Review of Peste des Petits Ruminants Occurrence and Spread in Tanzania
Abstract
:Simple Summary
Abstract
1. Introduction
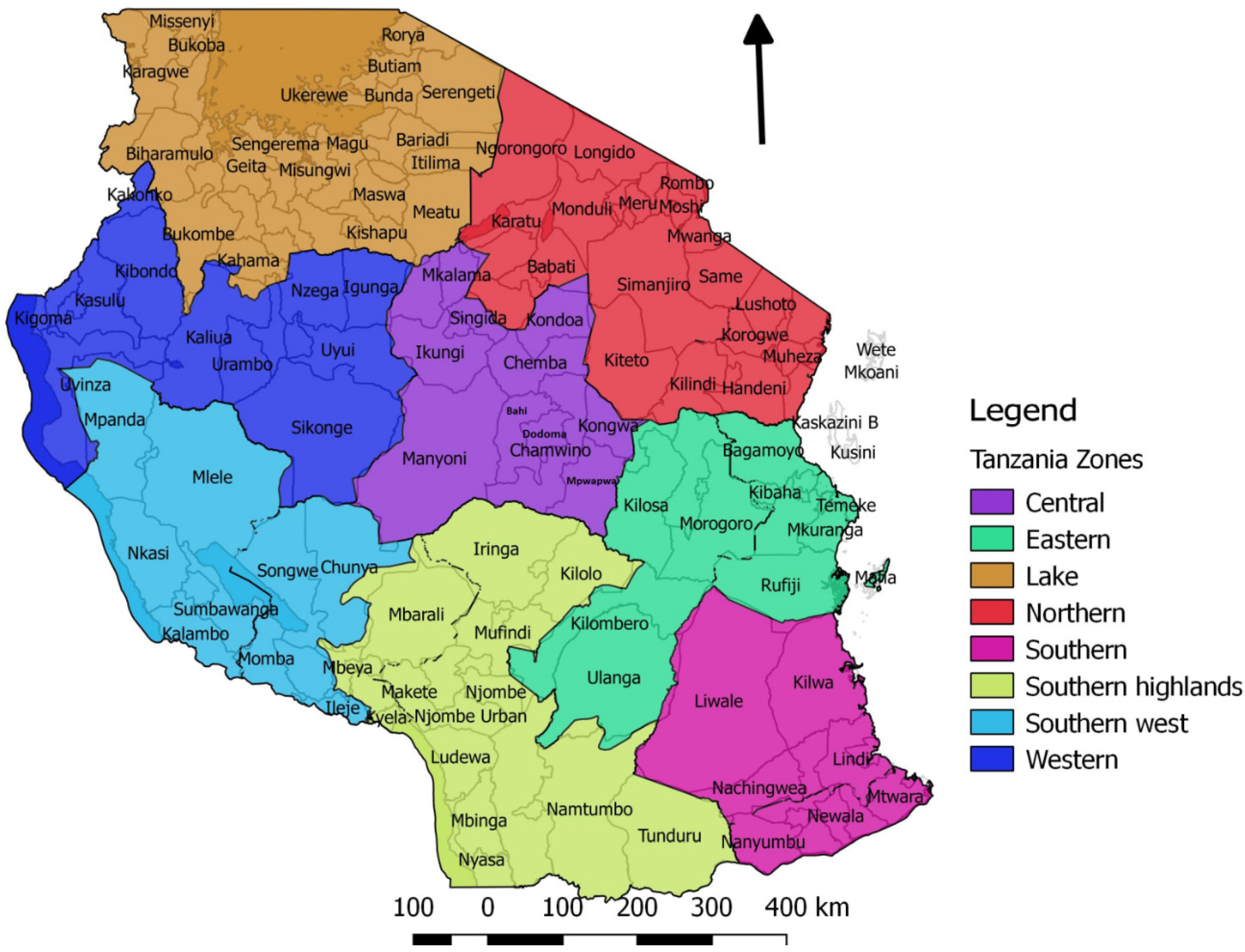
2. Materials and Methods
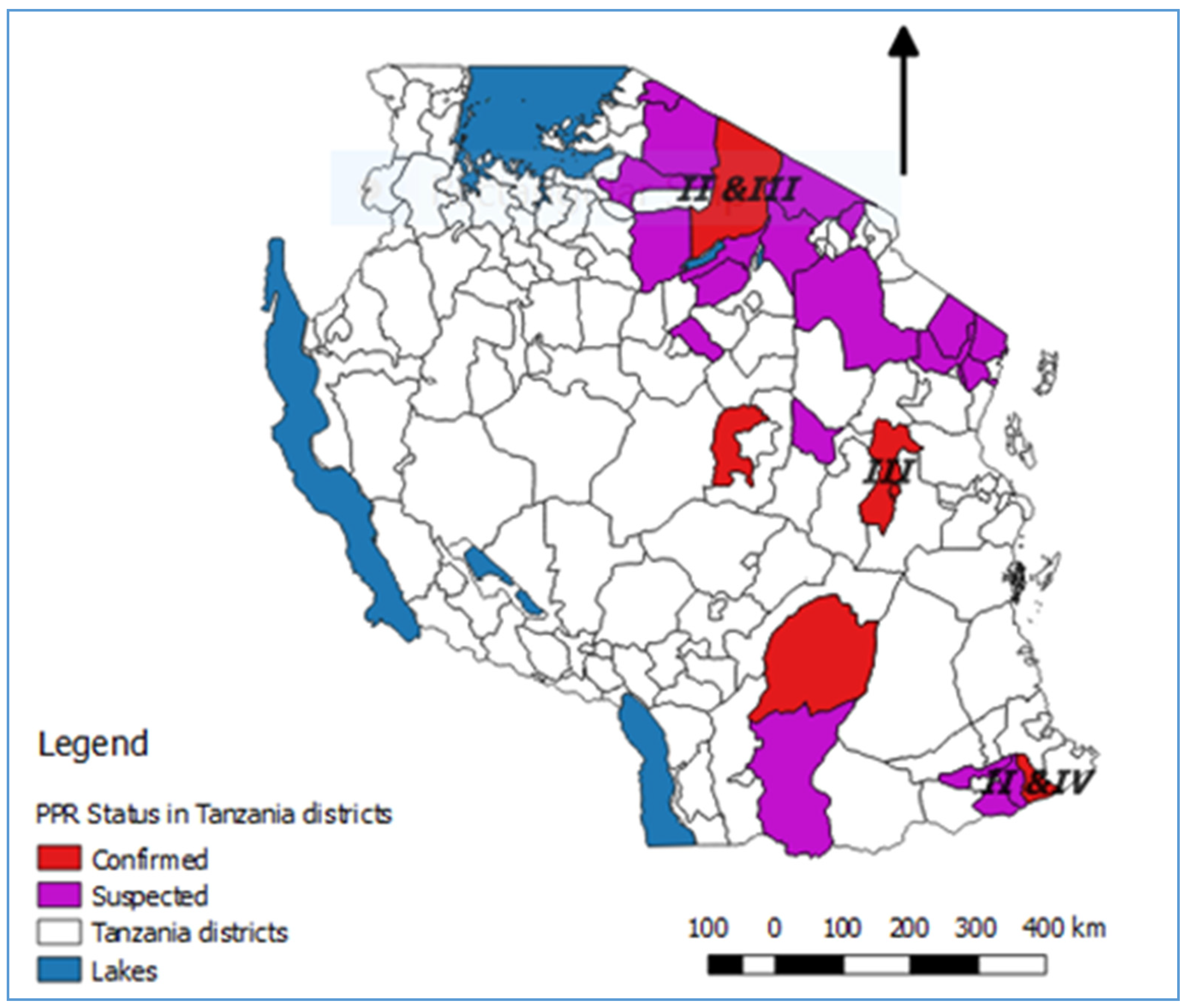
3. Results and Discussion
3.1. Evidence of PPRV in Tanzania Prior to Confirmation of Infection in 2008
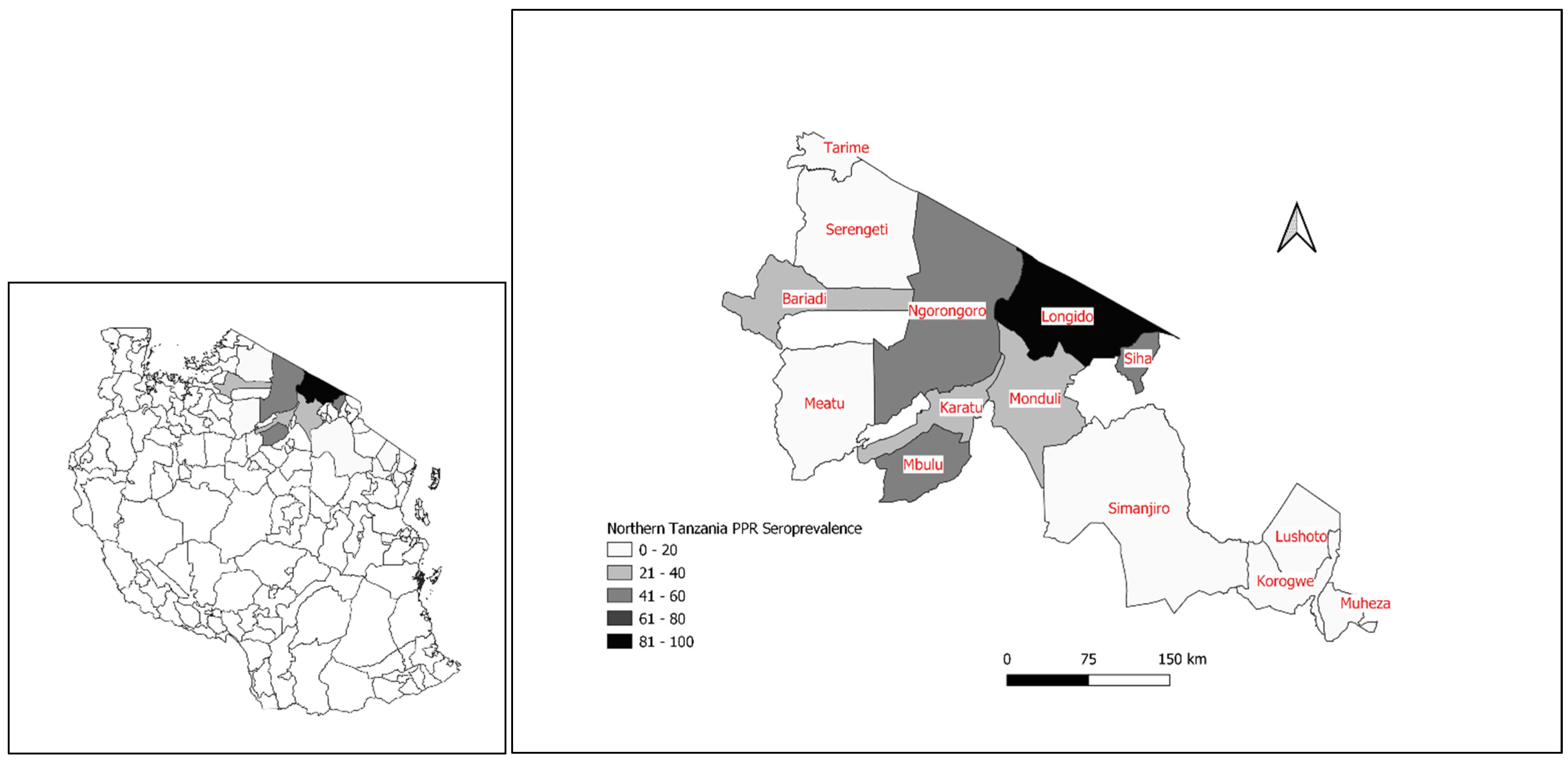
3.2. The First Confirmed PPRV Disease Cases in Northern Tanzania, 2008
3.3. Emergence of PPRV Disease in Southern Tanzania, 2009–2010
3.4. PPRV Disease in Eastern Tanzania, 2010
3.5. PPRV Disease in Central Tanzania, 2014
3.6. Evidence of PPRV Infection in Camels and Cattle
3.7. Evidence of PPRV Infection in Wildlife
3.8. Molecular Biology of PPRV in Tanzania
- Lineage III: KF939644 Tanzania Ngorongoro goat 2013 [55], KT989870 PPRV/TAN/Melela/2014 and KT989871 PPRV/TAN/Magadu/2014 [62], KF939643 Tanzania Dakawa Goat 2013 [55]. The six sequences marked by an asterisk (PPRV/TAN/Goat 3, 4, 10, 11 and Sheep 14, 15/2015 are GenBank accession numbers: MT181842-47 obtained from Ngorongoro 2015 [56].
- Lineage IV: KF 672,745 Tanzania, MM88 Goat 2011 [14].
3.9. PPRV Serological Surveys
3.10. PPRV Co-Infections with Other Pathogens
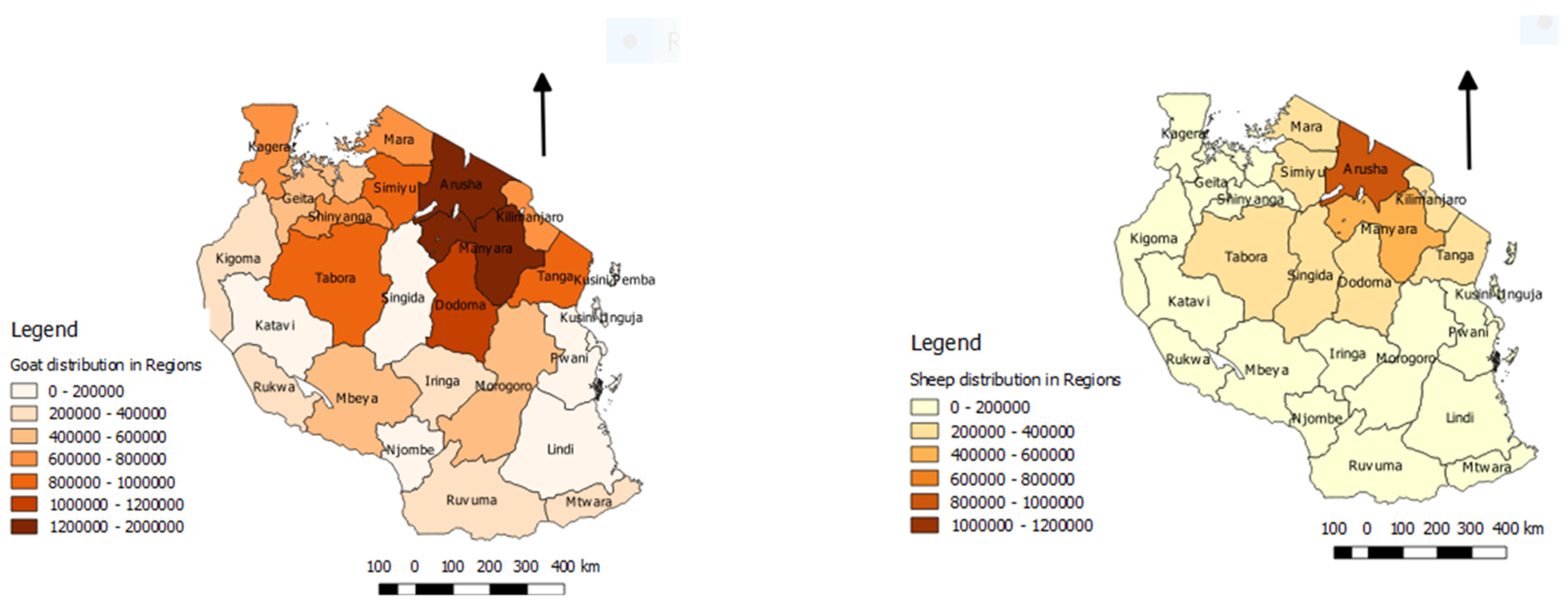
3.11. Socio-Economic Impact of PPR Disease
3.12. Challenges for the Control and Eradication of PPRV in Tanzania
3.12.1. Timely Diagnosis and Intervention
3.12.2. Diagnosis of PPR Disease
3.12.3. Extensive Production System with Mobility of Small Ruminants
3.12.4. Transboundary Small Ruminant Movements
3.12.5. Small Ruminant Trade
3.12.6. Wildlife
3.12.7. Strengthening of Veterinary Services
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO; OIE. Global strategy for the control and eradication of PPR. In Proceedings of the International Conference for the Control and Eradication of Peste des Petits Ruminants (PPR), Abidjan, Ivory Coast, 31 March–2 April 2015. [Google Scholar]
- Hoffmann, B.; Wiesner, H.; Maltzan, J.; Mustefa, R.; Eschbaumer, M.; Arif, F.A.; Beer, M. Fatalities in Wild Goats in Kurdistan Associated with Peste Des Petits Ruminants Virus. Transbound. Emerg. Dis. 2011, 59, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Munir, M. Role of Wild Small Ruminants in the Epidemiology of Peste des Petits Ruminants. Transbound. Emerg. Dis. 2013, 61, 411–424. [Google Scholar] [CrossRef] [PubMed]
