The Patterns and Causes of Dermatitis in Terrestrial and Semi-Aquatic Mammalian Wildlife
Abstract
Simple Summary
Abstract
1. Introduction
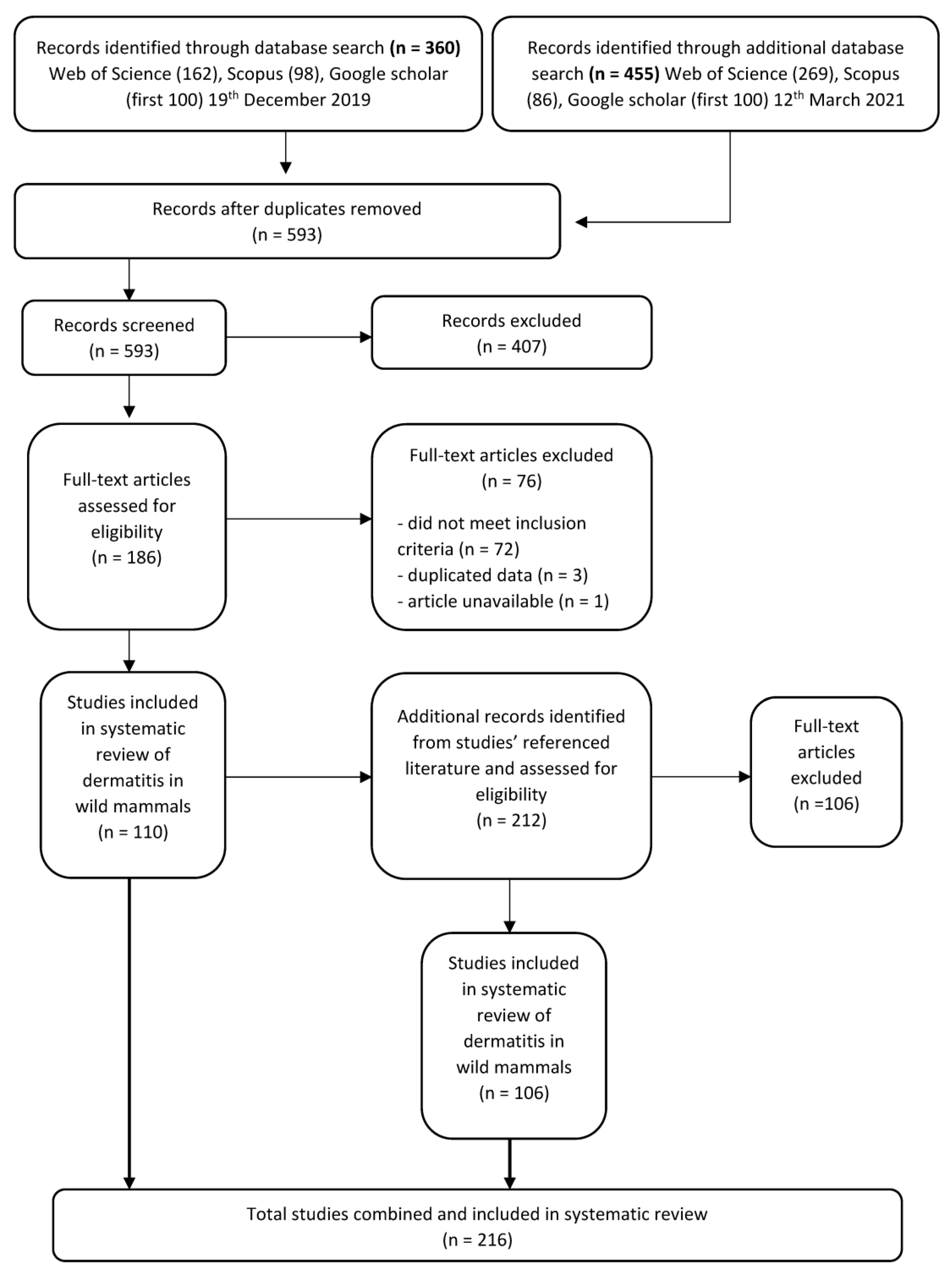
2. Database Search and Literature Screening
2.1. Inclusion and Exclusion Criteria
2.2. Data Extraction
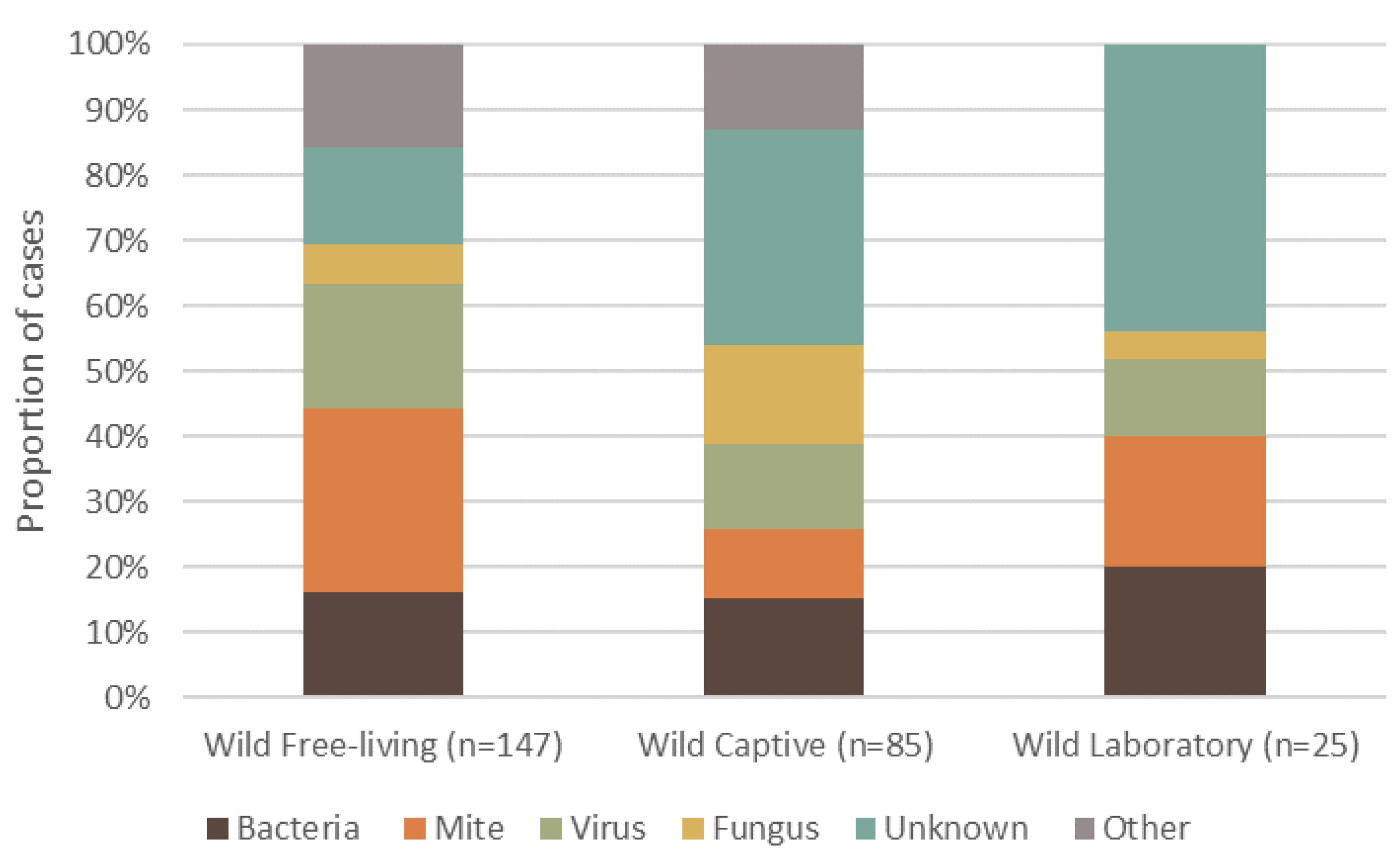
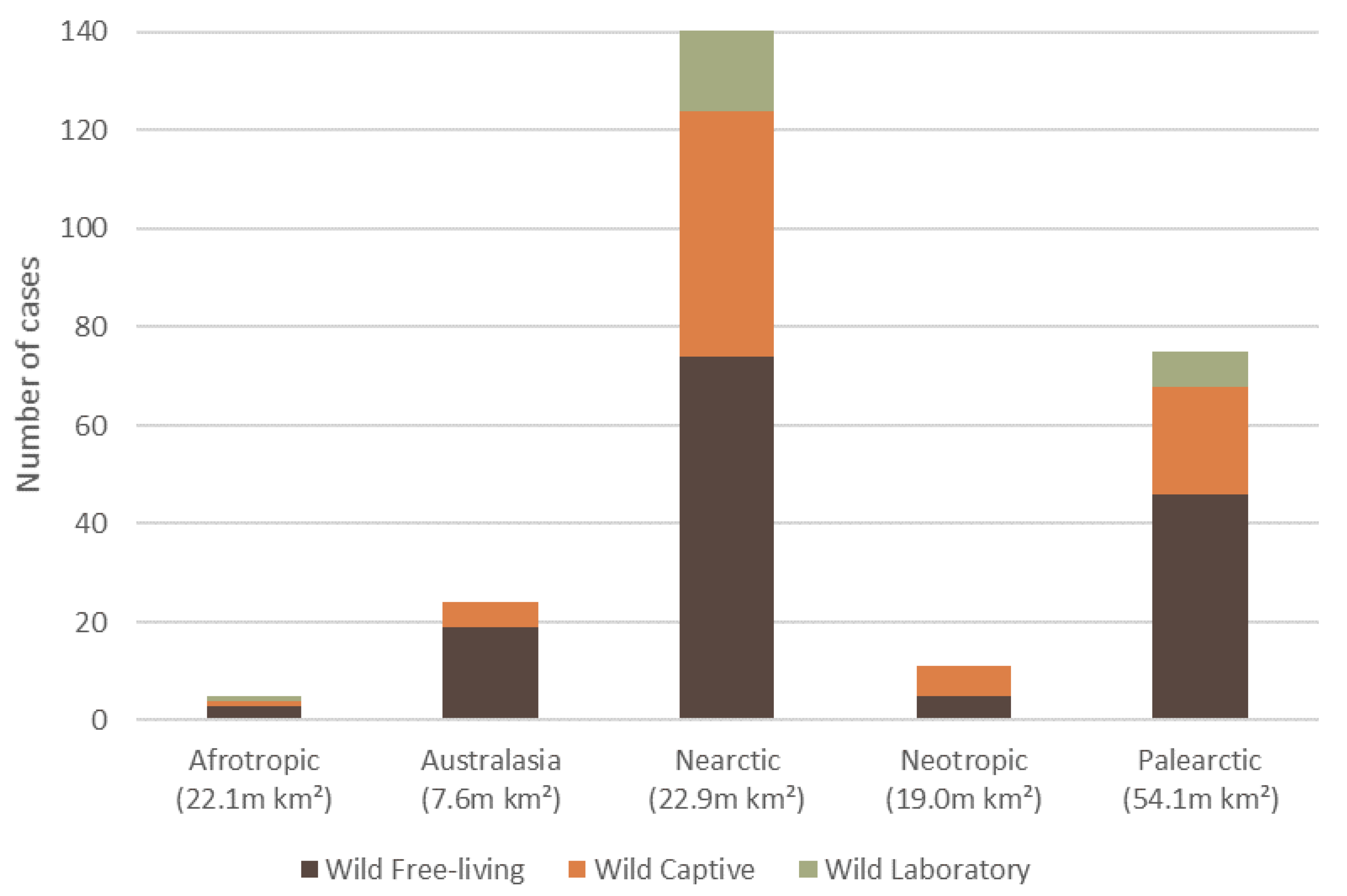
3. Overall Causes, Captivity Status, and Geographic Spread of Reported Dermatitis
4. Etiological Agents Responsible for the Causes of Dermatitis across Mammalian Wildlife Species
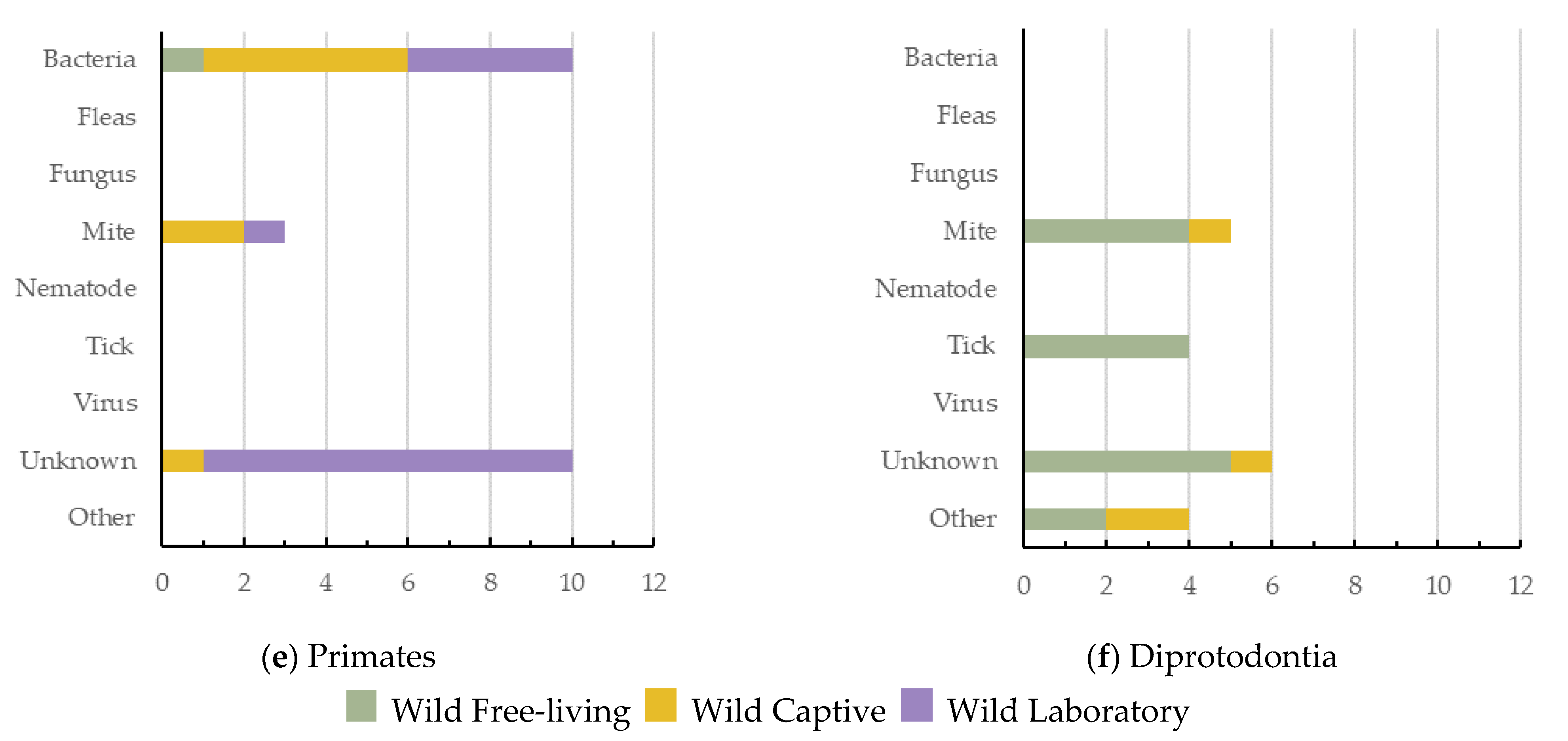
5. Causes of Dermatitis across Wildlife Orders
6. Threatened Species Reported with Dermatitis
7. Discussion
7.1. The Main Causes of Dermatitis in Mammalian Wildlife: Mites, Bacteria and Viruses
7.2. Captivity Status and Dermatitis in Wild Mammals
7.3. Threatened Species with Dermatitis
7.4. Reporting Bias for Orders of Mammalian Wildlife and across Ecozones
7.5. Limitations of Reviewing Clinical Signs of Diseases or Irritants
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Richard, J.L.; Shotts, E.B. Wildlife reservoirs of dermatophilosis. In Wildlife Diseases; Page, L.A., Ed.; Springer: Boston, MA, USA, 1976; pp. 205–214. ISBN 978-1-4757-1656-6. [Google Scholar]
- Parish, L.C.; Schwartzman, R.M. Zoonoses of dermatological interest. Semin. Dermatol. 1993, 12, 57–64. [Google Scholar]
- Linek, M.; Favrot, C. Impact of canine atopic dermatitis on the health-related quality of life of affected dogs and quality of life of their owners. Vet. Dermatol. 2010, 21, 456–462. [Google Scholar] [CrossRef]
- Rehal, B.; Armstrong, A.W. Health outcome measures in atopic dermatitis: A systematic review of trends in disease severity and quality-of-life instruments 1985–2010. PLoS ONE 2011, 6, e17520. [Google Scholar] [CrossRef]
- Munson, L.; Koehler, J.W.; Wilkinson, J.; Miller, R. Vesicular and ulcerative dermatopathy resembling superficial necrolytic dermatitis in captive black rhinoceroses (Diceros bicornis). Vet. Pathol. 1998, 35, 31–42. [Google Scholar] [CrossRef]
