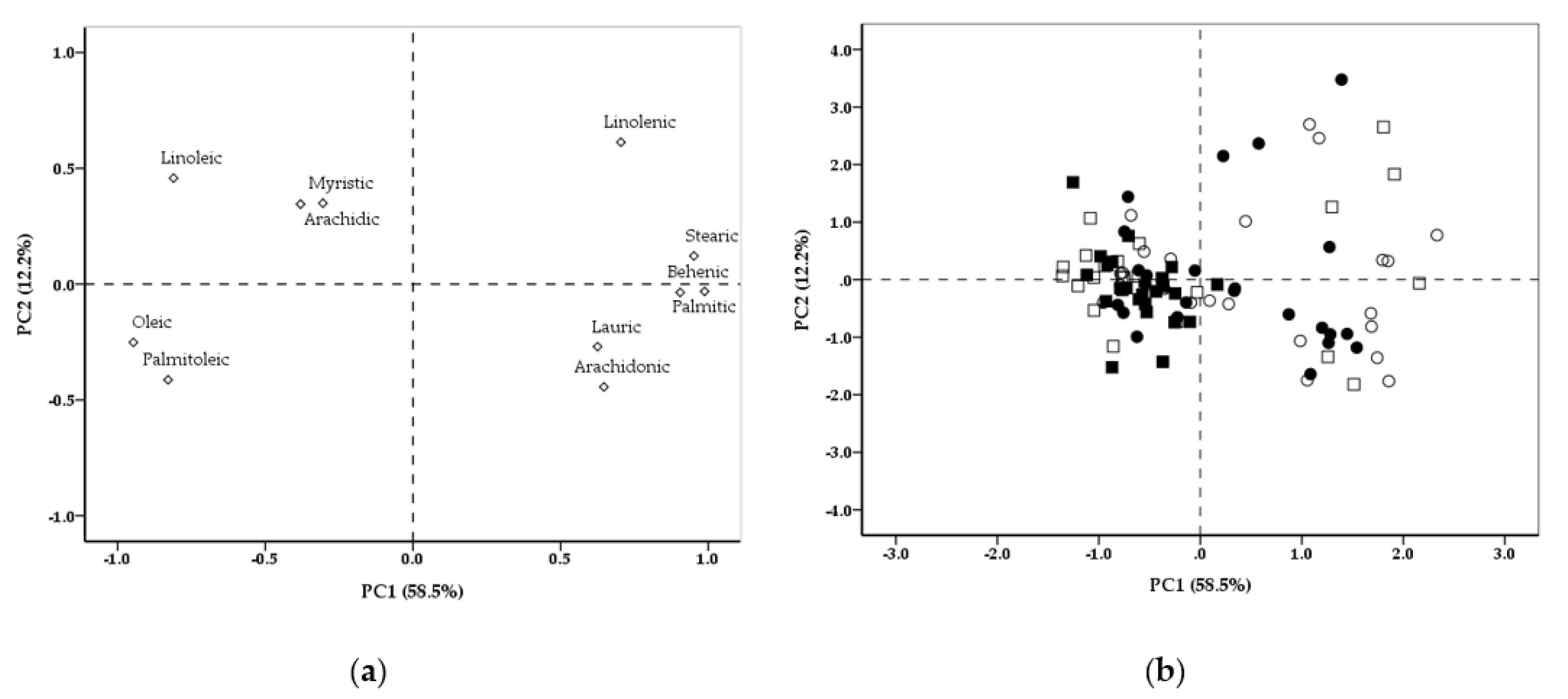
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Horsted, K.; Hammershøj, M.; Allesen-Holm, B.H. Effect of grass-clover forage and whole-wheat feeding on the sensory quality of eggs. J. Sci. Food Agric. 2010, 90, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Sossidou, E.N.; Dal Bosco, A.; Elson, H.A.; Fontes, C.M.G.A. Pasture based systems for poultry production: Implications and perspectives. World Poult. Sci. J. 2011, 67, 47–58. [Google Scholar] [CrossRef]
- Singh, M.; Cowieson, A.J. Range use and pasture consumption in free-range poultry production. Anim. Prod. Sci. 2013, 53, 1202–1208. [Google Scholar] [CrossRef]
- Hammershøj, M.; Johansen, N.F. Review: The effect of grass and herbs in organic egg production on egg fatty acid composition, egg yolk colour and sensory properties. Livest. Sci. 2016, 194, 37–43. [Google Scholar] [CrossRef]
- Rakonjac, S.; Bogosavljević-Bošković, S.; Pavlovski, Z.; Škrbić, Z.; Dosković, V.; Petrović, M.D.; Petričevi, V. Laying hen rearing systems: A review of major production results and egg quality traits. World Poult. Sci. J. 2014, 70, 93–104. [Google Scholar] [CrossRef]
- Zhao, Y.; Shepherd, T.A.; Swanson, J.C.; Mench, J.A.; Karcher, D.M.; Xin, H. Comparative evaluation of three egg production systems: Housing characteristics and management practices. Poult. Sci. 2015, 94, 475–484. [Google Scholar] [CrossRef]
- Tufarelli, V.; Ragni, M.; Laudadio, V. Feeding forage in poultry: A promising alternative for the future of production systems. Agriculture 2018, 8, 81. [Google Scholar] [CrossRef]
- Mugnai, C.; Dal Bosco, A.; Castellini, C. Effect of rearing system and season on the performance and egg characteristics of Ancona laying hens. Ital. J. Anim. Sci. 2009, 8, 175–188. [Google Scholar] [CrossRef]
- Mugnai, C.; Sossidou, E.N.; Dal Bosco, A.; Ruggeri, S.; Mattioli, S.; Castellini, C. The effects of husbandry system on the grass intake and egg nutritive characteristics of laying hens. J. Sci. Food Agric. 2014, 94, 459–467. [Google Scholar] [CrossRef]
- Mohammed, K.A.F.; Sarmiento-Franco, L.; Santos-Ricalde, R.; Solorio-Sanchez, J.F. Egg production, egg quality and crop content of Rhode Island Red hens grazing on natural tropical vegetation. Trop. Anim. Health Prod. 2013, 45, 367–372. [Google Scholar] [CrossRef]
- Skřivan, M.; Englmaierova, M. The deposition of carotenoids and alpha-tocopherol in hen eggs produced under a combination of sequential feeding and grazing. Anim. Feed Sci. Technol. 2014, 190, 79–86. [Google Scholar] [CrossRef]