- Pruvot, M.; Fine, A.E.; Hollinger, C.; Strindberg, S.; Damdinjav, B.; Buuveibaatar, B.; Chimeddorj, B.; Bayandonoi, G.; Khishgee, B.; Sandag, B.; et al. Outbreak of peste des petits ruminants virus among critically endangered Mongolian saiga and other wild ungulates, Mongolia, 2016–2017. Emerg. Infect. Dis. 2020, 26, 51. [Google Scholar] [CrossRef]
- Muniraju, M.; Munir, M.; Parthiban, A.R.; Banyard, A.C.; Bao, J.; Wang, Z.; Ayebazibwe, C.; Ayelet, G.; El Harrak, M.; Mahapatra, M.; et al. Molecular Evolution of Peste des Petits Ruminants Virus. Emerg. Infect. Dis. 2014, 20, 2023–2033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Libeau, G.; Diallo, A.; Parida, S. Evolutionary genetics underlying the spread of peste des petits ruminants virus. Anim. Front. 2014, 4, 14–20. [Google Scholar] [CrossRef] [Green Version]
- Clarke, B.D.; Islam, M.R.; Abu Yusuf, M.; Mahapatra, M.; Parida, S. Molecular detection, isolation and characterization of Peste-des-petits ruminants virus from goat milk from outbreaks in Bangladesh and its implication for eradication strategy. Transbound. Emerg. Dis. 2018, 65, 1597–1604. [Google Scholar] [CrossRef]
- Banyard, A.C.; Parida, S.; Batten, C.; Oura, C.; Kwiatek, O.; Libeau, G. Global distribution of peste des petits ruminants virus and prospects for improved diagnosis and control. J. Gen. Virol. 2010, 91, 2885–2897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, F.; Li, J.; Li, L.; Liu, Y.; Wu, X.; Wang, Z. Peste des petits ruminants in China since its first outbreak in 2007: A 10-year review. Transbound. Emerg. Dis. 2018, 65, 638–648. [Google Scholar] [CrossRef]
- Abu Elzein, E.M.E.; Housawi, F.M.T.; Bashareek, Y.; Gameel, A.A.; Al-Afaleq, A.I.; Anderson, E. Severe PPR Infection in gazelles kept under semi-free range conditions. J. Vet. Med. Ser. B Infect. Dis. Vet. Public Health 2004, 51, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Abubakar, M.; Munir, M. Peste des Petits Ruminants Virus: An Emerging Threat to Goat Farming in Pakistan. Transbound. Emerg. Dis. 2014, 61 (Suppl. 1), 7–10. [Google Scholar] [CrossRef]
