Maternal Supplementation with Polyphenols and Omega-3 Fatty Acids during Pregnancy: Prenatal Effects on Growth and Metabolism
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethic Statement
2.2. Animals and Experimental Procedures
2.3. Sampling of Fetuses and Placentas
2.4. Evaluation of Maternal and Fetal Anti/Prooxidant and Metabolic Status
2.5. Statistical Analysis
3. Results
3.1. Effects of Dietary Hydroxytyrosol and n-3 PUFA on the Sows
3.2. Effects of Dietary Hydroxytyrosol and n-3 PUFA on the Fetuses
3.2.1. Effects on Litter Features
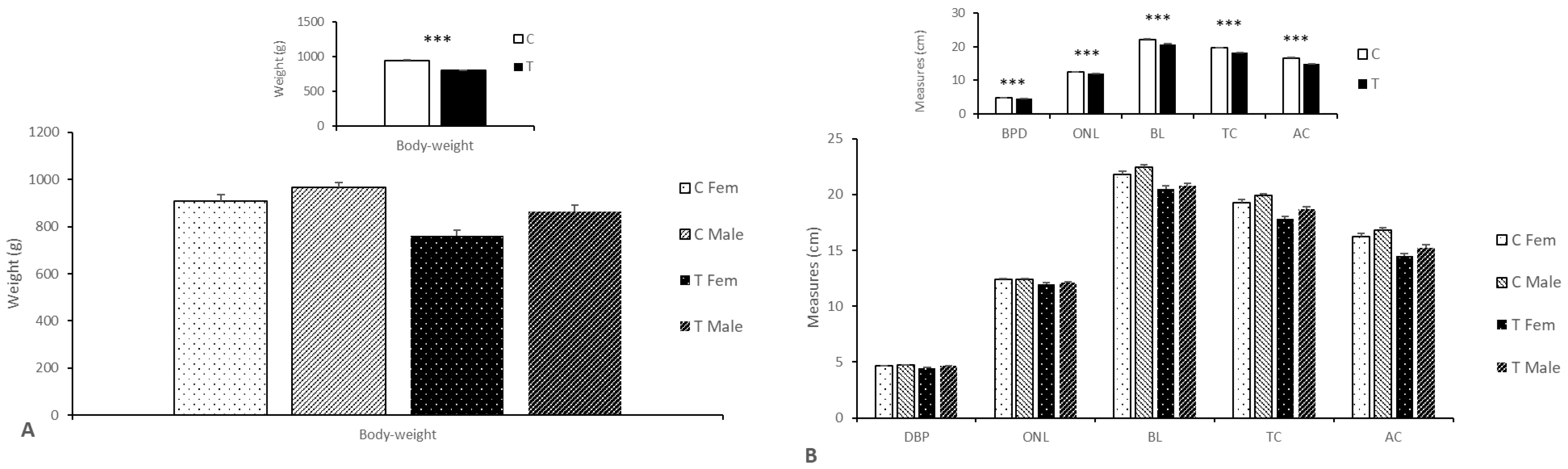
3.2.2. Effects on Body Weight, Size, and Composition
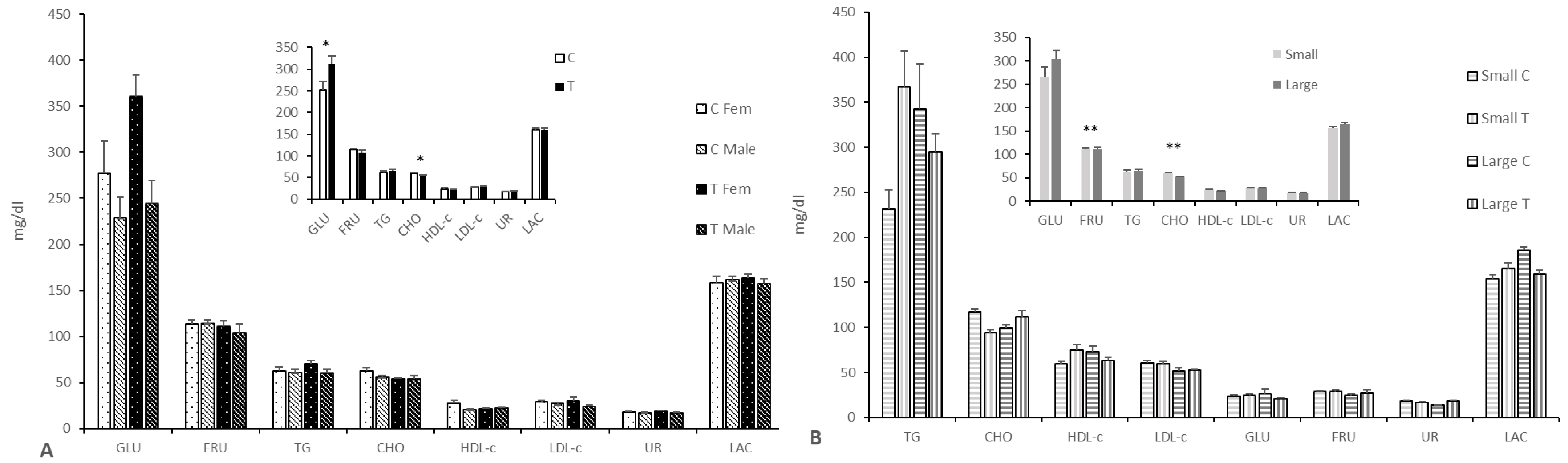
3.2.3. Effects on Fetal Pro-Antioxidant and Metabolic Status
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, G.; Bazer, F.W.; Cudd, T.A.; Meininger, C.J.; Spencer, T.E. Maternal Nutrition and Fetal Development. J. Nutr. 2004, 134, 2169–2172. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med. Cell Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Tundis, R.; Loizzo, M.; Menichini, F.F.; Statti, G.; Menichini, F.F. Biological and Pharmacological Activities of Iridoids: Recent Developments. Mini Rev. Med. Chem. 2008, 8, 399–420. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Contreras, C.; Vazquez-Gomez, M.; Pardo, Z.; Heras-Molina, A.; Pesantez, J.L.; Encinas, T.; Torres-Rovira, L.; Astiz, S.; Nieto, R.; Ovilo, C.; et al. Polyphenols and IUGR pregnancies: Effects of maternal hydroxytyrosol supplementation on hepatic fat accretion and energy and fatty acids profile of fetal tissues. Nutrients 2019, 11, 1534. [Google Scholar] [CrossRef]
- Garcia-Contreras, C.; Vazquez-Gomez, M.; Barbero, A.; Pesantez, J.; Zinellu, A.; Berlinguer, F.; Gonzalez-Añover, P.; Gonzalez, J.; Encinas, T.; Torres-Rovira, L.; et al. Polyphenols and IUGR Pregnancies: Effects of Maternal Hydroxytyrosol Supplementation on Placental Gene Expression and Fetal Antioxidant Status, DNA-Methylation and Phenotype. Int. J. Mol. Sci. 2019, 20, 1187. