Real-Time PCR as an Alternative Technique for Detection of Dermatophytes in Cattle Herds
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Standard
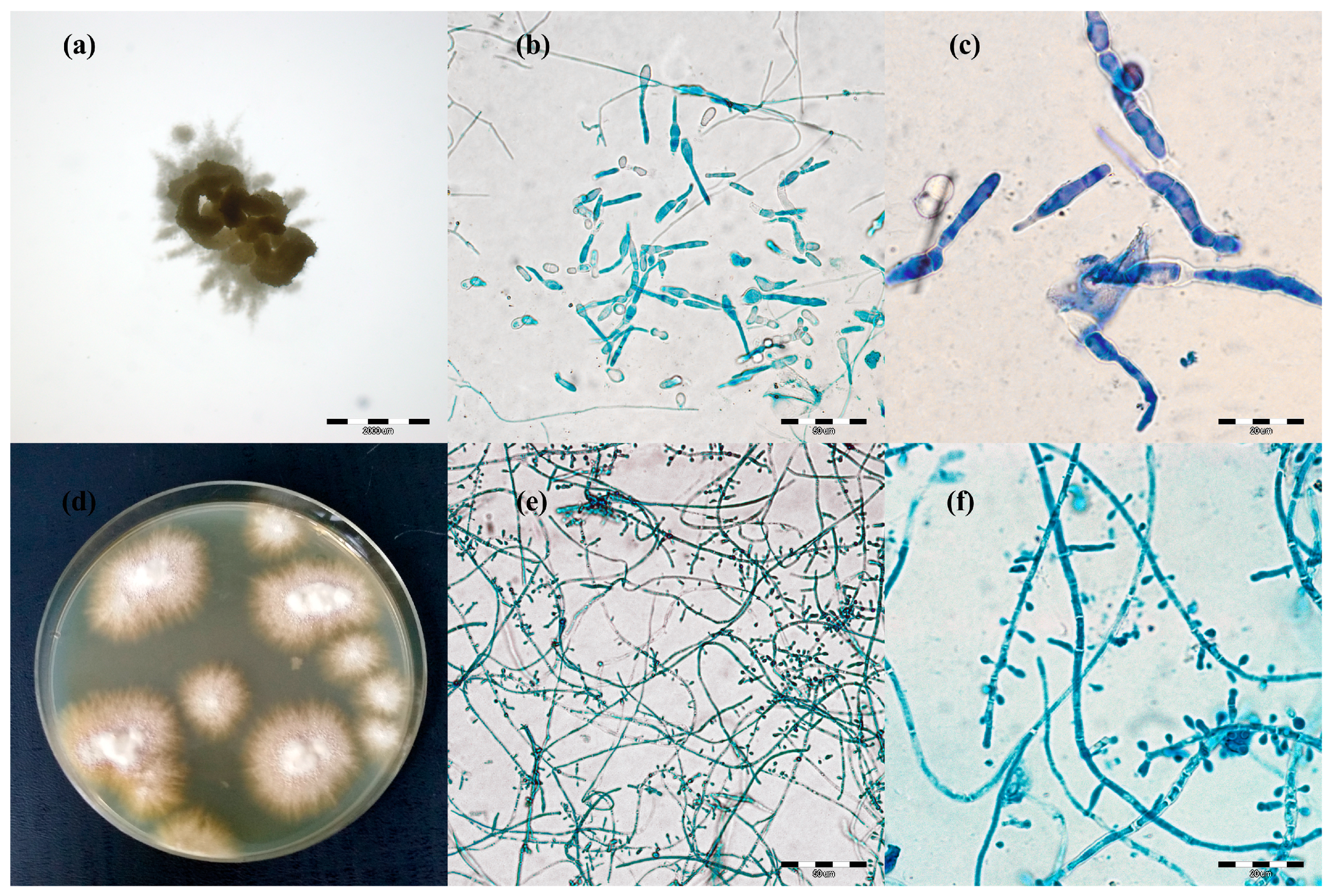
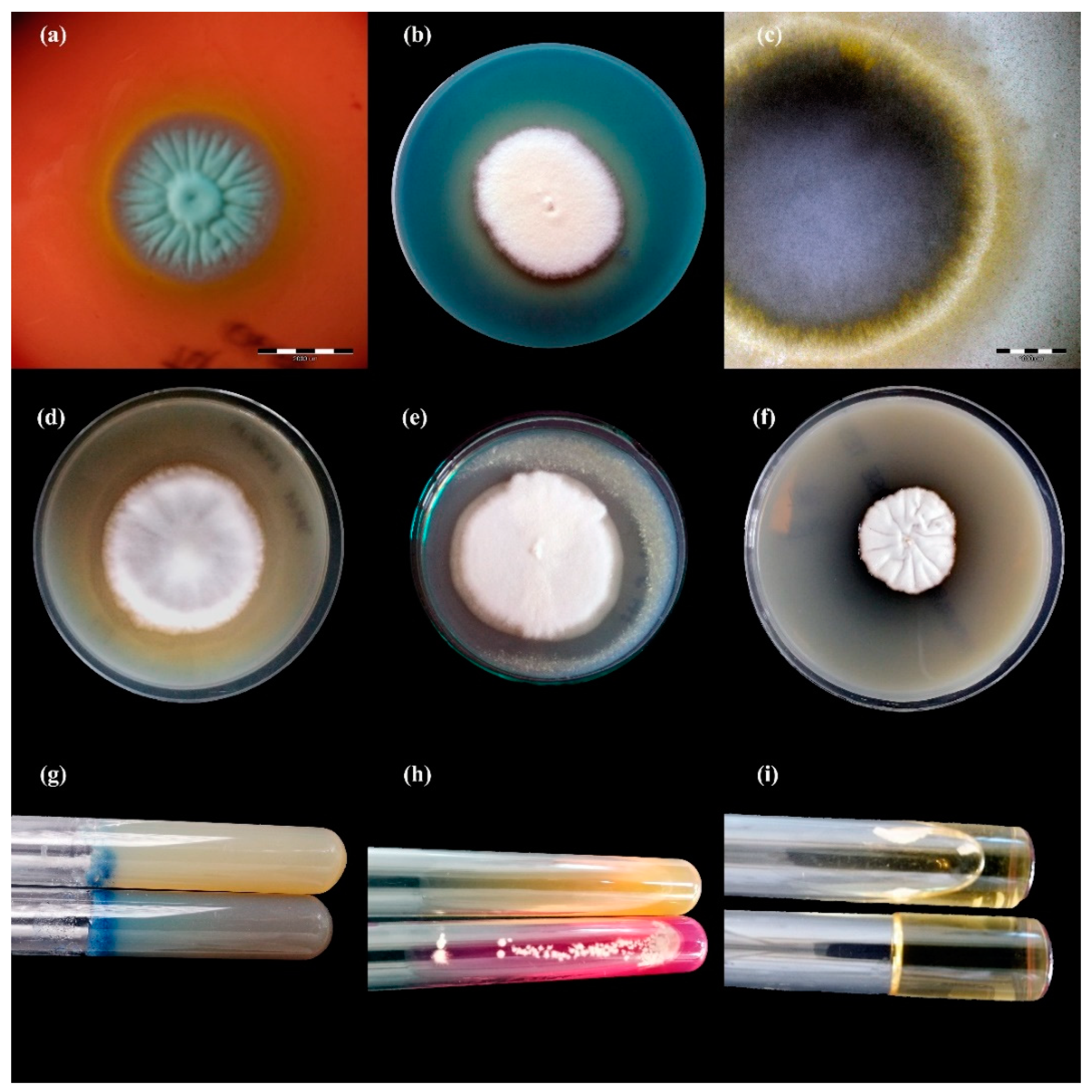
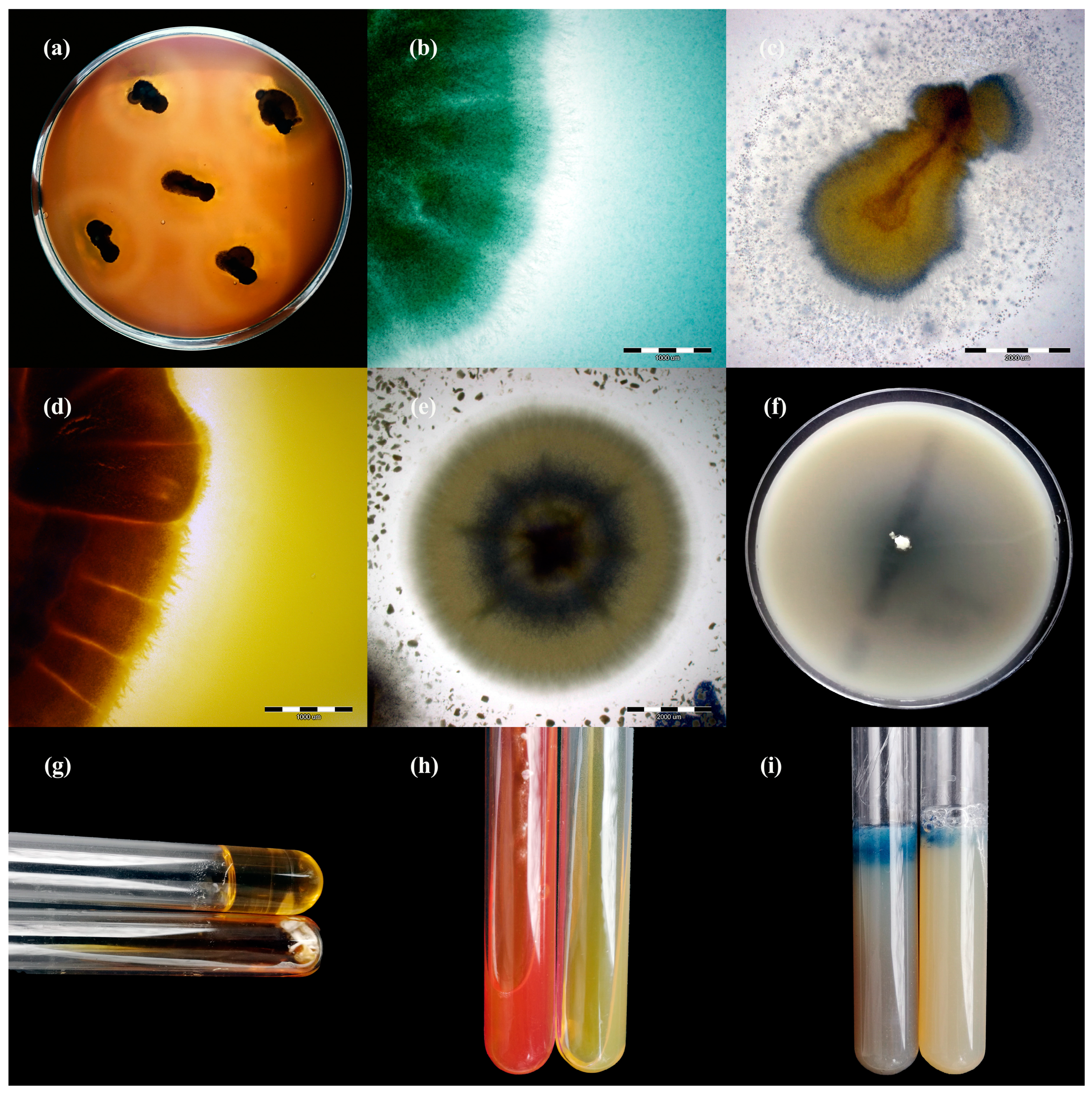
2.2. Dermatophyte Isolates
2.3. Laboratory Diagnostics Procedure
2.4. Enzymatic Activities
3. Results
3.1. Detection and Identification of Dermatophytes
3.2. Enzymatic Activities
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Weitzman, I.; Summerbell, R.C. The Dermatophytes. Clin. Microbiol. Rev. 1995, 8, 240–259. [Google Scholar] [CrossRef]
- Havlickova, B.; Czaika, V.A.; Friedrich, M. Epidemiological Trends in Skin Mycoses Worldwide. Mycoses 2008, 51, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Gnat, S.; Łagowski, D.; Nowakiewicz, A.; Trościańczyk, A.; Zięba, P. Infection of Trichophyton Verrucosum in Cattle Breeders, Poland: A 40-Year Retrospective Study on the Genomic Variability of Strains. Mycoses 2018, 61, 681–690. [Google Scholar] [CrossRef]
- Gnat, S.; Nowakiewicz, A.; Zięba, P. Taxonomy of Dermatophytes—The Classification Systems May Change But the Identification Problems Remain the Same. Postępy Mikrobiol. Adv. Microbiol. 2019, 58, 49–58. [Google Scholar] [CrossRef]
- Lund, A.; Bratberg, A.M.; Næss, B.; Gudding, R. Control of Bovine Ringworm by Vaccination in Norway. Vet. Immunol. Immunopathol. 2014, 158, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Gnat, S.; Nowakiewicz, A.; Lagowski, D.; Czyk, A.T.; Zieba, P. Multiple-Strain Trichophyton Mentagrophytes Infection in a Silver Fox (Vulpes Vulpes) from a Breeding Farm. Med. Mycol. 2019, 57, 171–180. [Google Scholar] [CrossRef]
- Maraki, S.; Tselentis, Y. Dermatophytoses in Crete, Greece, between 1992 and 1996. Mycoses 1998, 41, 175–178. [Google Scholar] [CrossRef]
- Mercantini, R.; Moretto, D.; Palamara, G.; Mercantini, P.; Marsella, R. Epidemiology of Dermatophytoses Observed in Rome, Italy, between 1985 and 1993. Mycoses 1995, 38, 415–419. [Google Scholar] [CrossRef]