- Rahman, M.Z.; Haider, N.; Gurley, E.S.; Ahmed, S.; Osmani, M.G.; Hossain, M.B.; Islam, A.; Khan, S.A.; Hossain, M.E.; Epstein, J.H.; et al. Epidemiology and genetic characterization of Peste des petits ruminants virus in Bangladesh. Vet. Med. Sci. 2018, 4, 161–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwiatek, O.; Ali, Y.H.; Saeed, I.; Khalafalla, A.I.; Mohamed, O.I.; Abu Obeida, A.; Abdelrahman, M.B.; Osman, H.M.; Taha, K.M.; Abbas, Z.; et al. Asian Lineage of Peste des Petits Ruminants Virus, Africa. Emerg. Infect. Dis. 2011, 17, 1223–1231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Misinzo, G.; Kgotlele, T.; Muse, E.A.; Van Doorsselaere, J.; Berg, M.; Munir, M. Peste des Petits Ruminants Virus Lineage II and IV From Goats in Southern Tanzania During an Outbreak in 2011. Br. J. Virol. 2015, 2, 1–4. [Google Scholar]
- Baron, M.D.; Parida, S.; Oura, C.A.L. Peste des petits ruminants: A suitable candidate for eradication? Vet. Rec. 2011, 169, 16–21. [Google Scholar] [CrossRef]
- Truong, T.; Boshra, H.; Embury-Hyatt, C.; Nfon, C.; Gerdts, V.; Tikoo, S.; Babiuk, L.A.; Kara, P.D.; Chetty, T.; Mather, A.; et al. Peste des Petits Ruminants Virus Tissue Tropism and Pathogenesis in Sheep and Goats following Experimental Infection. PLoS ONE 2014, 9, e87145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahapatra, M.; Sayalel, K.; Muniraju, M.; Eblate, E.; Fyumagwa, R.; Shilinde, S.; MaulidMdaki, M.; Keyyu, J.; Parida, S.; Kock, R. Spillover of Peste des Petits Ruminants Virus from Domestic to Wild Ruminants in the Serengeti Ecosystem, Tanzania. Emerg. Infect. Dis. 2015, 21, 2230–2234. [Google Scholar] [CrossRef] [Green Version]
- Abubakar, M.; Mahapatra, M.; Muniraju, M.; Arshed, M.J.; Khan, E.H.; Banyard, A.C.; Ali, Q.; Parida, S. Serological Detection of Antibodies to Peste des Petits Ruminants Virus in Large Ruminants. Transbound. Emerg. Dis. 2015, 64, 513–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lembo, T.; Oura, C.; Parida, S.; Hoare, R.; Frost, L.; Fyumagwa, R.; Kivaria, F.; Chubwa, C.; Kock, R.; Cleaveland, S.; et al. Peste des Petits Ruminants Infection among Cattle and