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Gomez, M.; Garcia-Contreras, C.; Torres-Rovira, L.; Pesantez, J.L.; Gonzalez-Añover, P.; Gomez-Fidalgo, E.; Sanchez-Sanchez, R.; Ovilo, C.; Isabel, B.; Astiz, S.; et al. Polyphenols and IUGR pregnancies: Maternal hydroxytyrosol supplementation improves prenatal and early-postnatal growth and metabolism of the offspring. PLoS ONE 2017, 12, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Gomez, M.; Heras-Molina, A.; Garcia-Contreras, C.; Pesantez-Pacheco, J.L.; Torres-Rovira, L.; Martinez-Fernandez, B.; Gonzalez, J.; Encinas, T.; Astiz, S.; Ovilo, C.; et al. Polyphenols and IUGR Pregnancies: Effects of Maternal Hydroxytyrosol Supplementation on Postnatal Growth, Metabolism and Body Composition of the Offspring. Antioxidants 2019, 8, 535. [Google Scholar] [CrossRef]
- Shrestha, N.; Sleep, S.L.; Cuffe, J.S.M.; Holland, O.J.; Perkins, A.V.; Yau, S.Y.; McAinch, A.J.; Hryciw, D.H. Role of omega-6 and omega-3 fatty acids in fetal programming. Clin. Exp. Pharm. Physiol. 2020, 47, 907–915. [Google Scholar] [CrossRef]
- Leskanich, C.O.; Noble, R.C. The comparative roles of polyunsaturated fatty acids in pig neonatal development. Br. J. Nutr. 1999, 81, 87–106. [Google Scholar] [CrossRef]
- Greenberg, J.A.; Bell, S.J.; Ausdal, W.V. Omega-3 Fatty Acid supplementation during pregnancy. Rev. Obstet. Gynecol. 2008, 1, 162–169. [Google Scholar]
- Haggarty, P. Fatty Acid Supply to the Human Fetus. Annu. Rev. Nutr. 2010, 30, 237–255. [Google Scholar] [CrossRef] [PubMed]
- Hachey, D.L. Benefits and risks of modifying maternal fat intake in pregnancy and lactation. Am. J. Clin. Nutr. 1994, 59, 454S–464S. [Google Scholar] [CrossRef] [PubMed]
- Amusquivar, E.; Rupérez, F.J.; Barbas, C.; Herrera, E. Low Arachidonic Acid Rather than α-Tocopherol Is Responsible for the Delayed Postnatal Development in Offspring of Rats Fed Fish Oil Instead of Olive Oil during Pregnancy and Lactation. J. Nutr. 2000, 130, 2855–2865. [Google Scholar] [CrossRef] [PubMed]
- Thorsdottir, I.; Birgisdottir, B.E.; Halldorsdottir, S.; Geirsson, R.T. Association of Fish and Fish Liver Oil Intake in Pregnancy with Infant Size at Birth among Women of Normal Weight before Pregnancy in a Fishing Community. Am. J. Epidemiol. 2004, 160, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Jump, D.B. The biochemistry of n-3 polyunsaturated fatty acids. J. Biol. Chem. 2002, 277, 8755–8758. [Google Scholar] [CrossRef] [PubMed]
- Chow, C.K. Fatty Acids in Foods and Their Health Implications; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Prostek, A.; Gajewska, M.; Kamola, D.; Bałasińska, B. The influence of EPA and DHA on markers of inflammation in 3T3-L1 cells at different stages of cellular maturation. Lipids Health Dis. 2014, 13, 3. [Google Scholar] [CrossRef]
