Early Nutrition with Different Diets Composition versus Fasting on Immunity-Related Gene Expression and Histomorphology of Digestive and Lymphoid Organs of Layer-Type Chicks
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Hatching Eggs, Experimental Treatments and Management
2.3. Lymphoid and Digestive Organs Histomorphology
2.4. Immunity-Related Gene Expression
2.5. Statistical Analysis
3. Results
3.1. Histomorphology of Lymphoid Organs
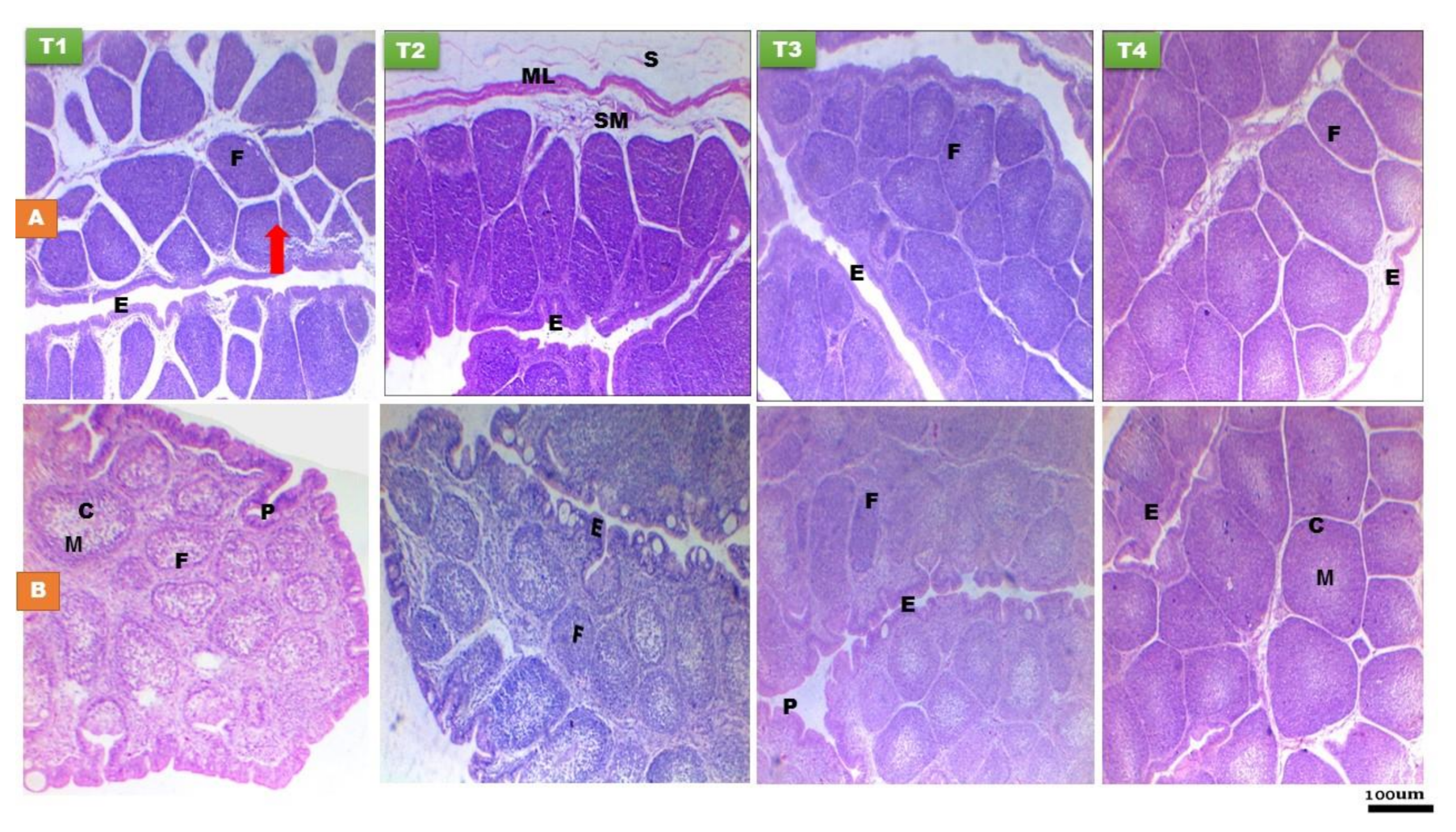
3.1.1. Thymus Gland
3.1.2. Bursa of Fabricius
3.1.3. Spleen
3.2. Liver and Proventriculus Histomorphology
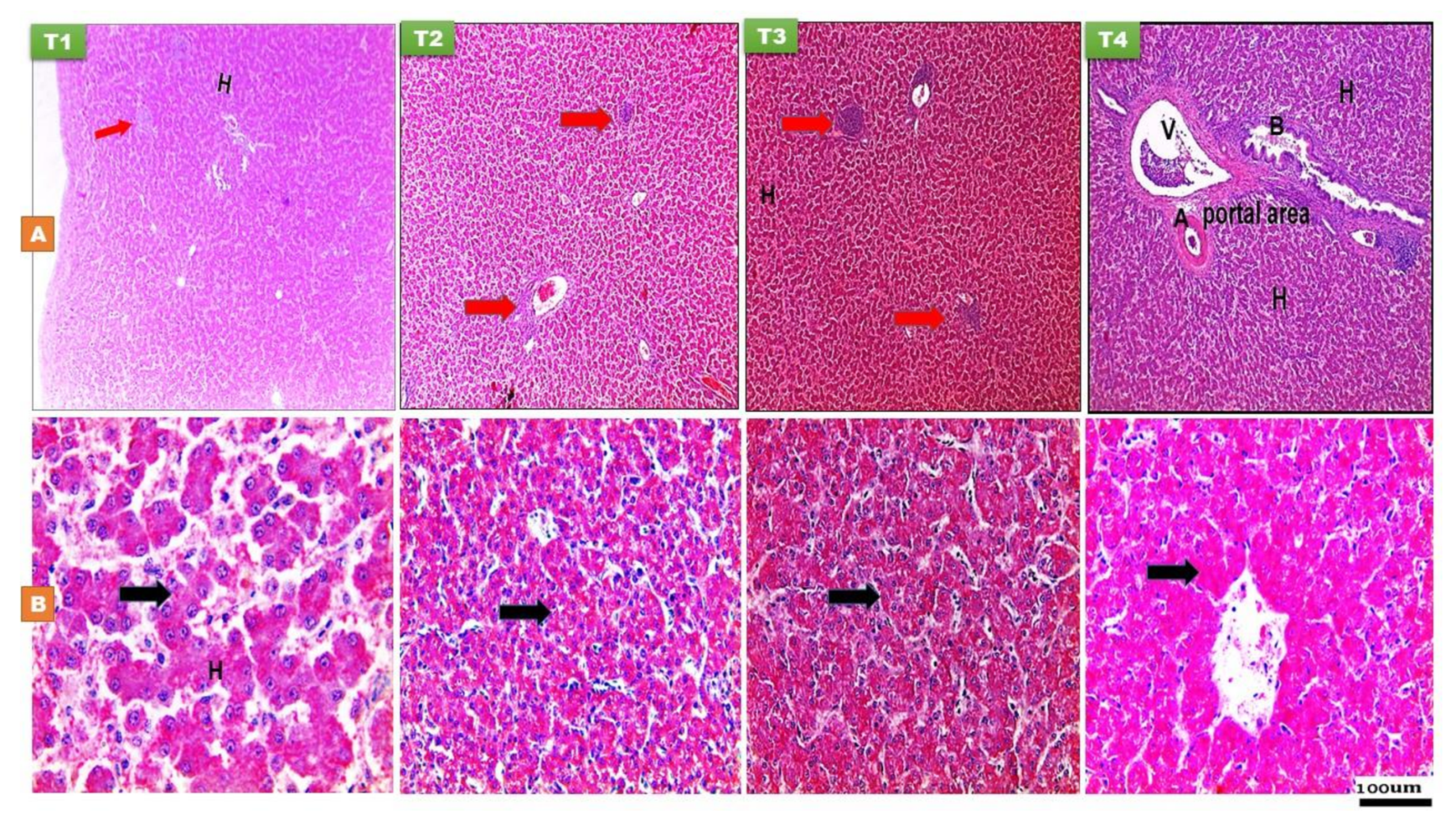
3.2.1. The Liver
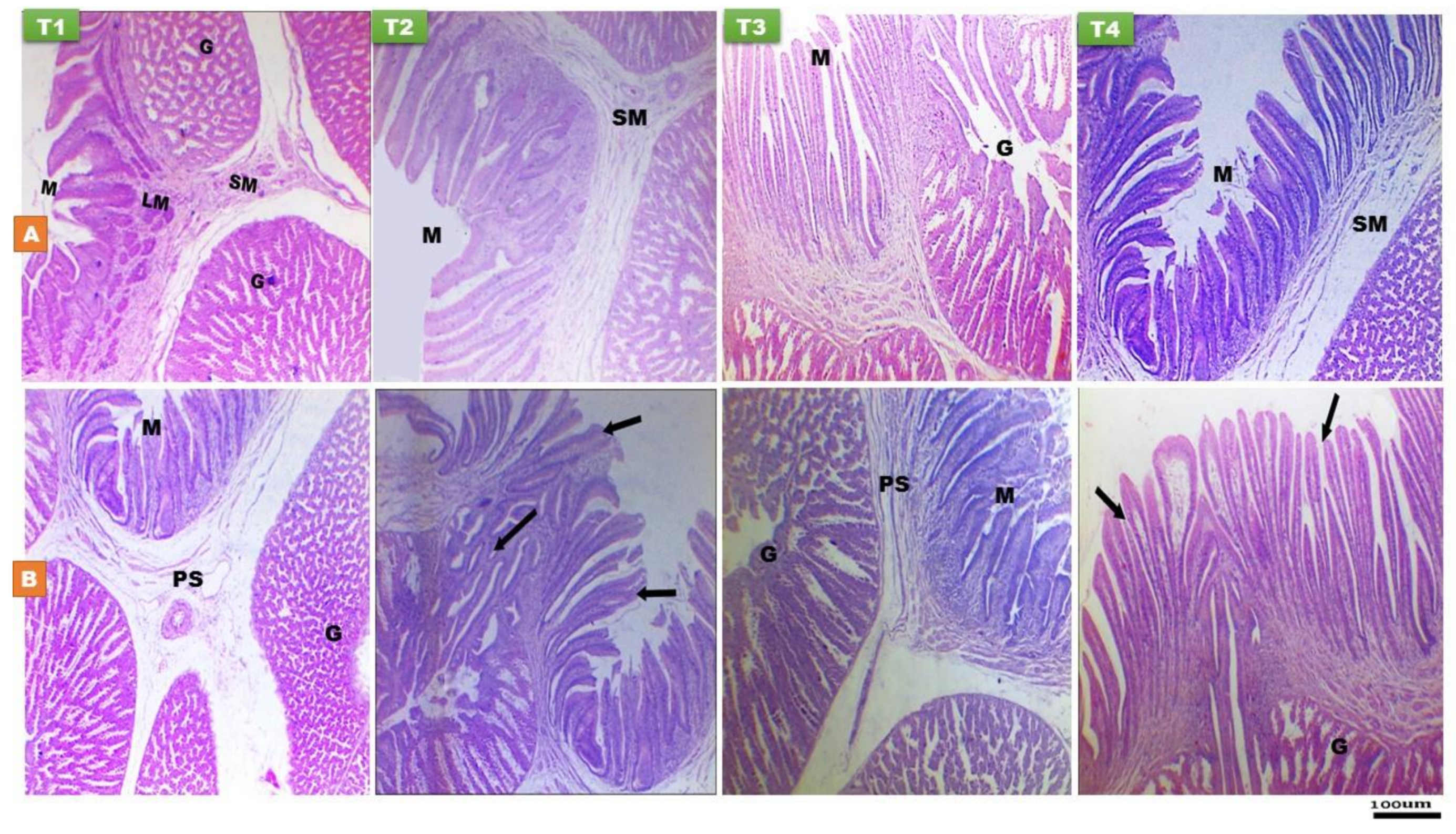
3.2.2. Proventriculus
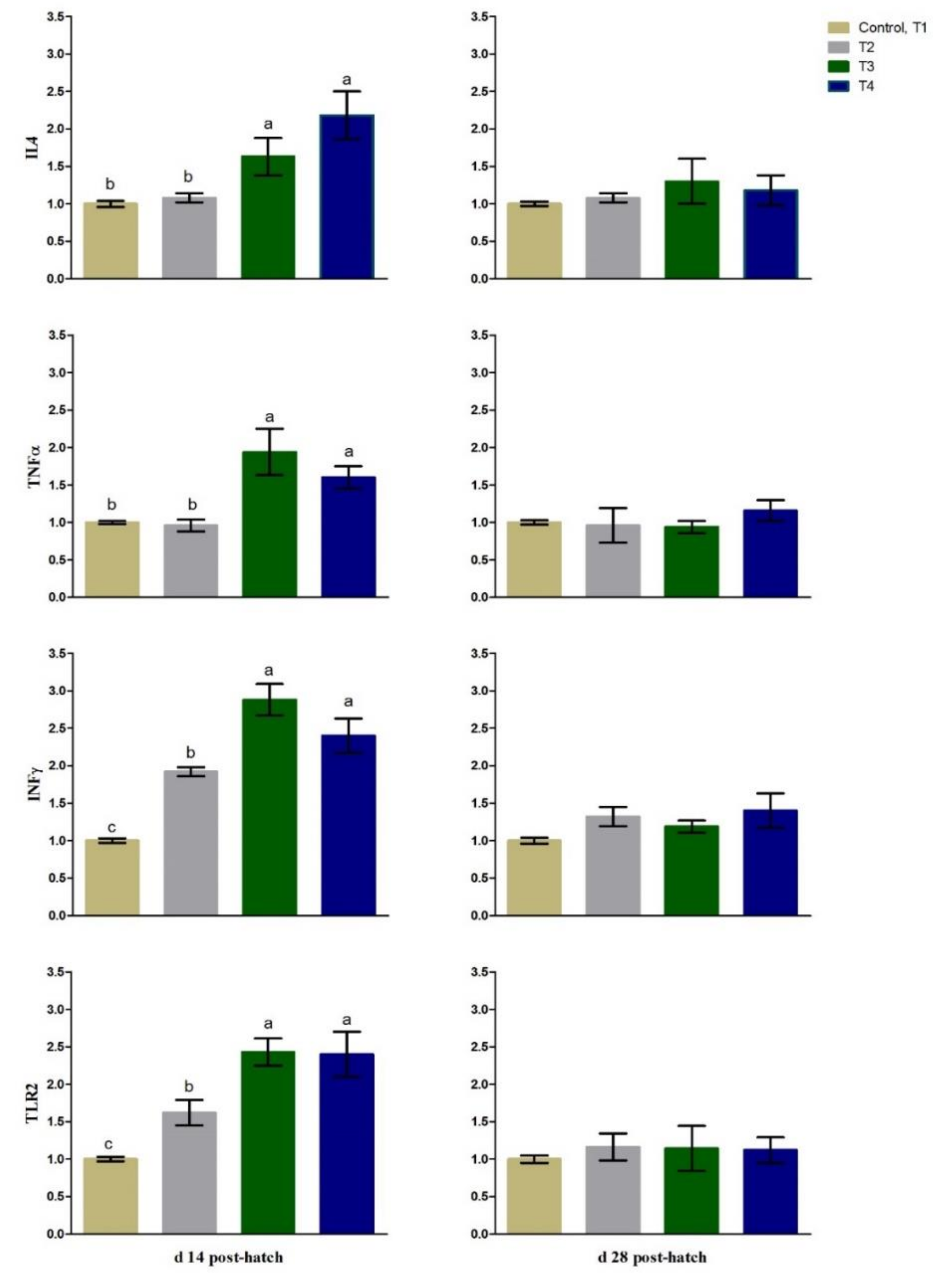
3.3. Immunity Related Gene Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Juul-Madsen, H.R.; Su, G.; Sørensen, P. Influence of early or late start of first feeding on growth and immune phenotype of broilers. Br. Poult. Sci. 2004, 45, 210–222. [Google Scholar] [CrossRef]
- Dibner, J.J.; Knight, C.D.; Kitchell, M.L.; Atwell, C.A.; Downs, A.C.; Ivey, F.J. Early feeding and development of the immune system in neonatal poultry. J. Appl. Poult. Res. 1998, 7, 425–436. [Google Scholar] [CrossRef]
- Bhanja, S.K.; Sudhagar, M.; Goel, A.; Pandey, N.; Mehra, M.; Agarwal, S.K.; Mandal, A. Differential expression of growth and immunity related genes influenced by in ovo supplementation of amino acids in broiler chickens. Czech J. Anim. Sci. 2014, 59, 399–408. [Google Scholar] [CrossRef]
- Panda, A.K.; Bhanja, S.K.; Shyam Sunder, G. Early post hatch nutrition on immune system development and function in broiler chickens. World Poult. Sci. J. 2015, 71, 285–296. [Google Scholar] [CrossRef]
- Batal, A.; Parsons, C. Effect of fasting versus feeding oasis after hatching on nutrient utilization in chicks. Poult. Sci. 2002, 81, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Bar Shira, E.; Sklan, D.; Friedman, A. Impaired immune responses in broiler hatchling hindgut following delayed access to feed. Vet. Immunol. Immunopathol. 2005, 105, 33–45. [Google Scholar] [CrossRef]
- Abou-Elnaga, M.K.; Selim, S. Influence of early feeding with different diet composition on performance and intestinal morphology of layer-type chicks during the brooding period. J. Anim. Feed Sci. 2018, 27, 268–275. [Google Scholar] [CrossRef]