Wildlife in Northern Tanzania. Emerg. Infect. Dis. 2013, 19, 2037–2040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madege. Peste des Petits Ruminants (PPR) and Contagious Pleuropneumonia (CBPP) Surveillance in Lake Zone, Presentation to the VACNADA Inception Workshop, Morogoro, 2011; Archived document at Zonal Veterinary Investigation Centre: Mwanza, Tanzania, 2011. [Google Scholar]
- Baron, J.; Bin-Tarif, A.; Herbert, R.; Frost, L.; Taylor, G.; Baron, M.D. Early changes in cytokine expression in peste des petits ruminants disease. Vet. Res. 2014, 45, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munir, M.; Zohari, S.; Berg, M.; Munir, M.; Zohari, S.; Berg, M. Replication and Virulence Determinants of Peste des Petits Ruminants Virus. In Molecular Biology and Pathogenesis of Peste des Petits Ruminants Virus; Springer: Berlin, Germany, 2013; pp. 23–32. [Google Scholar] [CrossRef]
- Parida, S.; Muniraju, M.; Mahapatra, M.; Muthuchelvan, D.; Buczkowski, H.; Banyard, A. Peste des petits ruminants. Vet. Microbiol. 2015, 181, 90–106. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.K.; Rajak, K.K.; Muthuchelvan, D.; Banyard, A.C.; Parida, S. Chapter 11 Vaccines against Peste des Petits ruminants virus. In Peste des Petits Ruminants Virus; Munir, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 51–67. [Google Scholar]
- Singh, R.P.; Bandyopadhyay, S.K. Peste des petits ruminants vaccine and vaccination in India: Sharing experience with disease endemic countries. Virus Dis. 2015, 26, 215–224. [Google Scholar] [CrossRef] [Green Version]
- Awa, D.N.; Ngagnou, A.; Tefiang, E.; Yaya, D.; Njoya, A. Post vaccination and colostral peste des petits ruminants antibody dynamics in research flocks of Kirdi goats and Foulbe sheep of north Cameroon. Prev. Vet. Med. 2002, 55, 265–271. [Google Scholar] [CrossRef]
- Balamurugan, V.; Sen, A.; Venkatesan, G.; Rajak, K.K.; Bhanuprakash, V.; Singh, R.K. Study on passive immunity: Time of vaccination in kids born to goats vaccinated against Peste des petits ruminants. Virol. Sin. 2012, 27, 228–233. [Google Scholar] [CrossRef]