- Heras-Molina, A.; Pesantez-Pacheco, J.L.; Astiz, S.; Garcia-Contreras, C.; Vazquez-Gomez, M.; Encinas, T.; Óvilo, C.; Isabel, B.; Gonzalez-Bulnes, A. Maternal Supplementation with Polyphenols and Omega-3 Fatty Acids during Pregnancy: Effects on Growth, Metabolism, and Body Composition of the Offspring. Animals 2020, 10, 1946. [Google Scholar] [CrossRef]
- Committee on Nutrient Requirements of Swine; Board on Agriculture and Natural Resources; Division on Earth and Life Studies; National Research Council of the National Academies. Nutrient Requirements of Swine: Eleventh Revised Edition; National Academies Press: Washington, DC, USA, 2012; ISBN 978-0-309-22423-9. [Google Scholar]
- Gonzalez-Bulnes, A.; Astiz, S.; Ovilo, C.; Lopez-Bote, C.J.; Torres-Rovira, L.; Barbero, A.; Ayuso, M.; Garcia-Contreras, C.; Vazquez-Gomez, M. Developmental Origins of Health and Disease in swine: Implications for animal production and biomedical research. Theriogenology 2016, 86, 110–119. [Google Scholar] [CrossRef]
- Lopez-Bote, C.; Rey, A.; Ruiz, J.; Isabel, B.; Sanz Arias, R. Effect of feeding diets high in monounsaturated fatty acids and α-tocopheryl acetate to rabbits on resulting carcass fatty acid profile and lipid oxidation. Anim. Sci. 1997, 64, 177–186. [Google Scholar] [CrossRef]
- Sukhija, P.S.; Palmquist, D.L. Rapid method for determination of total fatty acid content and composition of feedstuffs and feces. J. Agric. Food Chem. 1988, 36, 1202–1206. [Google Scholar] [CrossRef]
- Garcia-Contreras, C.; Vazquez-Gomez, M.; Astiz, S.; Torres-Rovira, L.; Sanchez-Sanchez, R.; Gomez-Fidalgo, E.; Gonzalez, J.; Isabel, B.; Rey, A.; Ovilo, C.; et al. Ontogeny of Sex-Related Differences in Foetal Developmental Features, Lipid Availability and Fatty Acid Composition. Int. J. Mol. Sci. 2017, 18, 1171. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Buege, J.A.; Aust, S.D. Microsomal Lipid Peroxidation. Methods Enzymol. 1978, 52, 302–310. [Google Scholar] [CrossRef]
- Konelab 20—Thermo Scientific—PDF Catalogs | Technical Documentation. Available online: https://pdf.medicalexpo.com/pdf/thermo-scientific/konelab-20/78678-85059.html (accessed on 21 January 2021).