- Tamboli, A.S.; Goel, A.; Mehra, M.; Rokade, J.J.; Bhadauria, P.; Yadav, A.S.; Majumdar, S.; Bhanja, S.K. Delayed post-hatch feeding affects the performance and immune competence differently in male and female broiler chickens. J. Appl. Anim. Res. 2018, 46, 306–313. [Google Scholar] [CrossRef]
- Simon, K.; de Vries Reilingh, G.; Kemp, B.; Lammers, A. Development of ileal cytokine and immunoglobulin expression levels in response to early feeding in broilers and layers. Poult. Sci. 2014, 93, 3017–3027. [Google Scholar] [CrossRef]
- Shinde, A.S.; Goel, A.; Mehra, M.; Rokade, J.; Bhadauria, P.; Mandal, A.B.; Bhanja, S.K. Delayed post hatch feeding affects performance, intestinal morphology and expression pattern of nutrient transporter genes in egg type chickens. J. Nutr. Food Sci. 2015, 5, 372. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, X.; Wang, T.; Chen, B.; Huang, Y.; Chen, H.; Chen, Q. The postembryonic development of the immunological barrier in the chicken spleens. J. Immunol. Res. 2019, 2019, 6279360. [Google Scholar] [CrossRef]
- Madej, J.P.; Stefaniak, T.; Bednarczyk, M. Effect of in ovo delivered prebiotics and symbiotic on lymphoid organs’ morphology in chickens. Poult. Sci. 2015, 94, 1209–1219. [Google Scholar] [CrossRef]
- Uni, Z.; Ferket, R.P. Methods for early nutrition and their potential. World. Poultry Sci. J. 2004, 60, 101–111. [Google Scholar] [CrossRef]
- Noy, Y.; Uni, Z. Early nutritional strategies. World. Poultry Sci. J. 2010, 66, 639–646. [Google Scholar] [CrossRef]
- Wang, A.; Anderson, D.; Rathgeber, B. Using different levels of glycerine, glucose, or sucrose in broiler starter diets to overcome negative effects of delayed feed access on growth performance. Can. J. Anim. Sci. 2018, 98, 311–324. [Google Scholar] [CrossRef]
- Waldroup, P.W. Use of molasses and sugar in poultry feeds. World. Poultry Sci. J. 1981, 37, 193. [Google Scholar] [CrossRef]
- Ivanovich, F.V.; Karlovich, O.A.; Mahdavi, R.; Afanasyevich, E.I. Nutrient density of prestarter diets from 1 to 10 days of age affects intestinal morphometry, enzyme activity, serum indices and performance of broiler chickens. Anim. Nutr. 2017, 3, 258–265. [Google Scholar] [CrossRef]