- Enchery, F.; Hamers, C.; Kwiatek, O.; Gaillardet, D.; Montange, C.; Brunel, H.; Goutebroze, S.; Philippe-Reversat, C.; Libeau, G.; Hudelet, P.; et al. Development of a PPRV challenge model in goats and its use to assess the efficacy of a PPR vaccine. Vaccine 2019, 37, 1667–1673. [Google Scholar] [CrossRef] [PubMed]
- FAO; OIE. Peste des Petits Ruminants Global Eradication Programme; FAO: Rome, Italy, 2016. [Google Scholar]
- Silanikove, N. Goat production under harsh environmental conditions: The physiological basis and the challenge. In The Opportunities and Challenges for Enhancing Goat Production in East Africa; Langston University: Langston, OK, USA, 2000. [Google Scholar]
- El Hag Ali, B.; Taylor, W.P. Isolation of peste des petits ruminants virus from the Sudan. Res. Vet. Sci. 1984, 36, 1–4. [Google Scholar] [CrossRef]
- Roeder, P.L.; Abraham, G.; Kenfe, G.; Barrett, T. Peste des petits ruminants in Ethiopian goats. Trop. Anim. Health Prod. 1994, 26, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Gitao, C.G.; Ithinji, D.G.; Gitari, R.; Ireri, G.R. The confirmation of Peste des petit ruminants (PPR) in Kenya and perception of the disease in West Pokot. Res. Opin. Anim. Vet. Sci. 2014, 4, 312–317. [Google Scholar]
- Wamwayi, H.M.; Rossiter, P.B.; Kariuki, D.P.; Wafula, J.S.; Barrett, T.; Anderson, J. Peste des petits ruminants antibodies in east Africa. Vet. Rec. 1995, 136, 199–200. [Google Scholar] [CrossRef] [PubMed]
- Kock, R.; Wambua, J.M.; Mwanzia, J.; Wamwayi, H.; Ndungu, E.K.; Barrett, T.; Kock, N.D.; Rossiter, P.B. Rinderpest epidemic in wild ruminants in Kenya 1993-97. Vet. Rec. 1999, 145, 275–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nkamwesiga, J.; Coffin-Schmitt, J.; Ochwo, S.; Mwiine, F.N.; Palopoli, A.; Ndekezi, C.; Isingoma, E.; Nantima, N.; Nsamba, P.; Adiba, R.; et al. Identification of Peste des Petits Ruminants Transmission Hotspots in the Karamoja Subregion of Uganda for Targeting of Eradication Interventions. Front. Vet. Sci. 2019, 6, 221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruhweza, S.; Ayebazibwe, C.; Mwiine, F.; Muhanguzi, D.; Olaho, W. Seroprevalence of Peste des Petits Ruminants (PPR) virus antibodies in goats and sheep in north-eastern Uganda. Bull. Anim. Health Prod. Afr. 2010, 58. [Google Scholar] [CrossRef]