- Rudolph, A.M. The fetal circulation and its response to stress. J. Dev. Physiol. 1984, 6, 11–19. [Google Scholar]
- Webel, S.K.; Otto, E.R.; Webel, D.M.; Moser, R.L.; Spencer, J.D.; Orr, D.E. Effect of protected n-3 polyunsaturated fatty acids (FertiliumTM) on litter size in sows. J. Anim. Sci. 2003, 81, 15000403. [Google Scholar]
- Rooke, J.A.; Sinclair, A.G.; Edwards, S.A.; Cordoba, R.; Pkiyach, S.; Penny, P.C.; Penny, P.; Finch, A.M.; Horgan, G.W. The effect of feeding salmon oil to sows throughout pregnancy on pre-weaning mortality of piglets. Anim. Sci. 2001, 73, 489–500. [Google Scholar] [CrossRef]
- Gonzalez-Añover, P.; Encinas, T.; Torres-Rovira, L.; Pallares, P.; Muñoz-Frutos, J.; Gomez-Izquierdo, E.; Sanchez-Sanchez, R.; Gonzalez-Bulnes, A. Ovulation rate, embryo mortality and intrauterine growth retardation in obese swine with gene polymorphisms for leptin and melanocortin receptors. Theriogenology 2011, 75, 34–41. [Google Scholar] [CrossRef]
- van der Lende, T.; de Jager, D. Death risk and preweaning growth rate of piglets in relation to the within-litter weight distribution at birth. Livest. Prod. Sci. 1991, 28, 73–84. [Google Scholar] [CrossRef]
- Lavery, A.; Lawlor, P.G.; Miller, H.M.; Magowan, E. The Effect of Dietary Oil Type and Energy Intake in Lactating Sows on the Fatty Acid Profile of Colostrum and Milk, and Piglet Growth to Weaning. Animals 2019, 9, 1092. [Google Scholar] [CrossRef]
- Luo, J.; Huang, F.; Xiao, C.; Fang, Z.; Peng, J.; Jiang, S. Responses of growth performance and proinflammatory cytokines expression to fish oil supplementation in lactation sows’ and/or weaned piglets’ diets. BioMed Res. Int. 2013, 2013. [Google Scholar] [CrossRef]
- Smit, M.N.; Spencer, J.D.; Patterson, J.L.; Dyck, M.K.; Dixon, W.T.; Foxcroft, G.R. Effects of dietary enrichment with a marine oil-based n-3 LCPUFA supplement in sows with predicted birth weight phenotypes on birth litter quality and growth performance to weaning. Animal 2015, 9, 471–480. [Google Scholar] [CrossRef]
- Grieger, J.A.; Clifton, V.L. A review of the impact of dietary intakes in human pregnancy on infant birthweight. Nutrients 2014, 7, 153–178. [Google Scholar] [CrossRef]
- Innis, S.M. Dietary (n-3) fatty acids and brain development. J. Nutr. 2007, 137, 855–859. [Google Scholar] [CrossRef]
- González-Correa, J.A.; Navas, M.D.; Lopez-Villodres, J.A.; Trujillo, M.; Espartero, J.L.; De La Cruz, J.P. Neuroprotective effect of hydroxytyrosol and hydroxytyrosol acetate in rat brain slices subjected to hypoxia–reoxygenation. Neurosci. Lett. 2008, 446, 143–146. [Google Scholar] [CrossRef]