- Steinmetz, H.W.; Kaumanns, W.; Dix, I.; Neimeier, K.A.; Kaup, F.J. Dermatologic investigation of alopecia in rhesus macaques (Macaca mulatta). J. Zoo Wildl. Med. 2005, 36, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Superina, M.; Garner, M.M.; Aguilar, R.F. Health evaluation of free-ranging and captive pichis (Zaedyus pichiy; Mammalia, Dasypodidae), in Mendoza Province, Argentina. J. Wildl. Dis. 2009, 45, 174–183. [Google Scholar] [CrossRef]
- Galli, E.; Cicconi, R.; Rossi, P.; Casati, A.; Brunetti, E.; Mancino, G. Atopic dermatitis: Molecular mechanisms, clinical aspects and new therapeutical approaches. Curr. Mol. Med. 2003, 3, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Dolma, N.; Andries, L.; Betiu, M. New aspects of the clinical and immunological approach in patients with atopic dermatitis. In Allergy, Asthma, Copd, Immunophysiology & Immunorehabilitology: Innovative Technologies; Sepiashvili, R., Ed.; Filodiritto Publisher: Barcelona, Spain, 2018; pp. 121–133. ISBN 978-88-85813-04-5. [Google Scholar]
- Zur, G.; Ihrke, P.J.; White, S.D.; Kass, P.H. Canine atopic dermatitis: A retrospective study of 266 cases examined at the University of California, Davis, 1992–1998. Part i. Clinical features and allergy testing results. Vet. Dermatol. 2002, 13, 89–102. [Google Scholar] [CrossRef]
- Kramer, J.; Fahey, M.; Santos, R.; Carville, A.; Wachtman, L.; Mansfield, K. Alopecia in rhesus macaques correlates with immunophenotypic alterations in dermal inflammatory infiltrates consistent with hypersensitivity etiology. J. Med. Primatol. 2010, 39, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Simpson, V.R.; Davison, N.J.; Kearns, A.M.; Pichon, B.; Hudson, L.O.; Koylass, M.; Blackett, T.; Butler, H.; Rasigade, J.P.; Whatmore, A.M. Association of a lukM-positive clone of Staphylococcus aureus with fatal exudative dermatitis in red squirrels (Sciurus vulgaris). Vet. Microbiol. 2013, 162, 987–991. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.; Etheridge, M. A case of suspected contact dermatitis in a juvenile cynomolgus monkey (Macaca fascicularis). J. Med. Primatol. 2008, 37, 56–59. [Google Scholar] [CrossRef] [PubMed]