- Gnat, S.; Łagowski, D.; Nowakiewicz, A.; Dyląg, M. Unusual Dermatomycoses Caused by Nannizzia Nana: The Geophilic Origin of Human Infections. Infection 2020, 48, 429–434. [Google Scholar] [CrossRef]
- Radostits, O.; Gay, C.; Hinchcliff, K.; Constable, P. Veterinary Medicine—A Textbook of the Diseases of Cattle, Horses, Sheep, Pigs and Goats, 10th ed.; Saunders: Edinburg, TX, USA, 2006; ISBN 9780702039911. [Google Scholar]
- Wawrzkiewicz, K.; Wawrzkiewicz, J. An Inactivated Vaccine against Ringworm. Comp. Immunol. Microbiol. Infect. Dis. 1992, 15, 31–40. [Google Scholar] [CrossRef]
- Courtellemont, L.; Chevrier, S.; Degeilh, B.; Belaz, S.; Gangneux, J.P.; Robert-Gangneux, F. Epidemiology of Trichophyton Verrucosum Infection in Rennes University Hospital, France: A 12-Year Retrospective Study. Med. Mycol. 2017, 55, 720–724. [Google Scholar] [CrossRef]
- Horvath, Z.; Gaal, T. Results of a Trial Using LFT 130 (USSR) Live Vaccine against Ringworm Infection of Cattle. Magy. Allatorv. Lapja 1977, 32, 452–454. [Google Scholar]
- Rotermund, V.H.; Franz, H.; Hausburg, G. Erste Erfahrungen Bei Der Anwendung Der Sowjetischen Trichophytievakzine Lft-130. Mon. Vet. 1977, 32, 576–577. [Google Scholar]
- Krdzalic, P.; Stojicevic, S.; Bresjanac, D. Practical possibility for immunoprophylaxis of trichoficia in cattle in industrial breeding. Vet. Glas. 1978, 32, 343–349. [Google Scholar]
- Stankushev, K.; Duparinova, M.; Kostov, G.; Gradinarski, I. Comparative immunological studies and the determination of the epizootiological effectiveness of Soviet vaccine LTF-130 in trichophytosis. Vet. Med. Nauk. 1979, 16, 67–73. [Google Scholar] [PubMed]
- Aamodt, O.; Naess, B.; Sandvik, O. Vaccination of Norwegian Cattle against Ringworm. Zent. Für Veterinärmedizin Reihe B 1982, 29, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Sarkisov, A.K.; Nikiforov, L.T. Specific Propylaxis and Immunogenic Vaccines in the Control of Dermatophytosis. In Proceedings of the Proceddings of the 10th ISHAM Congress, Barcelona, Spain, 26 June 1988; pp. 307–312. [Google Scholar]
- Rybnikář, A.; Vrzal, V.; Chumela, J. Protective Efficacy of Vaccines against Bovine Dermatophytosis after Double and Single Vaccination. Mycoses 1998, 41, 83–86. [Google Scholar] [CrossRef]