- López de las Hazas, M.-C.; Godinho-Pereira, J.; Macià, A.; Almeida, A.F.; Ventura, M.R.; Motilva, M.-J.; Santos, C.N. Brain uptake of hydroxytyrosol and its main circulating metabolites: Protective potential in neuronal cells. J. Funct. Foods 2018, 46, 110–117. [Google Scholar] [CrossRef]
- Schaffer, S.; Podstawa, M.; Visioli, F.; Bogani, P.; Müller, W.E.; Eckert, G.P. Hydroxytyrosol-rich olive mill wastewater extract protects brain cells in vitro and ex vivo. J. Agric. Food Chem. 2007, 55, 5043–5049. [Google Scholar] [CrossRef] [PubMed]
- Bell, A.W.; Hay, W.W., Jr.; Ehrhardt, R.A. Placental transport of nutrients and its implications for fetal growth. J. Reprod Fertil. Suppl. 1999, 54, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Hay, W.W., Jr. Placental transport of nutrients to the fetus. Horm. Res. 1994, 42, 215–222. [Google Scholar] [CrossRef]
- Hay, W.W., Jr. Recent observations on the regulation of fetal metabolism by glucose. J. Physiol. 2006, 572, 17–24. [Google Scholar] [CrossRef]
- Saintonge, J.; Côté, R. Brain development in relation to fetal weight and maternal glucose tolerance during normal gestation. Brain Dev. 1987, 9, 26–32. [Google Scholar] [CrossRef]
- Girard, J.; Ferre, P.; Pegorier, J.; Duee, P. Adaptations of glucose and fatty acid metabolism during perinatal period and suckling-weaning transition. Physiol. Rev. 1992, 72, 507–562. [Google Scholar] [CrossRef]
- Johnson, R.N.; Metcalf, P.A.; Baker, J.R. Fructosamine: A new approach to the estimation of serum glycosylprotein. An index of diabetic control. Clin. Chim. Acta 1983, 127, 87–95. [Google Scholar] [CrossRef]
- Segovia, S.A.; Vickers, M.H.; Zhang, X.D.; Gray, C.; Reynolds, C.M. Maternal supplementation with conjugated linoleic acid in the setting of diet-induced obesity normalises the inflammatory phenotype in mothers and reverses metabolic dysfunction and impaired insulin sensitivity in offspring. J. Nutr. Biochem. 2015, 26, 1448–1457. [Google Scholar] [CrossRef]
- Flachs, P.; Rossmeisl, M.; Bryhn, M.; Kopecky, J. Cellular and molecular effects of n-3 polyunsaturated fatty acids on adipose tissue biology and metabolism. Clin. Sci. 2009, 116, 1–16. [Google Scholar] [CrossRef]
- Yang, L.G.; Song, Z.X.; Yin, H.; Wang, Y.Y.; Shu, G.F.; Lu, H.X.; Wang, S.K.; Sun, G.J. Low n-6/n-3 PUFA Ratio Improves Lipid Metabolism, Inflammation, Oxidative Stress and Endothelial Function in Rats Using Plant Oils as n-3 Fatty Acid Source. Lipids 2016, 51, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Roche, H.M.; Gibney, M.J. Long-chain n-3 polyunsaturated fatty acids and triacylglycerol metabolism in the postprandial state. Lipids 1999, 34, S259–S265. [Google Scholar] [CrossRef]