- Tompkins, D.M.; Sainsbury, A.W.; Nettleton, P.; Buxton, D.; Gurnell, J. Parapoxvirus causes a deleterious disease in red squirrels associated with UK population declines. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2002, 269, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Connolly, J.; Obendorf, D.; Whittington, R. Haematological, serum biochemical and serological features of platypuses with and without mycotic granulomatous dermatitis. Aust. Vet. J. 1999, 77, 809–813. [Google Scholar] [CrossRef] [PubMed]
- Hemsley, S.; Canfield, P. Dermatitis in free-living common brushtail possums (Trichosurus-vulpecula). Aust. Vet. J. 1994, 24, 147–155. [Google Scholar]
- Yeruham, I.; Nyska, A. Acral lick dermatitis in a jackal (Canis aureus). J. Zoo Wildlife. Med. 1998, 29, 233. [Google Scholar]
- Pollock, C.G.; Rohrbach, B.; Ramsay, E.C. Fungal dermatitis in captive pinnipeds. J. Zoo Wildl. Med. 2000, 31, 374–379. [Google Scholar] [PubMed]
- Tomaselli, M.; Dalton, C.; Duignan, P.J.; Kutz, S.; van der Meer, F.; Kafle, P.; Surujballi, O.; Turcotte, C.; Checkley, S. Contagious ecthyma, Rangiferine brucellosis, and lungworm infection in a muskox (Ovibos moschatus) from the Canadian Arctic, 2014. J. Wildl. Dis. 2016, 52, 719–724. [Google Scholar] [CrossRef] [PubMed]
- Koh, L.P.; Dunn, R.R.; Sodhi, N.S.; Colwell, R.K.; Proctor, H.C.; Smith, V.S. Species coextinctions and the biodiversity crisis. Science 2004, 305, 1632–1634. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, S.; Allan, N.; Pesapane, R.; Brignolo, L.; Foley, J. Eradication of a tropical rat mite (Ornithonyssus bacoti) infestation from a captive colony of endangered Amargosa voles (Microtus californicus scirpensis). J. Zoo Wildl. Med. 2018, 49, 475–479. [Google Scholar] [CrossRef]
- Holz, P.H.; Lumsden, L.F.; Marenda, M.S.; Browning, G.F.; Hufschmid, J. Two subspecies of bent-winged bats (Miniopterus orianae bassanii and oceanensis) in southern Australia have diverse fungal skin flora but not Pseudogymnoascus destructans. PLoS ONE 2018, 13, e0204282. [Google Scholar] [CrossRef] [PubMed]
- Brian, L.C.; Rudd, J.L.; Westall, T.L.; Woods, L.W.; Stephenson, N.; Foley, J.E.; Richardson, D.; Clifford, D.L. Sarcoptic mange in endangered kit foxes (Vulpes macrotis mutica): Case histories, diagnoses, and implications for conservation. J. Wildl. Dis. 2017, 53, 46–53. [Google Scholar]