- Rybnikář, A.; Obořilová, E.; Hedbávný, R. Efficacy Test of Trichoben Vaccine Administered to Calves at Different Intervals between Vaccination and Re-Vaccination. Acta Vet. Brno 2008, 77, 239–243. [Google Scholar] [CrossRef][Green Version]
- Gudding, R.; Lund, A. Immunoprophylaxis of Bovine Dermatophytosis. Can. Vet. J. 1995, 36, 302–306. [Google Scholar]
- Ohst, T.; Kupsch, C.; Graser, Y.; Gräser, Y. Detection of Common Dermatophytes in Clinical Specimens Using a Simple Quantitative Real-Time TaqMan Polymerase Chain Reaction Assay. Br. J. Dermatol. 2016, 174, 602–609. [Google Scholar] [CrossRef]
- Gnat, S.; Łagowski, D.; Nowakiewicz, A.; Dyląg, M.; Osińska, M.; Sawicki, M. Detection and Identification of Dermatophytes Based on Currently Available Methods—A Comparative Study. J. Appl. Microbiol. 2021, 130, 278–291. [Google Scholar] [CrossRef]
- Tartor, Y.H.; Abo Hashem, M.E.; Enany, S. Towards a Rapid Identification and a Novel Proteomic Analysis for Dermatophytes from Human and Animal Dermatophytosis. Mycoses 2019, 62, 1116–1126. [Google Scholar] [CrossRef]
- Gnat, S.; Łagowski, D.; Nowakiewicz, A.; Dyląg, M. Molecular Methods for Diagnostics of Dermatomycoses—Review of Available Techniques and Evaluation of Their Advantages and Disadvantages in Implementation for in Routine Use. Postępy Mikrobiol. Adv. Microbiol. 2019, 58, 483–494. [Google Scholar] [CrossRef]
- Azrad, M.; Keness, Y.; Nitzan, O.; Pastukh, N.; Tkhawkho, L.; Freidus, V.; Peretz, A. Cheap and Rapid In-House Method for Direct Identification of Positive Blood Cultures by MALDI-TOF MS Technology. BMC Infect. Dis. 2019, 19, 72. [Google Scholar] [CrossRef] [PubMed]
- Gnat, S.; Łagowski, D.; Nowakiewicz, A.; Osińska, M.; Kopiński, Ł. Population Differentiation, Antifungal Susceptibility, and Host Range of Trichophyton Mentagrophytes Isolates Causing Recalcitrant Infections in Humans and Animals. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 2099–2113. [Google Scholar] [CrossRef]