- Hammershøj, M.; Steenfeldt, S. Organic egg production. II: The quality of organic eggs is influenced by hen genotype, diet and forage material analyzed by physical parameters, functional properties and sensory evaluation. Anim. Feed Sci. Technol. 2015, 208, 182–197. [Google Scholar] [CrossRef]
- Singh, M.; Ruhnke, I.; Koning, C.D.; Drake, K.; Skerman, A.G.; Hinch, G.N.; Glatz, P.C. Free-range poultry survey: Demographics and practices of semi-intensive free-range farming systems in Australia with an outdoor stocking density of ≤1500 hens/hectare. PLoS ONE 2017, 12. [Google Scholar] [CrossRef]
- Chen, S.; Xiang, H.; Zhu, X.; Zhang, H.; Wang, D.; Liu, H.; Wang, J.; Yin, T.; Liu, L.; Kong, M.; et al. Free dietary choice and free-range rearing improve the product quality, gait score, and microbial richness of chickens. Animals 2018, 8, 84. [Google Scholar] [CrossRef] [PubMed]
- Mierliță, D. Fatty acid profile and oxidative stability of egg yolks from hens under different production systems. S. Afr. J. Anim. Sci. 2020, 50, 196–206. [Google Scholar] [CrossRef]
- Popova, T.; Petkov, E.; Ayasan, T.; Ignatova, M. Quality of eggs from layers reared under alternative and conventional system. Braz. J. Poult. Sci. 2020, 22, 1–8. [Google Scholar] [CrossRef]
- Iqbal, Z.; Roberts, J.; Perez-Maldonado, R.A.; Goodarzi Boroojeni, F.; Swick, R.A.; Ruhnke, I. Pasture, multi-enzymes, benzoic acid and essential oils positively influence performance, intestinal organ weight and egg quality in free-range laying hens. Br. Poult. Sci. 2018, 59, 180–189. [Google Scholar] [CrossRef]
- Škrbić, Z.; Lukić, M.; Petričević, V.; Bogosavljević-Bošković, S.; Rakonjac, S.; Dosković, V.; Tolimir, N. Quality of eggs from pasture rearing layers of different genotypes. Biotechnol. Anim. Husb. 2020, 36, 181–190. [Google Scholar] [CrossRef]
- Guy, C.; Hennessy, D.; Gilliland, T.J.; Coughlan, F.; Mcclearn, B.; Dineen, M.; Mccarthy, B. Comparison of perennial ryegrass, Lolium perenne L., ploidy and white clover, Trifolium repens L., inclusion for herbage production, utilization and nutritive value. Grass Forage Sci. 2018, 73, 865–877. [Google Scholar] [CrossRef]
- Nwafor, I.C.; Shale, K.; Achilonu, M.C. Chemical composition and nutritive benefits of chicory (Cichorium intybus) as an ideal complementary and/or alternative livestock feed supplement. Sci. World J. 2017. [Google Scholar] [CrossRef]
- Dal Bosco, A.; Mugnai, C.; Rosati, A.; Paoletti, A.; Caporali, S.; Castellini, C. Effect of range enrichment on performance, behavior, and forage intake of free-range chickens. J. Appl. Poult. Res. 2017, 23, 137–145. [Google Scholar] [CrossRef]