- Zaria, L. Dermatophilus congolensis infection (dermatophilosis) in animals and man! An update. Comp. Immunol. Microb. 1993, 16, 179–222. [Google Scholar] [CrossRef]
- Kock, N.; Kock, M.D. Skin-lesions in free-ranging black rhinoceroses (Diceros-bicornis) in Zimbabwe. J. Zoo Wildl. Med. 1990, 21, 447–452. [Google Scholar]
- Brack, M.; Hochleithner, C.; Hochleithner, M.; Zenker, W. Suspected dermatophilosis in an adult orangutan (Pongo pygmaeus pygmaeus). J. Zoo Wildl. Med. 1997, 28, 336–341. [Google Scholar] [PubMed]
- Kearns, K.; Sleeman, J.; Frank, L.; Munson, L. Zinc-responsive dermatosis in a red wolf (Canis rufus). J. Zoo Wildl. Med. 2000, 31, 255–259. [Google Scholar] [PubMed]
- Cucchi-Stefanoni, K.; Juan-Sallés, C.; Parás, A.; Garner, M.M. Fatal anemia and dermatitis in captive agoutis (Dasyprocta mexicana) infested with Echidnophaga fleas. Vet. Parasitol. 2008, 155, 336–339. [Google Scholar] [CrossRef] [PubMed]
- Caron, T.J.; Artim, S.C.; Israelsen, W.J.; Holcombe, H.R.; Fox, J.G.; Bakthavatchalu, V. Cutaneous dermatophilosis in a meadow jumping mouse (Zapus hudsonius). Comp. Med. 2018, 68, 25–30. [Google Scholar]
- Hufschmid, J.; Handasyde, K.A.; Beveridge, I. The role of host and environmental factors in the epidemiology of rumpwear in brushtail possums. Aust. J. Zool 2010, 58, 250–262. [Google Scholar] [CrossRef]
- Phalen, D.; Mowbry, J.; Spielman, D. An investigation into exudative dermatitis in the common brushtail possum, Trichosurus vulpecula, and the effects of sex, age and season on the development of disease. In Kokako; Wildlife Society of NZVA: Wellington, New Zealand, 2010; Volume 17, p. 15. [Google Scholar]
- Olson, D.M.; Dinerstein, E. The global 200: A representation approach to conserving the earth’s most biologically valuable ecoregions. Conserv. Biol. 1998, 12, 502–515. [Google Scholar] [CrossRef]
- Olson, D.; Dinerstein, E.; Wikramanayake, E.; Burgess, N.; Powell, G.; Underwood, E.; D’Amico, J.; Itoua, I.; Strand, H.; Morrison, J.; et al. Terrestrial ecoregions of the world: A new map of life on earth. Bioscience 2001, 51, 933–938. [Google Scholar] [CrossRef]
- IUCN. The IUCN Red List of Threatened Species. Version 2021-1. 2021. Available online: http://www.iucnredlist.org (accessed on 12 March 2021).