- Sarlak, S.; Tabeidian, S.A.; Gheisari, A. Effects of time of initiation of feeding after hatching and diet composition on performance, carcass characteristics, digestive tract development and immune responses of broilers. Anim. Prod. Sci. 2016, 57, 1692–1701. [Google Scholar] [CrossRef]
- Longo, F.A.; Menten, J.F.M.; Pedroso, A.A.; Figueiredo, A.N.; Racanicci, A.M.C.; Gaiotto, J.B.; Sorbara, J.O.B. Carbohydrates in the diets of newly hatched chicks. Rev. Bras. Zootec. 2005, 34, 123–133. [Google Scholar] [CrossRef][Green Version]
- Jha, R.; Singh, A.K.; Yadav, S.; Berrocoso, J.F.D.; Mishra, B. Early nutrition programming (in ovo and post-hatch feeding) as a strategy to modulate gut health of poultry. Front. Vet. Sci. 2019, 6, 82. [Google Scholar] [CrossRef] [PubMed]
- Sad, S.; Mosmann, T.R. Single IL-2-secreting precursor CD4 T cell can develop into either Th1 or Th2 cytokine secretion phenotype. J. Immunol. 1994, 153, 3514–3522. [Google Scholar]
- Song, B.; Tang, D.; Yan, S.; Fan, H.; Li, G.; Shahid, M.S.; Mahmood, T.; Guo, Y. Effects of age on immune function in broiler chickens. J. Animal. Sci. Biotechnol. 2021, 12, 42. [Google Scholar] [CrossRef]
- Lammers, A.; Wieland, W.H.; Kruijt, L.; Jansma, A.; Straetemans, T.; Schots, A.; den Hartog, G.; Parmentier, H.K. Successive immunoglobulin and cytokine expression in the small intestine of juvenile chicken. Dev. Comp. Immunol. 2010, 34, 1254–1262. [Google Scholar] [CrossRef]
- Bancroft, J.D.; Stevens, A. Theory and Practice of Histological Techniques, 2nd ed.; Churchill Livingstone: New York, NY, USA, 2010. [Google Scholar]
- Sikandar, A.H.; Zaneb, M.; Younus, S.; Masood, A.; Aslam, A.; Shah, M.; Rehman, H. Growth performance, immune status and organ morphometry in broilers fed Bacillus subtilis-supplemented diet. S. Afr. J. Anim. Sci. 2017, 47, 378–388. [Google Scholar] [CrossRef]
- Sayrafi, R.; Aghagolzadeh, M. Histological and histochemical study of the proventriculus (Ventriculus glandularis) of common starling (Sturnus vulgaris). Anat. Histol. Embryol. 2019, 49, 105–111. [Google Scholar] [CrossRef]
- Rao, D.D.; Senzer, N.; Wang, Z.; Kumar, P.; Jay, C.M.; Nemunaitis, J. Bifunctional Short Hairpin RNA (bi-shRNA): Design and Pathway to Clinical Application. In siRNA Design; Springer: Clifton, NJ, USA, 2013; pp. 259–278. [Google Scholar]