- Aguilar, X.F.; Mahapatra, M.; Begovoeva, M.; Kalema-Zikusoka, G.; Driciru, M.; Ayebazibwe, C.; Adwok, D.S.; Kock, M.; Lukusa, J.-P.K.; Muro, J.; et al. Peste des Petits Ruminants at the Wildlife–Livestock Interface in the Northern Albertine Rift and Nile Basin, East Africa. Viruses 2020, 12, 293. [Google Scholar] [CrossRef] [Green Version]
- Kivaria, F.M.; Kwiatek, O.; Kapaga, A.M.; Swai, E.S.; Libeau, G.; Moshy, W.; Mbyuzi, A.O.; Gladson, J. The incursion, persistence and spread of peste des petits ruminants in Tanzania: Epidemiological patterns and predictions. Onderstepoort J. Vet. Res. 2013, 80, 10. [Google Scholar] [CrossRef] [PubMed]
- Niyokwishimira, A.; Baziki, J.D.D.; Dundon, W.G.; Nwankpa, N.; Njoroge, C.; Boussini, H.; Wamwayi, H.; Jaw, B.; Cattoli, G.; Nkundwanayo, C.; et al. Detection and molecular characterization of Peste des Petits Ruminants virus from outbreaks in Burundi. Transbound. Emerg. Dis. 2019, 66, 2067–2073. [Google Scholar] [CrossRef] [PubMed]
- Torsson, E.; Kgotlele, T.; Berg, M.; Mtui-Malamsha, N.; Swai, E.S.; Wensman, J.J.; Misinzo, G. History and current status of peste des petits ruminants virus in Tanzania. Infect. Ecol. Epidemiol. 2016, 6, 32701. [Google Scholar] [CrossRef] [PubMed]
- Shyaka, A.; Ugirabe, M.A.; Wensman, J.J. Serological Evidence of Exposure to Peste des Petits Ruminants in Small Ruminants in Rwanda. Front. Vet. Sci. 2021, 8, 186. [Google Scholar] [CrossRef] [PubMed]
- Britton, A.; Caron, A.; Bedane, B. Progress to Control and Eradication of Peste des Petits Ruminants in the Southern African Development Community Region. Front. Vet. Sci. 2019, 6, 6. [Google Scholar] [CrossRef]
- African Union. The Pan African Strategy for Control and Eradication of Peste des Petits Ruminants; African Union—Interafrican Bureau for Animal Resources (AU-IBAR): Nairobi, Kenya, September 2015; pp. 1–3. [Google Scholar]
- ICPALD. The IGAD Regional Peste des Petits Ruminants (PPR) Control and Eradication Strategy Presented at the Global Conference In Abijan (31 March–2 April 2015). 2016. Available online: http://icpald.org/wp-content/uploads/2016/01/Progressive-Control-and-Eradication-Strategy-1.pdf (accessed on 23 April 2019).