- Corrales-Retana, L.; Ciucci, F.; Conte, G.; Casarosa, L.; Mele, M.; Serra, A. Profile of fatty acid lipid fractions of omega-3 fatty acid-enriched table eggs. J. Anim. Physiol. Anim. Nutr. 2021, 105, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Cayan, H.; Erener, G. Effect of olive leaf (Olea europaea) powder on laying hens performance, egg quality and egg yolk cholesterol levels. Asian Australas. J. Anim. Sci. 2015, 28, 538–543. [Google Scholar] [CrossRef] [PubMed]
- Lantinga, E.A.; Neuteboom, J.H.; Meijs, J.A.C. Sward methods. In Herbage Intake Handbook, 2nd ed.; Penning, P.D., Ed.; The British Grassland Society: Reading, UK, 2004; pp. 23–52. [Google Scholar]
- AOAC International. Official Method of Analysis of AOAC International, 18th ed.; AOAC Int.: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Walczak, J.; Bocian, S.; Kowalkowski, T.; Trziszka, T.; Buszewski, B. Determination of omega fatty acid profiles in egg yolk by HILIC-LC-MS and GC-MS. Food Anal. Methods. 2017, 10, 1264–1272. [Google Scholar] [CrossRef]
- Boehringer Manheim Gmbh Biochemica. Methods of Biochemical Analysis and Food Analysis; Boehringer Manheim Gmbh Biochemica: Manheim, Germany, 1995; pp. 26–28. [Google Scholar]
- Jolliffe, I.T. Principal Component Analysis, 2nd ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Horsted, K.; Hammershoj, M.; Hermansen, J.E. Short-term effects on productivity and egg quality in nutrient-restricted versus non-restricted organic layers with access to different forage crops. Acta Agric. Scand. Sect. A Anim. Sci. 2006, 56, 42–54. [Google Scholar] [CrossRef]
- Lorenz, C.; Kany, T.; Grashorn, M.A. Method to estimate feed intake from pasture in broilers and laying hens. Arch. Geflügelk 2013, 77, 160–165. [Google Scholar]
- Breitsameter, L.; Gauly, M.; Isselstein, J. Sward botanical composition and sward quality affect the foraging behaviour of free-range laying hens. Appl. Anim. Behav. Sci. 2014, 150, 27–36. [Google Scholar] [CrossRef]
- Iqbal, Z.; Metzger, F.; Singh, M.; Morgan, N.; Swick, R.A.; Perez-Maldonado, R.A.; M’Sadeq, S.A.; Zentek, J.; Ruhnke, I. Enzymes and/or combination of organic acid and essential oils supplementation in pasture-fed free-range laying hens increased the digestibility of nutrients and non-starch polysaccharides. Poult. Sci. 2019, 98, 1410–1424. [Google Scholar] [CrossRef]
- Adedokun, S.A.; Adeola, O. Calcium and phosphorus digestibility: Metabolic limits. J. Appl. Poult. Res. 2013, 22, 600–608. [Google Scholar] [CrossRef]