- Arlian, L.G.; Morgan, M.S. A review of Sarcoptes scabiei: Past, present and future. Parasites Vectors 2017, 10, 297. [Google Scholar] [CrossRef] [PubMed]
- Escobar, L.E.; Carver, S.; Cross, P.C.; Rossi, L.; Almberg, E.S.; Yabsley, M.J.; Niedringhaus, K.D.; Van Wick, P.; Dominguez-Villegas, E.; Gakuya, F.; et al. Sarcoptic mange: An emerging panzootic in wildlife. Transbound Emerg. Dis. 2021. [Google Scholar] [CrossRef]
- Foley, J.; Serieys, L.; Stephenson, N.; Riley, S.; Foley, C.; Jennings, M.; Wengert, G.; Vickers, W.; Boydston, E.; Lyren, L.; et al. A synthetic review of notoedres species mites and mange. Parasitology 2016, 143, 1847–1861. [Google Scholar] [CrossRef]
- Salvadori, C.; Formenti, N.; Trogu, T.; Lanfranchi, P.; Papini, R.A.; Poli, A. Demodicosis in Chamois (Rupicapra rupicapra subsp. rupicapra) in the Italian Alps, 2013–2014. J. Wildl. Dis 2016, 52, 433–435. [Google Scholar]
- Gebreyohannes, M.; Gebresselassie, M. An overview on dermatophilosis of animals. J. Anim. Sci. Adv. 2013, 3, 337–344. [Google Scholar]
- Paez-Espino, D.; Eloe-Fadrosh, E.A.; Pavlopoulos, G.A.; Thomas, A.D.; Huntemann, M.; Mikhailova, N.; Rubin, E.; Ivanova, N.N.; and Kyrpides, N.C. Uncovering earth’s virome. Nature 2016, 536, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Tryland, M.; Beckmen, K.B.; Burek-Huntington, K.A.; Breines, E.M.; Klein, J. Orf virus infection in Alaskan mountain goats, Dall’s sheep, muskoxen, caribou and Sitka black-tailed deer. Acta. Vet. Scand 2018, 60, 12. [Google Scholar] [CrossRef] [PubMed]
- Chiller, K.; Selkin, B.A.; Murakawa, G.J. Skin microflora and bacterial infections of the skin. JIDSP 2001, 6, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Gordon, M.A.; Edwards, M.R. Micromorphology of Dermatophilus congolensis. J. Bacteriol. 1963, 86, 1101–1115. [Google Scholar] [CrossRef]
- Wobeser, G.; Gordon, M. Dermatophilus infection in Columbian ground squirrels (Citellus columbianus columbianus). Bull. Wildl. Dis. Assoc. 1969, 5, 31–32. [Google Scholar] [CrossRef]
- McClure, H.; Kaplan, W.; Bonner, W.; Keeling, M. Dermatophilosis in owl monkeys. Sabouraudia 1971, 9, 185–190. [Google Scholar] [CrossRef]
- Eo, K.Y.; Kwon, O.D. Dermatitis caused by Dermatophilus congolensis in a zoo polar bear (Ursus maritimus). Pak. Vet. J. 2014, 34, 560–562. [Google Scholar]
- Salkin, I.; Gordon, M.; Stone, W. Dermatophilosis among wild raccoons in New York State. J. Am. Vet. Med. A. 1976, 169, 949–951. [Google Scholar]
- Nemeth, N.M.; Ruder, M.G.; Gerhold, R.W.; Brown, J.D.; Munk, B.A.; Oesterle, P.T.; Kubiski, S.V.; Keel, M.K. Demodectic mange, dermatophilosis, and other parasitic and bacterial dermatologic diseases in free-ranging white-tailed deer (Odocoileus virginianus) in the United States from 1975 to 2012. Vet. Pathol. 2014, 51, 633–640. [Google Scholar] [CrossRef]
- Clubb, R.; Mason, G. Captivity effects on wide-ranging carnivores. Nature 2003, 425, 473–474. [Google Scholar] [CrossRef]
- Salas, M.; Manteca, X.; Abáigar, T.; Delclaux, M.; Enseñat, C.; Martínez-Nevado, E.; Quevedo, M.Á.; Fernández-Bellon, H. Using farm animal welfare protocols as a base to assess the welfare of wild animals in captivity—Case study: Dorcas Gazelles (Gazella dorcas). Animals 2018, 8, 111. [Google Scholar] [CrossRef] [PubMed]
- Miller, R. Quarantine protocols and preventive medicine procedures for reptiles, birds and mammals in zoos. Rev. Sci. Tech. 1996, 15, 183–190. [Google Scholar] [CrossRef]
- Barrows, M.; Killick, R.; Saunders, R.; Tahas, S.; Day, C.; Wyatt, K.; Horspool, T.; Lackey, L.B.; Cook, J. Retrospective analysis of elective health examinations as preventative medicine interventions at a zoological collection. J. Zoo Aquar. Res. 2017, 5, 25–32. [Google Scholar]
- Keesing, F.; Holt, R.D.; Ostfeld, R.S. Effects of species diversity on disease risk. Ecol. Lett. 2006, 9, 485–498. [Google Scholar] [CrossRef] [PubMed]
- Chabreck, R.H.; Thompson, R.B.; Ensminger, A.B. Chronic dermatitis in nutria in Louisiana. J. Wildl. Dis. 1977, 13, 333–335. [Google Scholar] [CrossRef] [PubMed]
- Barlow, A.; Jolliffe, T.; Tomlin, M.; Worledge, L.; Miller, H. Mycotic dermatitis in a vagrant parti-coloured bat (Vespertilio murinus) in Great Britain. Vet. Rec. 2011, 169, 614. [Google Scholar] [CrossRef]
- Van Horn, R.C.; Sutherland-Smith, M.; Bracho Sarcos, A.E.; Thomas, G.; Shanks, J.A.; Owen, M.A. The Andean bear alopecia syndrome may be caused by social housing. Zoo Biol. 2019, 38, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Howard, C.; Flather, C.H.; Stephens, P.A. A global assessment of the drivers of threatened terrestrial species richness. Nat. Commun. 2020, 11, 993. [Google Scholar] [CrossRef] [PubMed]