- Ciriaco, E.; Píñera, P.P.; Díaz-Esnal, B.; Laurà, R. Age-related changes in the avian primary lymphoid organs (thymus and bursa of Fabricius). Microsc. Res. Tech. 2003, 62, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Ma, C.; Sun, Z.; Wang, L.; Huang, S.; Su, X.; Xu, J.; Zhang, H. Feed-additive probiotics accelerate yet antibiotics delay intestinal microbiota maturation in broiler chicken. Microbiome 2017, 5, 91. [Google Scholar] [CrossRef]
- Elmore, S.A. Enhanced histopathology of the thymus. Toxicol. Pathol. 2006, 34, 656–665. [Google Scholar] [CrossRef]
- Villagrán-de la Mora, Z.; Vázquez-Paulino, O.; Avalos, H.; Ascencio, F.; Nuño, K.; Villarruel-López, A. Effect of a symbiotic mix on lymphoid organs of broilers infected with Salmonella typhimurium and Clostridium perfringens. Animals 2020, 10, 886. [Google Scholar] [CrossRef]
- Rooney, A.A.; Bermudez, D.S.; Guillette, L.J. Altered histology of the thymus and spleen in contaminant exposed juvenile American alligators. J. Morphol. 2003, 256, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Klasing, K.C. Nutrition and the immune system. Br. Poult. Sci. 2007, 48, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Madej, J.P.; Chrzazstek, K.; Piasecki, T.; Wieliczko, A. New insight into the structure, development, functions and popular disorders of bursa fabricii. Anat. Histol. Embryol. 2013, 42, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Ratcliffe, M.J. Antibodies, immunoglobulin genes and the bursa of Fabricius in chicken B cell development. Dev. Comp. Immunol. 2006, 30, 101–118. [Google Scholar] [CrossRef]
- Attia, Y.A.; Al-Khalaifah, H.; Abd El-Hamid, H.S.; Al-Harthi, M.A.; El-shafey, A.A. Effect of different levels of multienzymes on immune response, blood haematology and biochemistry, antioxidants status and organs histology of broiler chicks fed standard and low-density diets. Front. Vet. Sci. 2020, 6, 510. [Google Scholar] [CrossRef] [PubMed]
- John, J.L. The avian spleen: A neglected organ. Q. Rev. Biol. 1994, 69, 327–351. [Google Scholar] [CrossRef]
- Greiner, E.F.; Guppy, M.; Brand, K. Glucose is essential for proliferation and the glycolytic enzyme induction that provokes a transition to glycolytic energy production. J. Biol. Chem. 1994, 269, 31484–31490. [Google Scholar] [CrossRef]