- Gnat, S.; Nowakiewicz, A.; Ziółkowska, G.; Trościańczyk, A.; Majer-Dziedzic, B.; Zięba, P. Evaluation of Growth Conditions and DNA Extraction Techniques Used in the Molecular Analysis of Dermatophytes. J. Appl. Microbiol. 2017, 122, 1368–1379. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.D.; Lee, S.B.; Taylor, J.W.; Innis, M.A.; Gelfand, D.H.; Snisky, J. Amplification and direct sequencing of fungal ribosomal RNA Genes for phylogenetics. In CRC Protocols; Academic Press: Cambridge, MA, USA, 2007; pp. 315–322. ISBN 0-12-372180-6. [Google Scholar]
- Gnat, S.; Łagowski, D.; Nowakiewicz, A.; Zięba, P. Phenotypic Characterization of Enzymatic Activity of Clinical Dermatophyte Isolates from Animals with and without Skin Lesions and Humans. J. Appl. Microbiol. 2018, 125, 700–709. [Google Scholar] [CrossRef] [PubMed]
- De Hoog, G.S.; Guarro, J.; Gené, J.; Figueras, M.J.; de Hoog, G.S.; Guarro, J.; Gené, J.; Figueras, M.J. Atlas of Clinical Fungi, 3rd ed.; Centraalbureau Voor Schimmelcultures (CBS): Utrecht, The Netherlands, 2019; ISBN 90-70351-43-9. [Google Scholar]
- Łagowski, D.; Gnat, S.; Nowakiewicz, A.; Osińska, M.; Trościańczyk, A.; Zięba, P. In Search of the Source of Dermatophytosis: Epidemiological Analysis of Trichophyton Verrucosum Infection in Llamas and the Breeder (Case Report). Zoonoses Public Health 2019, 66, 982–989. [Google Scholar] [CrossRef] [PubMed]
- Siesenop, U.; Bohm, K.H.; Brandebusemeyer, E.; Conrad, P. Studies on Growth, Spore-Forming Ability and Virulence of the Vaccine Strain TV-M-310 of the Vaccine Bioveta against Ringworm. Mycoses 1994, 37, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Lund, A.; Deboer, D.J. Immunoprophylaxis of Dermatophytosis in Animals. Mycopathologia 2008, 166, 407–424. [Google Scholar] [CrossRef]
- Weitzman, I.; Chin, N.X.; Kunjukunju, N.; Della-Latta, P. A Survey of Dermatophytes Isolated from Human Patients in the United States from 1993 to 1995. J. Am. Acad. Dermatol. 1998, 39, 255–261. [Google Scholar] [CrossRef]
- Łagowski, D.; Gnat, S.; Nowakiewicz, A.; Osińska, M.; Zięba, P. Application of Genotyping Methods in the Investigation of Sources of Dermatophytosis Associated with Vaccination in Cattle. Ann. Appl. Biol. 2020, 177, 325–332. [Google Scholar] [CrossRef]