- Ketta, M.; Tůmová, E. Relationship between eggshell thickness and other eggshell measurements in eggs from litter and cages. Ital. J. Anim. Sci. 2018, 17, 234–239. [Google Scholar] [CrossRef]
- Samiullah, J.R.; Roberts, J.R.; Chousalkar, K. Effect of production system and flock age on egg quality and total bacterial load in commercial laying hens. J. Appl. Poult. Res. 2014, 23, 59–70. [Google Scholar] [CrossRef]
- Sokołowicz, Z.; Krawczyk, J.; Dykiel, M. Effect of alternative housing system and hen genotype on egg quality characteristics. Emir. J. Food Agric. 2018, 30, 695–703. [Google Scholar] [CrossRef]
- Kuźniacka, J.; Biesek, J.; Banaszak, M.; Grabowicz, M.; Adamski, M. The quality of eggs from rosa 1 hens fed diets containing seeds of legume plants (Lupinus luteus L., Lupinus angustifolius, and Pisum sativum) in two laying phases. Animals 2020, 10, 1942. [Google Scholar] [CrossRef]
- Yilmaz Dikmen, B.; Ipek, A.; Şahan, Ü.; Sözcü, A.; Baycan, S.C. Impact of different housing systems and age of layers on egg quality characteristics. Turk. J. Vet. Anim. Sci. 2017, 41, 77–84. [Google Scholar] [CrossRef]
- Nain, S.; Renema, R.A.; Korver, D.R.; Zuidhof, M.J. Characterization of the n-3 polyunsaturated fatty acid enrichment in laying hens fed an extruded flax enrichment source. Poult. Sci. 2012, 91, 1720–1732. [Google Scholar] [CrossRef]
- Małgorzata, G.; Krawczyk, J.; Otwinowska-Mindur, A. Effect of production cycle and season on quality and fatty acid profile of organic eggs from local Polish Greenleg Partridge hens. Europ. Poult. Sci. 2017, 81, 1–13. [Google Scholar] [CrossRef]
- Minelli, G.; Sirri, F.; Folegatti, E.; Meluzzi, A.; Franchini, A. Egg quality traits of laying hens reared in organic and conventional systems. Ital. J. Anim. Sci. 2007, 6, 728–730. [Google Scholar] [CrossRef]
- Zita, L.; Jeníková, M.; Härtlová, H. Effect of housing system on egg quality and the concentration of cholesterol in egg yolk and blood of hens of native resources of the Czech Republic and Slovakia. J. Appl. Poult. Res. 2018, 27, 380–388. [Google Scholar] [CrossRef]
| Ingredient | g/kg | Calculated Chemical Composition | % |
|---|---|---|---|
| Corn | 580.0 | ME (Mcal/kg) | 2.75 |
| Soybean meal (48% CP) | 209.0 | Crude protein | 17.80 |
| Sunflower meal (36% CP) | 20.0 | Ether extract | 6.42 |
| Full-fat soybean (37% CP) | 70.0 | Crude fibre | 2.88 |
| Limestone (38%) | 95.0 | Lysine | 0.84 |
| Dicalcium phosphate | 19.0 | Methionine + cysteine | 0.69 |
| Salt | 2.5 | Ash | 11.12 |
| Vitamin + mineral premix * | 2.5 | Calcium | 4.10 |
| DL-methionine | 1.5 | Available phosphorus | 0.32 |
| Lysine | 0.5 |