- Tabeidian, S.A.; Samie, A.; Poureza, A.J.; Sadeghi, G.H. Effect of fasting or post-hatch diet’s type on chick development. J. Anim. Vet. Adv. 2011, 9, 406–413. [Google Scholar] [CrossRef]
- Bakyaraj, S.; Bhanja, S.K.; Majumdar, S.; Dash, B.B. Post-hatch immunomodulation through in ovo-supplemented nutrients in broiler chickens. J. Sci. Food Agric. 2012, 92, 313–320. [Google Scholar] [CrossRef]
- Bhanja, S.K.; Goel, A.; Mehra, M.; Pandey, N.; Majumdar, S.; Mandal, A.B. In ovo carbohydrate, supplementation modulates growth and immunity related genes in broiler chickens. J. Anim. Physiol. Anim. Nutr. 2015, 99, 163–173. [Google Scholar] [CrossRef]
- He, H.; Mackinnon, K.M.; Genovese, K.J.; Kogut, M.H. CpG oligodeoxynucleotide and double-stranded RNA synergize to enhance nitric oxide production and mRNA expression of inducible nitric oxide synthase: Proinflammatory cytokines; and chemokines in chicken monocytes. Innate Immun. 2010, 17, 137–144. [Google Scholar]
- Denbow, D.M. Gastrointestinal Anatomy and Physiology. In Sturkie’s Avian Physiology, 5th ed.; Whittow, G.C., Ed.; Academic Press: New York, NY, USA, 2000; pp. 299–325. [Google Scholar]
- Vickery, K.; Tohidi-Esfahani, R.; Pouliopoulos, J.; Welschinger, R.; Dixon, R.; Deva, A.; Cossart, Y. The effect of surgical immunomodulation on liver inflammation and clearance of DHBV infection. J. Med. Virol. 2006, 78, 1572–1578. [Google Scholar] [CrossRef]
- Hünigen, H.; Mainzer, K.; Hirschberg, R.M.; Custodis, P.; Gemeinhardt, O.; Al Masri, S.; Richardson, K.C.; Hafez, H.M.; Plendl, J. Structure and age-dependent development of the turkey liver: A comparative study of a highly selected meat-type and a wild-type turkey line. Poult. Sci. 2016, 95, 901–911. [Google Scholar] [CrossRef] [PubMed]
- Olah, I.; Nagy, N.; Vervelde, L. Structure of the Avian Lymphoid System. In Avian Immunology, 2nd ed.; Schat, K., Kaspers, B., Kaiser, P., Eds.; Academic Press Elsevier: London, UK, 2013; pp. 11–45. [Google Scholar]
- Zaefarian, F.; Abdollahi, M.R.; Cowieson, A.; Ravindran, V. Avian liver: The forgotten organ. Animals 2019, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- Freeman, B.M. The mobilisation of hepatic glycogen in Gallus domesticus at the end of incubation. Comp. Biochem. Physiol. 1969, 28, 1169–1176. [Google Scholar] [CrossRef]