- Lund, A.; Bratberg, A.M.; Solbakk, I.T. In Vitro Release of Interferon-γ by Trichophytin-Stimulated Whole Blood Cell Cultures from Ringworm-Vaccinated and Control Calves Experimentally Inoculated with Trichophyton Verrucosum. Vet. Dermatol. 2001, 12, 75–80. [Google Scholar] [CrossRef]
- Faldyna, M.; Oborilova, E.; Krejci, J.; Tesarik, R.; Krejci, E.; Pavlova, B.; Rybnikar, A. A Correlation of in Vitro Tests for the Immune Response Detection: A Bovine Trichophytosis Model. Vaccine 2007, 25, 7948–7954. [Google Scholar] [CrossRef]
- Dolenc-Voljc, M. Dermatophyte Infections in the Ljubljana Region, Slovenia, 1995-2002. Mycoses 2005, 48, 181–186. [Google Scholar] [CrossRef]
- Kuklová, I.; Kuĉerová, H. Dermatophytoses in Prague, Czech Republic, between 1987 and 1998. Mycoses 2001, 44, 493–496. [Google Scholar] [CrossRef]
- Tietz, H.-J.; Kunzelmann, V.; Schönian, G. Wandel des dermatomykologischen Erregerspektrums. Mycoses 1995, 38, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Sabou, M.; Denis, J.; Boulanger, N.; Forouzanfar, F.; Glatz, I.; Lipsker, D.; Poirier, P.; Candolfi, E.; Letscher-Bru, V. Molecular Identification of Trichophyton Benhamiae in Strasbourg, France: A 9-Year Retrospective Study. Med. Mycol. 2018, 56, 723–734. [Google Scholar] [CrossRef]
- Nakamura, Y.; Kano, R.; Nakamura, E.; Saito, K.; Watanabe, S.; Hasegawa, A. Case Report. First Report on Human Ringworm Caused by Arthroderma Benhamiae in Japan Transmitted from a Rabbit. Mycoses 2002, 45, 129–131. [Google Scholar] [CrossRef] [PubMed]
- Drouot, S.; Mignon, B.; Fratti, M.; Roosje, P.; Monod, M. Pets as the Main Source of Two Zoonotic Species of the Trichophyton Mentagrophytes Complex in Switzerland, Arthroderma Vanbreuseghemii and Arthroderma Benhamiae. Vet. Dermatol. 2009, 20, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Symoens, F.; Jousson, O.; Packeu, A.; Fratti, M.; Staib, P.; Mignon, B.; Monod, M. The Dermatophyte Species Arthroderma Benhamiae: Intraspecies Variability and Mating Behaviour. J. Med. Microbiol. 2013, 62, 377–385. [Google Scholar] [CrossRef]