| Nutrient | CI | TR | MIX |
|---|---|---|---|
| Dry matter | 18.36 | 16.96 | 17.73 |
| Crude protein | 16.63 | 23.73 | 19.48 |
| Ether extract | 2.39 | 1.94 | 2.06 |
| Acid detergent fibre | 30.64 | 33.77 | 32.60 |
| Neutral detergent fibre | 47.66 | 35.16 | 42.58 |
| Ash | 0.93 | 0.98 | 0.97 |
| FA (g/100 g FA) | CD | CI | TR | MIX |
|---|---|---|---|---|
| Myristic acid (C14:0) | 0.05 | 0.52 | 0.47 | 0.50 |
| Palmitic acid (C16:0) | 12.31 | 20.88 | 27.01 | 24.84 |
| Stearic acid (18:0) | 2.08 | 5.30 | 7.42 | 6.48 |
| Σ Saturated fatty acids | 14.44 | 26.70 | 34.90 | 31.82 |
| Palmitoleic acid (C16:1) n-7 | 0.13 | 1.51 | 3.04 | 2.92 |
| Oleic acid (18:1) n-9 | 30.11 | 10.00 | 24.38 | 18.51 |
| Σ Monounsaturated fatty acids | 30.24 | 11.51 | 27.42 | 21.43 |
| Linolenic acid (18:3) n-3 | 1.49 | 39.18 | 15.99 | 26.57 |
| Linoleic acid (18:2) n-6 | 52.40 | 22.61 | 21.69 | 20.18 |
| Σ Polyunsaturated fatty acids | 53.89 | 61.79 | 37.68 | 46.75 |
| Parameter 1 | C | CI | TR | MIX | SEM | p-Value |
|---|---|---|---|---|---|---|
| Initial body weight (g/hen) | 1792 | 1753 | 1775 | 1786 | 4.8 | |
| Final body weight (g/hen) | 1950 | 1927 | 1937 | 1936 | 10.3 | 0.282 |
| Egg-laying rate (%) | 95.8 | 94.5 | 94.5 | 95.2 | 1.25 | 0.673 |
| Concentrate intake (g/hen/day) | 130.4 a | 126.8 b | 124.5 c | 125.7 bc | 4.17 | 0.018 |
| Herbage intake (g/hen/day) | - | 13.7 c | 18.0 a | 15.5 b | 0.24 | <0.001 |
| Egg weight (g) | 63.0 | 63.0 | 63.2 | 63.2 | 0.22 | 0.782 |
| Egg mass (g/hen/day) | 60.4 | 59.5 | 59.7 | 60.1 | 0.18 | 0.328 |
| FCR (g feed: g egg mass) | 2.16 | 2.13 | 2.08 | 2.09 | 0.011 | 0.195 |
| Cracked egg ratio (%) | 1.79 | 1.73 | 2.39 | 2.90 | 0.766 | 0.677 |
| Parameter 1 | C | CI | TR | MIX | SEM | p-Value |
|---|---|---|---|---|---|---|
| Haugh units | 80.24 | 81.92 | 82.68 | 80.40 | 1.047 | 0.825 |
| Yolk ratio (g/100 g egg) | 27.29 | 27.54 | 28.37 | 27.97 | 0.243 | 0.695 |
| Albumen ratio (g/100 g egg) | 58.49 | 58.62 | 57.97 | 57.93 | 0.300 | 0.793 |
| Eggshell ratio (g/100 g egg) | 13.70 | 13.82 | 13.63 | 14.09 | 0.147 | 0.728 |
| Yolk/albumen ratio | 0.47 | 0.47 | 0.49 | 0.48 | 0.006 | 0.756 |
| Eggshell strength (kg/cm2) | 4.01 | 4.12 | 3.89 | 3.23 | 0.249 | 0.616 |
| Eggshell thickness (μm) | 0.46 a | 0.40 b | 0.41 b | 0.41 b | 0.001 | 0.013 |
| Albumen index | 8.75 | 9.05 | 8.69 | 8.35 | 0.321 | 0.900 |
| Yolk index | 44.17 | 44.25 | 43.67 | 48.22 | 0.748 | 0.094 |
| Shape index | 77.09 | 76.77 | 77.28 | 79.06 | 0.503 | 0.394 |
| Yolk colour (grade 1–16) | 12.1 | 12.0 | 11.8 | 12.0 | 0.095 | 0.583 |
| Variables 1 | C | CI | TR | MIX | SEM | p-Value |