- Kornasio, R.; Halevy, O.; Kedar, O.; Uni, Z. Effect of in ovo feeding and its interaction with timing of first feed on glycogen reserves, muscle growth, and body weight. Poult. Sci. 2011, 90, 1467–1477. [Google Scholar] [CrossRef]
- Hassan, S.A.; Moussa, E.A. Gross and microscopic studies on the stomach of domestic duck (Anas platyrhynchos) and domestic pigeon (Columba livia domestica). J. Vet. Anat. 2012, 5, 105–127. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, J.; Shi, J.; Hu, X.D.; Chen, J.G. Histological observation of the stomach of the yellow-billed grosbeak. Int. J. Morphol. 2013, 31, 512–515. [Google Scholar] [CrossRef]
- Akter, K.; Mussa, M.T.; Sayeed, M.A.; Hai, M.A.; Uddin, M.M. Study on postnatal growth and development of crop and proventriculus of digestive tract of broiler. Bangl. J. Vet. Med. 2018, 16, 7–11. [Google Scholar] [CrossRef]
- Selvan, P.S.; Ushakumary, S.; Ramesh, G. Studies on the histochemistry of the proventriculus and gizzard of post-hatch guinea fowl (Numida meleagris). Int. J. Poult. Sci. 2008, 7, 1112–1116. [Google Scholar] [CrossRef]
- van de Ven, L.J.F.; van Wagenberg, A.V.; Decuypere, E.; Kemp, B.; van den Brand, H. Perinatal broiler physiology between hatching and chick collection in 2 hatching systems. Poult. Sci. 2013, 92, 1050–1061. [Google Scholar] [CrossRef]
- Maiorka, A.; Santin, E.; Dahlke, F.; Boleli, I.C.; Furlan, R.L.; Macari, M. Posthatching water and feed deprivation affect the gastrointestinal tract and intestinal mucosa development of broiler chicks. J. Appl. Poult. Res. 2003, 12, 483–492. [Google Scholar] [CrossRef]


| Gene 1 | Sequence (5′-3′) | Annealing Temperature (°C) | Product Size (bp) | Accession Number | Reference |
|---|---|---|---|---|---|
| IL4 | F-AATGACATCCAGGGAGAGGTTTC R-GCTAGTTGGTGGAAGAAGGTACG | 55 | 219 | JN639847 | [3] |
| TNFα | F-AGACCAGATGGGAAGGGAATGAA R-GAAGAGGCCACCACACGACAG | 55 | 219 | JN942589 | [8] |
| INFγ | F-AGCTGACGGTGGACCTATTATTGT R-CGGCTTTGCGCTGGATTC | 58 | 260 | JN942588 | [3] |
| TLR2 | F-GTGGCCATGTCGATCAGCAGAAAC R-TCAGCGGAGAGTCACAGATGTAGC | 56 | 202 | NM_204278 1 | [8] |
| 28S rRNA | F-CAGGTGCAGATCTTGGTGGTAGTA R- GCTCCCGCTGGCTTCTCC | 58 | 273 | JN639848 | [3] |
| Items 2 | Treatments 1 | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| CON-T1 | T2 | T3 | T4 | |||