- Brasch, J.; Wodarg, S. Morphological and Physiological Features of Arthroderma Benhamiae Anamorphs Isolated in Northern Germany. Mycoses 2015, 58, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, A.; Mueller, R.S.S.; Werckenthin, C.; Straubinger, R.K.K.; Hein, J. Dermatophytes in Pet Guinea Pigs and Rabbits. Vet. Microbiol. 2012, 157, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Contet-Audonneau, N.; Leyer, C. Émergence d’un Dermatophyte Transmis Par Le Cochon d’Inde et Proche de Trichophyton Mentagrophytes Var. Erinacei: T. Mentagrophytes Var. Porcellae. J. Mycol. Med. 2010, 20, 321–325. [Google Scholar] [CrossRef]
- Khettar, L.; Contet-Audonneau, N. Cochon d’Inde et dermatophytose. Ann. Dermatol. Venereol. 2012, 139, 631–635. [Google Scholar] [CrossRef] [PubMed]
- El-Heis, S.; Borman, A.M.; Szekely, A.; Godfrey, K.M. Tinea Corporis Caused by Arthroderma Benhamiae in a Child. Clin. Exp. Dermatol. 2016, 41, 955–957. [Google Scholar] [CrossRef][Green Version]
- Nenoff, P.; Krüger, C.; Ginter-Hanselmayer, G.; Tietz, H.J. Mykologie-Ein Update. Teil 1: Dermatomykosen: Erreger, Epidemiologie Und Pathogenese. JDDG—J. Ger. Soc. Dermatol. 2014, 12, 188–212. [Google Scholar] [CrossRef]
- Mayser, P.; Budihardja, D. A Simple and Rapid Method to Differentiate Arthroderma Benhamiae from Microsporum Canis. J. Dtsch. Dermatol. Ges. 2013, 11, 322–327. [Google Scholar] [CrossRef]
- Sieklucki, U.; Oh, S.-H.H.; Hoyer, L.L. Frequent Isolation of Arthroderma Benhamiae from Dogs with Dermatophytosis. Vet. Dermatol. 2014, 25, 39-e14. [Google Scholar] [CrossRef]
- Takahashi, H.; Takahashi-kyuhachi, H.; Takahashi, Y.; Yarita, K.; Takayama, A.; Inomata, T.; Sano, A.; Nishimura, K.; Kamei, K. An Intrafamilial Transmission of Arthroderma Benhamiae in Canadian Porcupines (Erethizon Dorsatum) in a Japanese Zoo. Med. Mycol. 2008, 46, 465–473. [Google Scholar] [CrossRef][Green Version]
- Łagowski, D.; Gnat, S.; Nowakiewicz, A.; Osińska, M.; Trościańczyk, A.; Zięba, P. Dermatophytosis with Concurrent Trichophyton Verrucosum and T. Benhamiae in Calves after Long-Term Transport. Vet. Dermatol. 2020, 31, 414-e111. [Google Scholar] [CrossRef]