- Kubo, M.; Sato, H.; Hattori, S.; Kuraishi, T. Dermatitis associated with infestation of a trombiculid mite, Leptotrombidium miyajimai, in an Amami rabbit (Pentalagus furnessi). J. Wildl. Dis. 2014, 50, 416–418. [Google Scholar] [CrossRef] [PubMed]
- Witte, C.L.; Lamberski, N.; Rideout, B.A.; Vaida, F.; Citino, S.B.; Barrie, M.T.; Haefele, H.J.; Junge, R.E.; Murray, S.; Hungeford, L.L. Epidemiology of clinical feline herpesvirus infection in zoo-housed cheetahs (Acinonyx jubatus). J. Am. Vet. Med. Assoc. 2017, 251, 946–956. [Google Scholar] [CrossRef] [PubMed]
- LaRose, J.; Meredith, A.; Everest, D.; Fiegna, C.; McInnes, C.; Shaw, D.; Milne, E. Epidemiological and postmortem findings in 262 red squirrels (Sciurus vulgaris) in Scotland, 2005 to 2009. Vet. Rec. 2010, 167, 297–302. [Google Scholar] [CrossRef] [PubMed]
- McInnes, C.; Coulter, L.; Dagleish, M.; Deane, D.; Gilray, J.; Percival, A.; Willoughby, K.; Scantlebury, M.; Marks, N.; Graham, D. The emergence of squirrelpox in Ireland. Anim. Conserv. 2013, 16, 51–59. [Google Scholar] [CrossRef]
- Munson, L.; Wack, R.; Duncan, M.; Montali, R.J.; Boon, D.; Stalis, I.; Crawshaw, G.J.; Cameron, K.N.; Mortenson, J.; Citino, S.; et al. Chronic eosinophilic dermatitis associated with persistent feline herpes virus infection in cheetahs (Acinonyx jubatus). Vet. Pathol. 2004, 41, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Frankham, R. Stress and adaptation in conservation genetics. J. Evol. Biol. 2005, 18, 750–755. [Google Scholar] [CrossRef]
- Spielman, D.; Brook, B.W.; Briscoe, D.A.; Frankham, R. Does inbreeding and loss of genetic diversity decrease disease resistance? Conserv. Genet. 2004, 5, 439–448. [Google Scholar] [CrossRef]
- Munson, L.; Terio, K.A.; Worley, M.; Jago, M.; Bagot-Smith, A.; Marker, L. Extrinsic factors significantly affect patterns of disease in free-ranging and captive cheetah (Acinonyx jubatus) populations. J. Wildl. Dis. 2005, 41, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Whiteman, N.K.; Matson, K.D.; Bollmer, J.L.; Parker, P.G. Disease ecology in the Galapagos hawk (Buteo galapagoensis): Host genetic diversity, parasite load and natural antibodies. Proc. Roy. Soc. B Biol. Sci. 2006, 273, 797–804. [Google Scholar] [CrossRef]
- O’Brien, S.J.; Wildt, D.E.; Goldman, D.; Merril, C.R.; Bush, M. The cheetah is depauperate in genetic variation. Science 1983, 221, 459–462. [Google Scholar] [CrossRef]
- Jameson, E.; Brennan, J.M. An environmental analysis of some ectoparasites of small forest mammals in the Sierra Nevada, California. Ecol. Monogr. 1957, 27, 45–54. [Google Scholar] [CrossRef]
- Lyles, A.M.; Dobson, A.P. Infectious disease and intensive management: Population dynamics, threatened hosts, and their parasites. J. Zoo Wildl. Med. 1993, 24, 315–326. [Google Scholar]
- LeJeune, J.T.; Davis, M.A. Outbreaks of zoonotic enteric disease associated with animal exhibits. J. Am. Vet. Med. Assoc. 2004, 224, 1440–1445. [Google Scholar] [CrossRef] [PubMed]
- Peeler, E.J.; Oidtmann, B.C.; Midtlyng, P.J.; Miossec, L.; Gozlan, R.E. Non-native aquatic animals introductions have driven disease emergence in Europe. Biol. Invasions 2011, 13, 1291–1303. [Google Scholar] [CrossRef]
- Fèvre, E.M.; Bronsvoort, B.M.d.C.; Hamilton, K.A.; Cleaveland, S. Animal movements and the spread of infectious diseases. Trends Microbiol. 2006, 14, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Titeux, E.; Gilbert, C.; Briand, A.; Cochet-Faivre, N. From feline idiopathic ulcerative dermatitis to feline behavioral ulcerative dermatitis: Grooming repetitive behaviors indicators of poor welfare in cats. Front. Vet. Sci. 2018, 5, 81. [Google Scholar] [CrossRef]
- Sacco, K.A.; Milner, J.D. Gene–environment interactions in primary atopic disorders. Curr. Opin. Immunol. 2019, 60, 148–155. [Google Scholar] [CrossRef]
- Broom, D.M. Behaviour and welfare in relation to pathology. Appl. Anim. Behav. 2006, 97, 73–83. [Google Scholar] [CrossRef]
- Bauwens, L.; De Vroey, C.; De Meurichy, W. A case of exfoliative dermatitis in a captive southern white rhinoceros (Ceratotherium simum simum). J. Zoo Wildl. Med. 1996, 27, 271–274. [Google Scholar]
- Williams, E.S.; Pier, A.; Wilson, R.W. Dermatophilosis in a mule deer, Odocoileus hemionus (rafinesque), from Wyoming. J. Wildl. Dis. 1984, 20, 236–238. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.; Cano, J. Demodectic mange in a white-tailed deer from Walker County, Texas. J. Med. Entomol. 2014, 45, 572–575. [Google Scholar] [CrossRef]
- van Strien, A.J.; van Swaay, C.A.M.; Termaat, T. Opportunistic citizen science data of animal species produce reliable estimates of distribution trends if analysed with occupancy models. J. Appl. Ecol. 2013, 50, 1450–1458. [Google Scholar] [CrossRef]
- Kuussaari, M.; Heliölä, J.; Pöyry, J.; Saarinen, K. Contrasting trends of butterfly species preferring semi-natural grasslands, field margins and forest edges in northern Europe. J. Insect Conserv. 2007, 11, 351–366. [Google Scholar] [CrossRef]