- Wambura, P.N. Serological evidence of the absence of peste des petits ruminants in Tanzania. Vet. Rec. 2000, 146, 473–474. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.; McKay, J.A. The detection of antibodies against peste des petits ruminants virus in cattle, sheep and goats and the possible implications to rinderpest control programmes. Epidemiol. Infect. 1994, 112, 225–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kock, R.A. The Role of Wildlife in the Epidemiology of Rinderpest in East and Central Africa 1994–2004: A Study Based on Serological Surveillance and Disease Investigation. Ph.D. Thesis, University of Cambridge, Cambridge, UK, 2008. [Google Scholar]
- Karimuribo, E.D.; Loomu, P.M.; Mellau, L.S.B.; Swai, E.S. Retrospective study on sero-epidemiology of peste des petits ruminants before its official confirmation in northern Tanzania in 2008. Res. Opin. Anim. Vet. Sci. 2010, 1, 184–187. [Google Scholar]
- Swai, E.S.; Kapaga, A.; Kivaria, F.; Tinuga, D.; Joshua, G.; Sanka, P. Prevalence and distribution of Peste des petits ruminants virus antibodies in various districts of Tanzania. Vet. Res. Commun. 2009, 33, 927–936. [Google Scholar] [CrossRef] [PubMed]
- Taylor, W.P.; Roeder, P.L.; Rweyemamu, M.M.; Melewas, J.N.; Majuva, P.; Kimaro, R.T.; Mollel, J.N.; Mtei, B.J.; Wambura, P.; Anderson, J.; et al. The control of rinderpest in Tanzania between 1997 and 1998. Trop. Anim. Health Prod. 2002, 34, 471–487. [Google Scholar] [CrossRef] [PubMed]
- Diallo, A.; Okuthe, S.; Kamata, A. Rapid Assessment for the Prevention and Control of Peste des Petits Ruminants, United Republic of Tanzania, Mission Report September 2010; Crisis Management Centre Animal Health (CMC-AH), Food and Agriculture Organization of the United Nations (FAO) and World Organization for Animal Health (OIE), 2011; pp. 1–37, (Unpublished report). [Google Scholar]
- Kivaria, F.M. Foot and mouth disease in Tanzania: An overview of its national status. Vet. Q. 2003, 25, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Mdetele, D.; Mwakabumbe, S.; Seth, M.; Madege, M. Evaluation of effectiveness of pest des petits ruminants vaccine in Northern Tanzania. Res. Opin. Anim. Vet. Sci. 2015, 5, 401–405. [Google Scholar]
- Kgotlele, T.; Macha, E.S.; Kasanga, C.J.; Kusiluka, L.J.M.; Karimuribo, E.D.; Van Doorsselaere, J.; Wensman, J.J.; Munir, M.; Misinzo, G. Partial Genetic Characterization of Peste Des Petits Ruminants Virus from Goats in Northern and Eastern Tanzania. Transbound. Emerg. Dis. 2014, 61, 56–62. [Google Scholar] [CrossRef]
- Jones, B.A.; Mahapatra, M.; Chubwa, C.; Clarke, B.; Batten, C.; Hicks, H.; Henstock, M.; Keyyu, J.; Kock, R.; Parida, S. Characterisation of Peste Des Petits Ruminants Disease in Pastoralist Flocks in Ngorongoro District of Northern Tanzania and Bluetongue Virus Co-Infection. Viruses 2020, 12, 389. [Google Scholar] [CrossRef] [Green Version]
- Kgotlele, T.; Chota, A.; Chubwa, C.C.; Nyasebwa, O.; Lyimo, B.; Torsson, E.; Karimuribo, E.; Kasanga, C.J.; Wensman, J.J.; Misinzo, G.; et al. Detection of peste des petits ruminants and concurrent secondary diseases in sheep and goats in Ngorongoro district, Tanzania. Comp. Haematol. Int. 2019, 28, 755–759. [Google Scholar] [CrossRef] [Green Version]
- Muse, E.A.; Karimuribo, E.D.; Gitao, G.C.; Misinzo, G.; Mellau, L.S.B.; Msoffe, P.L.M.; Swai, E.S.; Albano, M.O. Epidemiological investigation into the introduction and factors for spread of Peste des Petits Ruminants, southern Tanzania. Onderstepoort J. Vet. Res. 2012, 79, 6. [Google Scholar] [CrossRef] [Green Version]
- Muse, E.A.; Matondo, R.B.; Karimuribo, E.D.; Misinzo, G.; Albano, M.O.; Gitao, G.C. Clinico-pathological findings of the 2011 outbreak of Peste des Petits (PPR) in Tandahimba district, southern Tanzania. Res. Opin. Anim. Vet. Sci. 2012, 2, 256–262. [Google Scholar]
- Mbyuzi, A.O.; Komba, E.V.; Kimera, S.I.; Kambarage, D.M. Sero-prevalence and associated risk factors of peste des petits ruminants and contagious caprine pleuro-pneumonia in goats and sheep in the Southern Zone of Tanzania. Prev. Vet. Med. 2014, 116, 138–144. [Google Scholar] [CrossRef]
- Kgotlele, T.; Kasanga, C.J.; Kusiluka, L.J.; Misinzo, G. Preliminary investigation on presence of peste des petits ruminants in Dakawa, Mvomero district, Morogoro region, Tanzania. Onderstepoort J. Vet. Res. 2014, 81, 3. [Google Scholar] [CrossRef] [PubMed]
- Namtimba, A.M. Seroprevalence and Genetic Characterisation of Peste Des Petit Ruminants Virus in Selected areas of Tanzania. MSc Thesis, Sokoine University of Agriculture, Morogoro, Tanzania, 2015. [Google Scholar]
- Swai, E.; Moshy, W.; Mbise, E.; Kaaya, J.; Bwanga, S. Serological evidence of camels exposure to peste des petits ruminants virus in Tanzania. Res. Opin. Anim. Vet. Sci. 2011, 1, 325–329. [Google Scholar]