| Lymphoid organs | ||||||
| Thymus gland | ||||||
| Relative weight | 0.215 | 0.241 | 0.253 | 0.243 | 0.011 | 0.11 |
| Cortex | 263.31 b | 263.97 b | 348.38 a | 258.95 b | 38.70 | 0.03 |
| Medulla | 182.19 | 184.26 | 218.07 | 218.07 | 12.85 | 0.13 |
| Cortex:Medulla | 1.45 | 1.43 | 1.60 | 1.19 | 0.142 | 0.11 |
| Bursa of Fabricius | ||||||
| Relative weight | 0.340 b | 0.415 a | 0.440 a | 0.495 a | 0.018 | 0.003 |
| Pelicae height | 23.37 c | 57.70 b,c | 88.87 b | 144.40 a | 12.57 | <0.001 |
| Follicle width | 123.63 | 172.01 | 191.56 | 174.22 | 22.53 | 0.08 |
| Cortex | 232.18 b | 397.96 a | 377.40 a | 298.00 a,b | 32.55 | 0.003 |
| Medulla | 133.28 | 171.13 | 148.71 | 144.61 | 16.28 | 0.21 |
| Cortex:Medulla | 1.74 | 2.35 | 2.55 | 2.13 | 0.309 | 0.13 |
| Spleen | ||||||
| Relative weight | 0.125 b | 0.130 b | 0.145 a | 0.140 a | 0.003 | 0.04 |
| Red pulp | 145.27 | 173.23 | 132.89 | 158.64 | 12.88 | 0.09 |
| White pulp | 141.30 b | 161.44 b | 154.11 b | 223.59 a | 25.17 | 0.03 |
| GCA | 0.21 b | 0.40 a,b | 0.50 a | 0.52 a | 0.082 | 0.04 |
| Digestive organs | ||||||
| Proventriculus | ||||||
| Relative weight | 0.950 b | 1.05 a,b | 1.180 a | 1.170 a | 0.032 | 0.01 |
| Fold height | 303.56 b | 700.76 a | 565.50 a | 580.69 a | 71.39 | 0.003 |
| Mucosa | 496.22 b | 600.92 b | 1035.28 a | 1210.45 a | 89.09 | <0.001 |
| Gland | 695.54 b | 886.75 a | 834.37 a | 829.36 a | 64.19 | 0.04 |
| Lumen | 94.46 b | 218.51 a | 186.91 a | 126.33 b | 17.87 | <0.001 |
| Liver | ||||||
| Glycogen density | 1.42 c | 2.41 b | 2.75 b | 3.42 a | 0.288 | 0.001 |
| Items 2 | Treatments 1 | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| CON-T1 | T2 | T3 | T4 | |||
| Lymphoid organs | ||||||
| Thymus gland | ||||||
| Relative weight | 0.230 | 0.250 | 0.265 | 0.260 | 0.013 | 0.33 |
| Cortex | 278.20 b | 353.38 a | 396.71 a | 408.38 a | 31.13 | 0.01 |
| Medulla | 217.23 | 227.10 | 226.25 | 209.74 | 35.48 | 0.98 |
| Cortex:Medulla | 1.28 b | 1.55 a,b | 1.75 a | 1.95 a | 0.11 | 0.03 |
| Bursa of Fabricius | ||||||
| Relative weight | 0.240 | 0.253 | 0.250 | 0.251 | 0.016 | 0.36 |
| Pelicae height | 99.20 c | 135.39 b | 169.81 a | 161.33 a | 15.76 | 0.008 |
| Follicle width | 189.71 c | 163.78 c | 233.15 b | 314.91 a | 21.06 | <0.001 |