- Monsarrat, S.; Kerley, G.I.H. Charismatic species of the past: Biases in reporting of large mammals in historical written sources. Biol. Conserv. 2018, 223, 68–75. [Google Scholar] [CrossRef]
- Pandey, G.S.; Mweene, A.; Suzuki, A.K.; Nambota, A.; Kaji, T. Dermatophilosis (Cutaneous streptothricosis) in Kafue-lechwe (Kobus-leche-kafuensis). J. Wildl. Dis. 1994, 30, 586–588. [Google Scholar] [CrossRef][Green Version]
- Osman, S.A. Camel dermatophilosis: Clinical signs and treatment outcomes. J. Camel Pract. Res. 2014, 21, 199–204. [Google Scholar] [CrossRef]
- Clegg, S.R.; Mansfield, K.G.; Newbrook, K.; Sullivan, L.E.; Blowey, R.W.; Carter, S.D.; Evans, N.J. Isolation of digital dermatitis treponemes from hoof lesions in wild North American elk (Cervus elaphus) in Washington State, USA. J. Clin. Microbiol. 2015, 53, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Scagliarini, A.; Vaccari, F.; Turrini, F.; Bianchi, A.; Cordioli, P.; Lavazza, A. Parapoxvirus infections of red deer, Italy. Emerg. Infect Dis. 2011, 17, 684–687. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, C.B.; Burgess, N.D.; Coad, L.; Belokurov, A.; Besançon, C.; Boisrobert, L.; Campbell, A.; Fish, L.; Gliddon, D.; Humphries, K.; et al. Global analysis of the protection status of the world’s forests. Biol. Conserv. 2009, 142, 2122–2130. [Google Scholar] [CrossRef]
- Ennen, J.R.; Agha, M.; Sweat, S.C.; Matamoros, W.A.; Lovich, J.E.; Rhodin, A.G.J.; Iverson, J.B.; Hoagstrom, C.W. Turtle biogeography: Global regionalization and conservation priorities. Biol. Conserv. 2020, 241, 108323. [Google Scholar] [CrossRef]
- Buitenwerf, R.; Higgins, S.I. Convergence among global biogeographical realms in the physiological niche of evergreen and deciduous vegetation. Glob. Ecol. Biogeogr. 2016, 25, 704–715. [Google Scholar] [CrossRef]
- Krasnov, B.R.; Shenbrot, G.I.; van der Mescht, L.; Khokhlova, I.S. Drivers of compositional turnover are related to species’ commonness in flea assemblages from four biogeographic realms: Zeta diversity and multi-site generalised dissimilarity modelling. Int. J. Parasitol. 2020, 50, 331–344. [Google Scholar] [CrossRef] [PubMed]
- Stephens, P.R.; Altizer, S.; Smith, K.F.; Alonso Aguirre, A.; Brown, J.H.; Budischak, S.A.; Byers, J.E.; Dallas, T.A.; Jonathan Davies, T.; Drake, J.M.; et al. The macroecology of infectious diseases: A new perspective on global-scale drivers of pathogen distributions and impacts. Ecol. Lett. 2016, 19, 1159–1171. [Google Scholar] [CrossRef] [PubMed]
- Murray, K.A.; Olivero, J.; Roche, B.; Tiedt, S.; Guégan, J.F. Pathogeography: Leveraging the biogeography of human infectious diseases for global health management. Ecography 2018, 41, 1411–1427. [Google Scholar] [CrossRef]
- Biondi, M.; D’Alessandro, P. Biogeographical analysis of the flea beetle genus Chaetocnema in the afrotropical region: Distribution patterns and areas of endemism. J. Biogeogr. 2006, 33, 720–730. [Google Scholar] [CrossRef]
- Sánchez-Fernández, D.; Lobo, J.M.; Abellán, P.; Ribera, I.; Millán, A. Bias in freshwater biodiversity sampling: The case of Iberian water beetles. Divers. Distrib. 2008, 14, 754–762. [Google Scholar] [CrossRef]
- Dennis, R.L.H.; Sparks, T.H.; Hardy, P.B. Bias in butterfly distribution maps: The effects of sampling effort. J. Insect Conserv. 1999, 3, 33–42. [Google Scholar] [CrossRef]
- Hortal, J.; Jiménez-Valverde, A.; Gómez, J.F.; Lobo, J.M.; Baselga, A. Historical bias in biodiversity inventories affects the observed environmental niche of the species. Oikos 2008, 117, 847–858. [Google Scholar] [CrossRef]
- Kéry, M.; Royle, J.A.; Schmid, H.; Schaub, M.; Volet, B.; Häfliger, G.; Zbinden, N. Site-occupancy distribution modeling to correct population-trend estimates derived from opportunistic observations. Conserv. Biol. 2010, 24, 1388–1397. [Google Scholar] [CrossRef] [PubMed]
- Gedon, N.K.Y.; Mueller, R.S. Atopic dermatitis in cats and dogs: A difficult disease for animals and owners. Clin Transl Allergy 2018, 8, 41. [Google Scholar] [CrossRef] [PubMed]
- Palmer, M.A.; O’Connell, N.E. Digital dermatitis in dairy cows: A review of risk factors and potential sources of between-animal variation in susceptibility. Animals 2015, 5, 512–535. [Google Scholar] [CrossRef] [PubMed]
- Donahoe, S.L.; Lindsay, S.A.; Krockenberger, M.; Phalen, D.; Šlapeta, J. A review of neosporosis and pathologic findings of Neospora caninum infection in wildlife. IJP-PAW 2015, 4, 216–238. [Google Scholar] [CrossRef] [PubMed]
- Heaton, C.J.; Gerbig, G.R.; Sensius, L.D.; Patel, V.; Smith, T.C. Staphylococcus aureus epidemiology in wildlife: A systematic review. Antibiotics 2020, 9, 89. [Google Scholar] [CrossRef]