- Peyrol, J.; Riva, C.; Amiot, M.J. Hydroxytyrosol in the Prevention of the Metabolic Syndrome and Related Disorders. Nutrients 2017, 9, 306. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.T. Fetal metabolism and fetal growth. J. Reprod. Fertil. 1976, 47, 189–201. [Google Scholar] [CrossRef] [PubMed][Green Version]
| g/100 g Total Fatty Acids | ||||||||
|---|---|---|---|---|---|---|---|---|
| Estimated Analysis g/kg | Group C | Group T | Ingredient g/kg Dry Matter Basis | Group C | Group T | Fatty Acids | Group C | Group T |
| Dry Matter (DM) | 910.7 | 910.3 | Barley | 234.0 | 299.0 | C14:0 | 0.463 | 0.198 |
| EM (Kcal/kg) | 2910.4 | 2909.1 | Wheat | 300.1 | 192.5 | C16:0 | 20.64 | 11.83 |
| Crude protein | 122.8 | 123.4 | Wheat bran | 245.5 | 300.0 | C16:1 n-9 | 0.203 | 0.161 |
| Crude fat | 35.5 | 62.3 | Cookie Flour | 100.0 | 0.0 | C16:1 n-7 | 0.867 | 0.192 |
| Crude fiber | 62.1 | 73.0 | Beet pulp | 50.0 | 50.0 | C17:0 | 0.469 | 0.234 |
| Sunflower flour (28%) | 31.5 | 68.0 | C18:0 | 3.695 | 3.232 | |||
| Soybean oil | 7.0 | 0.0 | C18:1 n-9 | 19.26 | 18.38 | |||
| Linseed oil | 0.0 | 40.0 | C18:1 n-7 | 4.736 | 3.101 | |||
| Sepiolite | 0.0 | 18.1 | C18:2 n-6 | 41.51 | 32.26 | |||
| Salt | 5.0 | 5.0 | C18:3 n-3 | 4.132 | 28.96 | |||
| Phosfate Monocalcium | 2.5 | 2.5 | C20:0 | 0.349 | 0.288 | |||
| Calcium carbonate | 16.5 | 17.5 | C20:1 n-9 | 0.904 | 0.442 | |||
| L-Lysine (500 g/kg) | 4.2 | 3.8 | C20:5 n-3 | 0.234 | 0.105 | |||
| L-Threonine | 0.7 | 0.6 | C22:4 n-6 | 0.122 | 0.093 | |||
| MVP | 3.0 | 3.0 | C22:5 n-3 | 1.248 | 0.287 | |||
| C22:6 n-3 | 1.151 | 0.225 | ||||||
| SFA | 25.62 | 15.78 | ||||||
| MUFA | 25.97 | 22.28 | ||||||
| PUFA | 48.39 | 61.93 | ||||||
| n-6 | 41.63 | 32.35 | ||||||
| n-3 | 6.766 | 29.58 | ||||||
| n-6/n-3 | 6.153 | 1.094 | ||||||
| Treatment | Litter Size | ||||
|---|---|---|---|---|---|
| Control | Treated | Small | Large | ||
| Weights (g) | |||||
| Head | 207.4 E ± 2.94 | 183.4 F ± 3.17 | 200.5 A ± 3.33 | 188.4 B ± 3.37 | |
| Carcass | 525.3 E ± 10.1 | 442.3 F ± 10.8 | 500.8 A ± 11.4 | 460.2 B ± 11.6 | |
| Viscera | 165.8 E ± 3.81 | 138.8 F ± 3.46 | 156.7 ± 4.16 | 145.7 ± 3.71 | |
| Brain | 29.9 ± 0.23 | 29.3 ± 0.23 | 29.5 ± 0.19 | 29.7 ± 0.28 | |
| Heart | 9.09 E ± 0.24 | 7.70 F ± 0.19 | 8.63 ± 0.25 | 8.0 ± 0.20 | |
| Lungs | 31.8 E± 0.80 | 27.9 F ± 0.76 | 30.4 ± 0.78 | 29.0 ± 0.84 | |
| Liver | 28.4 E ± 0.81 | 24.9 F ± 0.72 | 27.3 ± 0.82 | 25.6 ± 0.76 | |