- Asil, R.M.; Ludlow, M.; Ballal, A.; Alsarraj, S.; Ali, W.H.; Mohamed, B.A.; Mutwakil, S.M.; Osman, N.A. First detection and genetic characterization of peste des petits ruminants virus from dorcas gazelles “Gazella dorcas” in the Sudan, 2016–2017. Arch. Virol. 2019, 164, 2537–2543. [Google Scholar] [CrossRef]
- Dundon, W.G.; Diallo, A.; Cattoli, G. Peste des petits ruminants in Africa: A review of currently available molecular epidemiological data, 2020. Arch. Virol. 2020, 165, 2147–2163. [Google Scholar] [CrossRef]
- Mdetele, D.; Seth, M.; Kabululu, M.; Misinzo, G.; Komba, E. A Comparative study of the Sero-prevalence of Peste Des Petits Ruminants Virus among Districts of Different Agro-Ecological Zones in Tanzania. East Afr. J. Sci. Technol. Innov. 2020, 1. [Google Scholar] [CrossRef]
- George, J. Economic impact of Contagious Caprine Pleuropneumonia and Peste Des Petits Ruminants In Pastoral communities of Ngorongoro and Coastal Districts, Tanzania. MSc Thesis, Open University of Tanzania, Dar es Salaam, Tanzania, 2017. [Google Scholar]
- Jones, B.A.; Rich, K.M.; Mariner, J.C.; Anderson, J.; Jeggo, M.; Thevasagayam, S.; Cai, Y.; Peters, A.R.; Roeder, P. The Economic Impact of Eradicating Peste des Petits Ruminants: A Benefit-Cost Analysis. PLoS ONE 2016, 11, e0149982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kisoza, J. The role of local institutions in the management of agro-pastoral and pastoral systems: A case study of Mkata plains, Kilosa district and Ngorongoro Conservation Area, Ngorongoro district, Tanzania. MSc Thesis, The Sokoine University of Agriculture, Morogoro, Tanzania, 2007. [Google Scholar]
- Mdetele, D.; Kassanga, C.; Seth, M.; Kayunze, K. Seroprevalence of foot and mouth disease in the wildlife-livestock interface and non-interface areas in Tanzania. Res. Opin. Anim. Vet. Sci. 2014, 4, 208–211. [Google Scholar]
- Mwambene, P.L.; Mbwile, R.P.; Udo, H.F.; Kimbi, E.C.; Materu, J.; Mwaiganju, A.; Madoffe, S. Assessing dynamics of forced livestock movements, livelihoods and future development options for pastoralists/agro-pastoralists in Ruvuma and Lindi Regions, in the Southern Tanzania. Livest. Res. Rural Dev. 2014, 26. Available online: http://www.lrrd.org/lrrd26/1/mwam26004.htm (accessed on 14 June 2020).
- Pingo’s Formu; Land Rights Research and Resources Institute (HAKIARDHI). Report on Eviction and Resettlement of Pastoralists from Ihefu and Usangu-Mbarali District to Kilwa and Lindi Districts. 2008. Available online: https://www.tnrf.org/en/node/7180 (accessed on 25 June 2019).
- Given, M.M.; Zebedayo, K.M.; Kizito, M.; Msomba, G.M.; Mvena, Z.K.; Mwajombe, K. Adaptive capacity of evicted agro-pastoralists from Ihefu Basin in Tanzania. J. Agric. Ext. Rural Dev. 2016, 8, 56–61. [Google Scholar] [CrossRef]
- Munson, L.; Terio, K.A.; Kock, R.; Mlengeya, T.; Roelke, M.E.; Dubovi, E.; Summers, B.; Sinclair, A.R.E.; Packer, C. Climate Extremes Promote Fatal Co-Infections during Canine Distemper Epidemics in African Lions. PLoS ONE 2008, 3, e2545. [Google Scholar] [CrossRef] [PubMed]
- Stermshorn, B.; Hargreaves, S.; Pacer, R.; Samake, F. PVS Gap Analysis: Preparation of a Plan to Strengthen the Veterinary Services of Tanzania. OIE Report—France, 2009. Available online: https://rr-africa.oie.int/en/our-mission/promotion-of-veterinary-services-improving-the-legal-framework-and-resources-of-national-veterinary-services/performance-of-veterinary-services-pvs-pathway-missions-status-of-implementation-in-africa/ (accessed on 21 June 2020).
- Stermshorn, B.; Carron, M.; Munstermann, S.; Wakhusama, S. PVS Evaluation Follow-Up Mission Report. OIE Report France, 2016. Available online: https://rr-africa.oie.int/en/our-mission/promotion-of-veterinary-services-improving-the-legal-framework-and-resources-of-national-veterinary-services/performance-of-veterinary-services-pvs-pathway-missions-status-of-implementation-in-africa/ (accessed on 21 June 2020).

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mdetele, D.P.; Komba, E.; Seth, M.D.; Misinzo, G.; Kock, R.; Jones, B.A. Review of Peste des Petits Ruminants Occurrence and Spread in Tanzania. Animals 2021, 11, 1698. https://doi.org/10.3390/ani11061698
Mdetele DP, Komba E, Seth MD, Misinzo G, Kock R, Jones BA. Review of Peste des Petits Ruminants Occurrence and Spread in Tanzania. Animals. 2021; 11(6):1698. https://doi.org/10.3390/ani11061698
Chicago/Turabian StyleMdetele, Daniel Pius, Erick Komba, Misago Dimson Seth, Gerald Misinzo, Richard Kock, and Bryony Anne Jones. 2021. "Review of Peste des Petits Ruminants Occurrence and Spread in Tanzania" Animals 11, no. 6: 1698. https://doi.org/10.3390/ani11061698
APA StyleMdetele, D. P., Komba, E., Seth, M. D., Misinzo, G., Kock, R., & Jones, B. A. (2021). Review of Peste des Petits Ruminants Occurrence and Spread in Tanzania. Animals, 11(6), 1698. https://doi.org/10.3390/ani11061698