- Wawrzkiewicz, K.; Wolski, T.; Łobarzewski, J. Screening the Keratinolytic Activity of Dermatophytes in Vitro. Mycopathologia 1991, 114, 1–8. [Google Scholar] [CrossRef]
- de A. Peres, N.T.; Maranhão, F.C.A.; Rossi, A.; Martinez-Rossi, N.M. Dermatophytes: Host-Pathogen Interaction and Antifungal Resistance. An. Bras. Dermatol. 2010, 85, 657–667. [Google Scholar] [CrossRef]
- Kaufman, G.; Horwitz, B.A.; Duek, L.; Ullman, Y.; Berdicevsky, I. Infection Stages of the Dermatophyte Pathogen Trichophyton: Microscopic Characterization and Proteolytic Enzymes. Med. Mycol. 2007, 45, 149–155. [Google Scholar] [CrossRef]
- Gnat, S.; Łagowski, D.; Nowakiewicz, A.; Zięba, P. The Host Range of Dermatophytes, It Is at All Possible? Phenotypic Evaluation of the Keratinolytic Activity of Trichophyton Verrucosum Clinical Isolates. Mycoses 2019, 62, 274–283. [Google Scholar] [CrossRef]
- Sharma, M.; Sharma, M.; Rao, V.M. In Vitro Biodegradation of Keratin by Dermatophytes and Some Soil Keratinophiles. Afr. J. Biochem. Res. 2011, 5, 1–6. [Google Scholar]
- Mercer, D.K.; Stewart, C.S. Keratin Hydrolysis by Dermatophytes. Med. Mycol. 2019, 57, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Gnat, S.; Łagowski, D.; Nowakiewicz, A.; Osińska, M.; Dyląg, M. Identification of Emerging Trends in the Prevalence of Dermatophytoses in Alpacas (Vicugna Pacos) Farmed in Poland. Transbound. Emerg. Dis. 2020, 67, 2702–2712. [Google Scholar] [CrossRef] [PubMed]
- Nenoff, P.; Uhrlaß, S.; Krüger, C.; Erhard, M.; Hipler, U.C.; Seyfarth, F.; Herrmann, J.; Wetzig, T.; Schroedl, W.; Gräser, Y. Trichophyton Spezies von Arthroderma Benhamiae—Ein Neuer Infektionserreger in Der Dermatologie. JDDG—J. Ger. Soc. Dermatol. 2014, 12, 571–582. [Google Scholar] [CrossRef]
- Ziółkowska, G.; Nowakiewicz, A.; Gnat, S.; Trościańczyk, A.; Zieba, P.; Majer Dziedzic, B. Molecular Identification and Classification of Trichophyton Mentagrophytes Complex Strains Isolated from Humans and Selected Animal Species. Mycoses 2015, 58, 119–126. [Google Scholar] [CrossRef] [PubMed]

| Localization on Farms | Statistical Data on Farms (Number of Individuals) | ||||
|---|---|---|---|---|---|