|---|---|---|---|---|---|---|
| Palmitic acid (16:0) | 26.76 a | 25.74 b | 27.70 a | 27.39 a | 0.180 | <0.001 |
| Stearic acid (18:0) | 10.31 b | 9.92 b | 12.04 a | 11.58 a | 0.226 | 0.001 |
| Behenic acid (22:0) | 0.26 a | 0.02 b | 0.10 b | 0.07 b | 0.021 | <0.001 |
| Arachidic acid (20:0) | 0.90 a | 0.94 a | 0.91 a | 0.79 b | 0.014 | 0.001 |
| Σ SFA | 38.17 a | 37.09 ab | 37.92 a | 36.39 b | 0.213 | 0.010 |
| Palmitoleic acid (16:1) | 2.68 a | 2.65 ab | 2.33 c | 2.42 bc | 0.046 | 0.014 |
| Oleic acid (18:1) | 37.17 ab | 38.29 a | 35.28 c | 36.19 bc | 0.272 | <0.001 |
| Σ MUFA | 41.12 a | 40.49 ab | 39.92 b | 40.61 ab | 0.135 | 0.017 |
| Linoleic acid (18:2) | 19.35 | 21.10 | 20.79 | 21.52 | 0.178 | 0.155 |
| Linolenic acid (18:3) | 1.22 | 1.28 | 1.31 | 1.48 | 0.054 | 0.191 |
| Arachidonic acid (20:4) | 0.13 a | 0.05 b | 0.06 b | 0.03 b | 0.011 | 0.006 |
| Σ PUFA | 20.72 c | 22.43 ab | 22.17 b | 23.05 a | 0.156 | <0.001 |
| Σ n-3 | 1.22 | 1.28 | 1.31 | 1.48 | 0.054 | 0.191 |
| Σ n-6 | 19.48 b | 21.15 a | 20.85 a | 21.55 a | 0.152 | <0.001 |
| n-6/n-3 | 15.91 b | 16.55 a | 15.90 b | 14.56 c | 0.110 | <0.001 |
| Cholesterol | 225.0 | 226.0 | 230.4 | 237.4 | 2.44 | 0.256 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kop-Bozbay, C.; Akdag, A.; Bozkurt-Kiraz, A.; Gore, M.; Kurt, O.; Ocak, N. Laying Performance, Egg Quality Characteristics, and Egg Yolk Fatty Acids Profile in Layer Hens Housed with Free Access to Chicory- and/or White Clover-Vegetated or Non-Vegetated Areas. Animals 2021, 11, 1708. https://doi.org/10.3390/ani11061708
Kop-Bozbay C, Akdag A, Bozkurt-Kiraz A, Gore M, Kurt O, Ocak N. Laying Performance, Egg Quality Characteristics, and Egg Yolk Fatty Acids Profile in Layer Hens Housed with Free Access to Chicory- and/or White Clover-Vegetated or Non-Vegetated Areas. Animals. 2021; 11(6):1708. https://doi.org/10.3390/ani11061708
Chicago/Turabian StyleKop-Bozbay, Canan, Ahmet Akdag, Ayfer Bozkurt-Kiraz, Merve Gore, Orhan Kurt, and Nuh Ocak. 2021. "Laying Performance, Egg Quality Characteristics, and Egg Yolk Fatty Acids Profile in Layer Hens Housed with Free Access to Chicory- and/or White Clover-Vegetated or Non-Vegetated Areas" Animals 11, no. 6: 1708. https://doi.org/10.3390/ani11061708
APA StyleKop-Bozbay, C., Akdag, A., Bozkurt-Kiraz, A., Gore, M., Kurt, O., & Ocak, N. (2021). Laying Performance, Egg Quality Characteristics, and Egg Yolk Fatty Acids Profile in Layer Hens Housed with Free Access to Chicory- and/or White Clover-Vegetated or Non-Vegetated Areas. Animals, 11(6), 1708. https://doi.org/10.3390/ani11061708