| Cortex | 212.67 b | 223.21 b | 246.24 b | 521.65 a | 37.68 | <0.001 |
| Medulla | 167.12 | 137.83 | 159.93 | 147.84 | 16.69 | 0.37 |
| Cortex:Medulla | 1.30 b | 1.62 b | 1.56 b | 3.65 a | 0.462 | 0.003 |
| Spleen | ||||||
| Relative weight | 0.115 | 0.120 | 0.125 | 0.135 | 0.009 | 0.28 |
| Red pulp | 179.41 | 165.20 | 181.99 | 169.73 | 19.13 | 0.79 |
| White pulp | 126.51 c | 180.41 b | 163.61 b | 231.20 a | 20.35 | 0.009 |
| GCA | 0.37 c | 0.55 b | 0.66 a | 0.63 a,b | 0.038 | <0.001 |
| Digestive organs | ||||||
| Proventriculus | ||||||
| Relative weight | 0.895 | 0.935 | 1.015 | 0.965 | 0.048 | 0.17 |
| Fold height | 493.88 b | 584.23 b | 597.53 b | 1182.03 a | 59.41 | <0.001 |
| Mucosa | 973.42 | 953.84 | 944.59 | 1118.86 | 125.54 | 0.34 |
| Gland | 624.79 b | 892.82 a | 1039.14 a | 855.09 a | 84.93 | 0.008 |
| Lumen | 179.03 | 271.63 | 288.64 | 261.50 | 33.07 | 0.20 |
| Liver | ||||||
| Glycogen density | 1.75 c | 2.43 b,c | 3.08 a,b | 3.50 a | 0.317 | 0.003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Selim, S.; Abdel-Megeid, N.S.; Abou-Elnaga, M.K.; Mahmoud, S.F. Early Nutrition with Different Diets Composition versus Fasting on Immunity-Related Gene Expression and Histomorphology of Digestive and Lymphoid Organs of Layer-Type Chicks. Animals 2021, 11, 1568. https://doi.org/10.3390/ani11061568
Selim S, Abdel-Megeid NS, Abou-Elnaga MK, Mahmoud SF. Early Nutrition with Different Diets Composition versus Fasting on Immunity-Related Gene Expression and Histomorphology of Digestive and Lymphoid Organs of Layer-Type Chicks. Animals. 2021; 11(6):1568. https://doi.org/10.3390/ani11061568
Chicago/Turabian StyleSelim, Shaimaa, Nazema S. Abdel-Megeid, Manal K. Abou-Elnaga, and Samy F. Mahmoud. 2021. "Early Nutrition with Different Diets Composition versus Fasting on Immunity-Related Gene Expression and Histomorphology of Digestive and Lymphoid Organs of Layer-Type Chicks" Animals 11, no. 6: 1568. https://doi.org/10.3390/ani11061568
APA StyleSelim, S., Abdel-Megeid, N. S., Abou-Elnaga, M. K., & Mahmoud, S. F. (2021). Early Nutrition with Different Diets Composition versus Fasting on Immunity-Related Gene Expression and Histomorphology of Digestive and Lymphoid Organs of Layer-Type Chicks. Animals, 11(6), 1568. https://doi.org/10.3390/ani11061568