| Animals | Importing * | Vaccinated (Total) | Symptomatic Ringworm/(%) ** | Asymptomatic Carriers *** | |
| south-eastern Poland | 760 | 50 | 690 | 9/1.18 | 8 |
| south-western Poland | 810 | 32 | 700 | 3/0.37 | 12 |
| north-western Poland | 700 | 45 | 670 | 9/1.29 | 4 |
| central Poland | 750 | 38 | 600 | 5/0.67 | 7 |
| eastern Poland | 720 | 65 | 150 | 12 (9 ****)/1.67 | 20 (8 ****) |
| central-eastern Poland | 830 | 76 | 754 | 2/0.24 | 3 |
| Type of Infection | Method (Number of Positive Animals/% of Positive Results) | Compatible (Number of Animals/% of Consistent Results) | |||||
|---|---|---|---|---|---|---|---|
| qPCR (P) | Microscopy (M) | Cultures (C) | (P)-(M) | (P)-(C) | (M)-(C) | (P)-(M)-(C) | |
| symptomatic | 40/100% | 40/100% | 40/100% | 40/100% | 40/100% | 40/100% | 40/100% |
| asymptomatic | 54/45% ST | 41/34.17% | 27/22.5% | 41/75.93% | 27/50% | 16/39.02% | 14/25.93% |
| Isolates | Keratinase | Phospholipase | Lipase | Elastase | Protease | DNase | Urease | Gelatinase | Haemolysis |
|---|---|---|---|---|---|---|---|---|---|
| T. verrucosum | + | + | ++ | + | + | + | + | + | +double |
| T. benhamiae | + | + | + | + | ++ | + | ++ | + | +single |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łagowski, D.; Gnat, S.; Nowakiewicz, A.; Trościańczyk, A. Real-Time PCR as an Alternative Technique for Detection of Dermatophytes in Cattle Herds. Animals 2021, 11, 1662. https://doi.org/10.3390/ani11061662
Łagowski D, Gnat S, Nowakiewicz A, Trościańczyk A. Real-Time PCR as an Alternative Technique for Detection of Dermatophytes in Cattle Herds. Animals. 2021; 11(6):1662. https://doi.org/10.3390/ani11061662
Chicago/Turabian StyleŁagowski, Dominik, Sebastian Gnat, Aneta Nowakiewicz, and Aleksandra Trościańczyk. 2021. "Real-Time PCR as an Alternative Technique for Detection of Dermatophytes in Cattle Herds" Animals 11, no. 6: 1662. https://doi.org/10.3390/ani11061662
APA StyleŁagowski, D., Gnat, S., Nowakiewicz, A., & Trościańczyk, A. (2021). Real-Time PCR as an Alternative Technique for Detection of Dermatophytes in Cattle Herds. Animals, 11(6), 1662. https://doi.org/10.3390/ani11061662