| Intestines | 54.6 E ± 1.50 | 44.5 F ± 1.17 | 51.8 C ± 1.58 | 46.4 D ± 1.26 | |
| Kidneys | 7.90 E ± 0.19 | 6.61 F ± 0.17 | 7.55 C ± 0.20 | 6.84 D ± 0.18 | |
| Spleen | 1.98 C ± 0.05 | 1.78 D ± 0.05 | 1.97 A ± 0.05 | 1.78 B ± 0.05 | |
| Ratios | |||||
| Head | /Total | 0.398 C ± 0.002 | 0.231 D ± 0.002 | 0.226 ± 0.002 | 0.228 ± 0.002 |
| Carcass | 0.315 E ± 0.003 | 0.549 F ± 0.002 | 0.559 C ± 0.002 | 0.549 D ± 0.003 | |
| Viscera | 0.176 A ± 0.002 | 0.172 B ± 0.001 | 0.174 ± 0.001 | 0.174 ± 0.001 | |
| Brain | /Head | 0.146 E ± 0.002 | 0.162 F± 0.003 | 0.149 C ± 0.002 | 0.160 D ± 0.003 |
| Heart | /Viscera | 0.055 ± 0.001 | 0.056 ± 0.001 | 0.055 ± 0.001 | 0.056 ± 0.001 |
| Lungs | 0.193 A ± 0.003 | 0.202 B ± 0.003 | 0.196 ± 0.003 | 0.200 ± 0.003 | |
| Liver | 0.173 ± 0.003 | 0.179 ± 0.003 | 0.175 ± 0.002 | 0.178 ± 0.004 | |
| Intestines | 0.330 A ± 0.004 | 0.321 B ± 0.003 | 0.329 A ± 0.003 | 0.320 B ± 0.003 | |
| Kidneys | 0.048 A ± 0.001 | 0.048 B ± 0.001 | 0.048 ± 0.001 | 0.048 ± 0.001 | |
| Spleen | 0.012 A ± 0.000 | 0.013 B ± 0.000 | 0.013 ± 0.000 | 0.012 ± 0.000 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heras-Molina, A.; Pesántez-Pacheco, J.L.; Garcia-Contreras, C.; Vázquez-Gómez, M.; López, A.; Benítez, R.; Núñez, Y.; Astiz, S.; Óvilo, C.; Isabel, B.; et al. Maternal Supplementation with Polyphenols and Omega-3 Fatty Acids during Pregnancy: Prenatal Effects on Growth and Metabolism. Animals 2021, 11, 1699. https://doi.org/10.3390/ani11061699
Heras-Molina A, Pesántez-Pacheco JL, Garcia-Contreras C, Vázquez-Gómez M, López A, Benítez R, Núñez Y, Astiz S, Óvilo C, Isabel B, et al. Maternal Supplementation with Polyphenols and Omega-3 Fatty Acids during Pregnancy: Prenatal Effects on Growth and Metabolism. Animals. 2021; 11(6):1699. https://doi.org/10.3390/ani11061699
Chicago/Turabian StyleHeras-Molina, Ana, José Luis Pesántez-Pacheco, Consolación Garcia-Contreras, Marta Vázquez-Gómez, Adrián López, Rita Benítez, Yolanda Núñez, Susana Astiz, Cristina Óvilo, Beatriz Isabel, and et al. 2021. "Maternal Supplementation with Polyphenols and Omega-3 Fatty Acids during Pregnancy: Prenatal Effects on Growth and Metabolism" Animals 11, no. 6: 1699. https://doi.org/10.3390/ani11061699
APA StyleHeras-Molina, A., Pesántez-Pacheco, J. L., Garcia-Contreras, C., Vázquez-Gómez, M., López, A., Benítez, R., Núñez, Y., Astiz, S., Óvilo, C., Isabel, B., & González-Bulnes, A. (2021). Maternal Supplementation with Polyphenols and Omega-3 Fatty Acids during Pregnancy: Prenatal Effects on Growth and Metabolism. Animals, 11(6), 1699. https://doi.org/10.3390/ani11061699