- Jones, D.M.; Thomsett, L.R. A short review of the diseases of rhinoceros skin with case reports on an exudative dermatitis of the white rhinoceros (Ceratotherium simum). VerhBer. Erkrank. Zootier 1972, 14, 227–231. [Google Scholar]
- McAlpine, D.F.; McBurney, S.; Sabine, M.; Vanderwolf, K.J.; Park, A.; Cai, H.Y. Molecular detection of Pseudogymnoascus destructans (Ascomycota: Pseudeurotiaceae) and unidentified fungal dermatitides on big brown bats (Eptesicus fuscus) overwintering inside buildings in Canada. J. Wildl. Dis. 2016, 52, 902–906. [Google Scholar] [CrossRef][Green Version]

| Manuscript | Mammal (Per Species) | Dermatitis (Per Species) |
|---|---|---|
| Title | Number of individuals | Type |
| Author(s) | Captivity status | Location of dermatitis |
| Year | Country | Clinical signs |
| Journal | Conservation status | Definitive Cause |
| Key terms |
| Cause | Percentage Definitive Diagnosis (n) | Species per Cause | Ecozone |
|---|---|---|---|
| Apicomplexan * | 1.2% (3) | 2 | 1 |
| Bacteria | 16.3% (42) | 29 | 6 |
| Bacteria & Fungus * | 0.8% (2) | 1 | 1 |
| Diptera * | 1.2% (3) | 3 | 2 |
| Ectoparasite (unknown) * | 0.4% (1) | 1 | 1 |
| Fleas | 0.8% (2) | 2 | 1 |
| Fungus | 9.0% (23) | 15 | 3 |
| Louse * | 0.8% (2) | 2 | 2 |
| Mineral deficiency * | 0.4% (1) | 1 | 1 |
| Mite | 21.4% (55) | 34 | 5 |
| Mite & Fungus * | 0.4% (1) | 1 | 1 |
| Mite & Nematode * | 0.4% (1) | 1 | 1 |
| Nematode | 2.7% (7) | 6 | 2 |
| Plant * | 1.6% (4) | 4 | 2 |
| Protozoa * | 0.4% (1) | 1 | 1 |
| Tick | 2.3% (6) | 6 | 2 |
| Unknown | 23.7% (61) | 35 | 5 |
| Virus | 16.3% (42) | 15 | 4 |
| Causal Agent | Category | Species | Orders |
|---|---|---|---|
| Dermatophilus congolensis | Bacteria | 18 | 6 |
| Parapoxvirus (genus) | Virus | 13 | 2 |
| Demodex sp. (genus) | Mite | 8 | 4 |
| Notoedres sp. (genus) | Mite | 8 | 3 |
| Sarcoptes scabiei | Mite | 7 | 2 |
| Staphylococcus sp. (genus) | Bacteria | 7 | 6 |
| Malassezia sp. (genus) | Fungus | 6 | 2 |
| IUCN (2021) Threatened Species | Threatened Category | Order | Total Cases | Endemic Ecozone Cases | Non-Endemic Ecozone Cases * | ||
|---|---|---|---|---|---|---|---|
| WFL | WC | WL | WC | ||||
| Canis rufus (red wolf) | CR | terrestrial Carnivora | 1 | 1 | |||
| Dasyprocta mexicana (Mexican agouti) | CR | Rodentia | 1 | 1 | |||
| Diceros bicornis (black rhinoceros) | CR | Perissodactyla | 5 | 2 | 3 | ||
| Mustela lutreola (European Mink) | CR | terrestrial Carnivora | 1 | 1 | |||
| Pongo pygmaeus pygmaeus (Northwest Bornean orangutan) | CR | Primates | 1 | 1 | |||
| Ailurus fulgens (red panda) | EN | terrestrial Carnivora | 2 | 2 | |||
| Elephas maximus (Asian Elephant) | EN | Other (Elephantidae) | 2 | 1 | 1 | ||
| Pentalagus furnessi (Amami rabbit) | EN | Lagomorpha | 1 | 1 | |||
| Petrogale persephone (Proserpine rock wallaby) | EN | Diprotodontia | 1 | 1 | |||
| Symphalangus syndactylus (siamang) | EN | Primates | 1 | 1 | |||
| Acinonyx jubatus (cheetah) | VU | terrestrial Carnivora | 5 | 1 | 4 | ||
| Alouatta palliata (Mantled Howler Monkey) | VU | Primates | 1 | 1 | |||
| Budorcas taxicolor tibetana (Sichuan takin) | VU | Artiodactyla | 1 | 1 | |||
| Hippopotamus amphibius (Nile hippopotamus) | VU | Artiodactyla | 3 | 3 | |||
| Lagothrix lagotricha (woolly monkey) | VU | Primates | 1 | 1 | |||
| Macaca fascicularis (crab-eating macaque) | VU | Primates | 2 | 2 * | |||
| Petrogale penicillata (brush-tailed rock-wallaby) | VU | Diprotodontia | 1 | 1 | |||
| Phascolarctos cinereus (koala) | VU | Diprotodontia | 1 | 1 | |||
| Rangifer tarandus (caribou) | VU | Artiodactyla | 4 | 1 | 2 | 1 | |
| Rhinoceros unicornis (Indian rhinoceros) | VU | Perissodactyla | 2 | 2 | |||
| Rusa unicolor (Sambar) | VU | Artiodactyla | 1 | 1 | |||
| Tremarctos ornatus (Andean bear) | VU | terrestrial Carnivora | 3 | 3 | |||
| Ursus maritimus (polar bear) | VU | terrestrial Carnivora | 4 | 2 | 1 | 1 | |
| Total | 45 | 10 | 7 | 1 | 26(*1) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ringwaldt, E.M.; Brook, B.W.; Carver, S.; Buettel, J.C. The Patterns and Causes of Dermatitis in Terrestrial and Semi-Aquatic Mammalian Wildlife. Animals 2021, 11, 1691. https://doi.org/10.3390/ani11061691
Ringwaldt EM, Brook BW, Carver S, Buettel JC. The Patterns and Causes of Dermatitis in Terrestrial and Semi-Aquatic Mammalian Wildlife. Animals. 2021; 11(6):1691. https://doi.org/10.3390/ani11061691
Chicago/Turabian StyleRingwaldt, Elise M., Barry W. Brook, Scott Carver, and Jessie C. Buettel. 2021. "The Patterns and Causes of Dermatitis in Terrestrial and Semi-Aquatic Mammalian Wildlife" Animals 11, no. 6: 1691. https://doi.org/10.3390/ani11061691
APA StyleRingwaldt, E. M., Brook, B. W., Carver, S., & Buettel, J. C. (2021). The Patterns and Causes of Dermatitis in Terrestrial and Semi-Aquatic Mammalian Wildlife. Animals, 11(6), 1691. https://doi.org/10.3390/ani11061691






