Cinnamon Extract and Probiotic Supplementation Alleviate Copper-Induced Nephrotoxicity via Modulating Oxidative Stress, Inflammation, and Apoptosis in Broiler Chickens
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Plant Extract Preparation
2.3. Experimental Birds
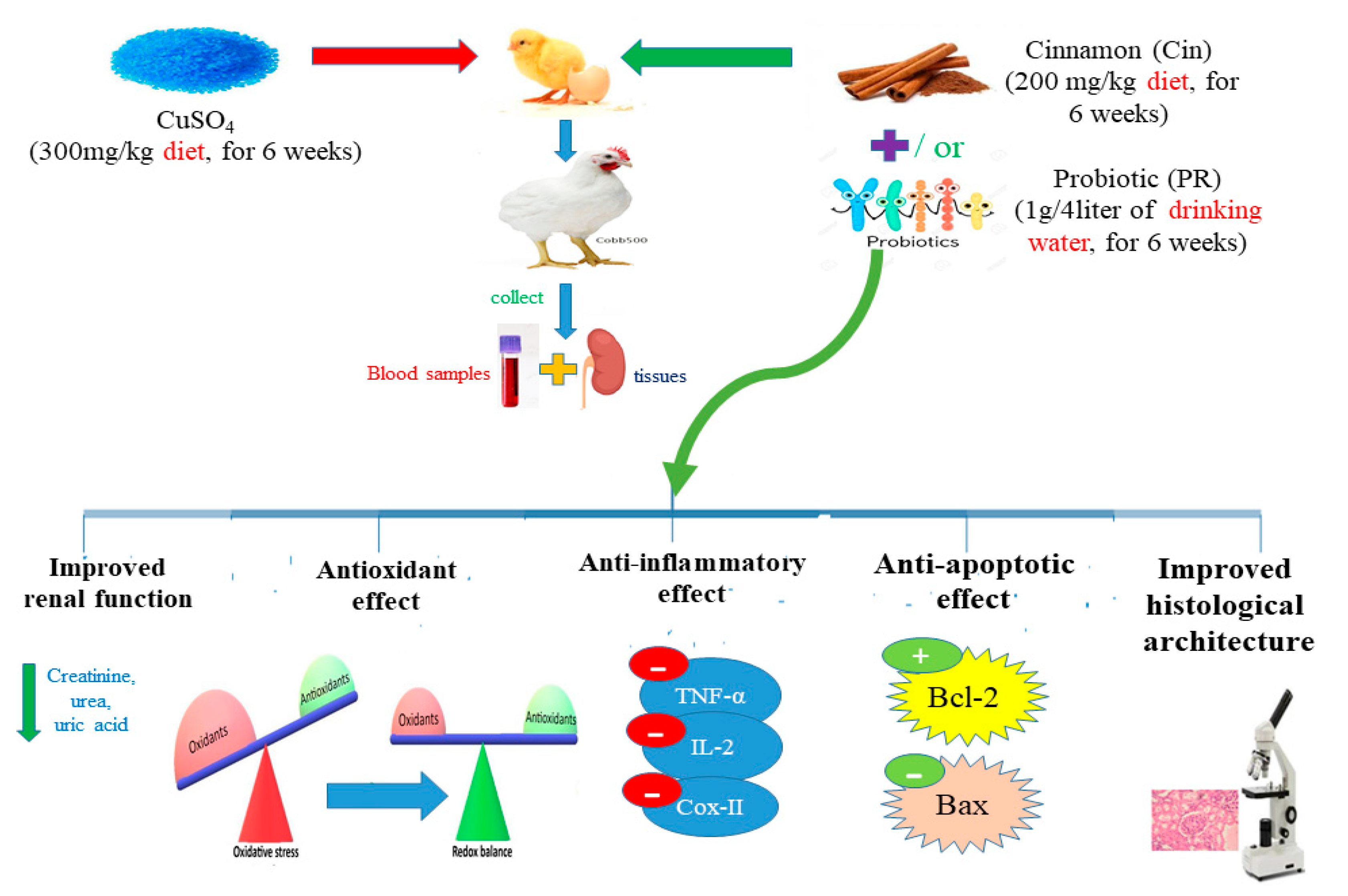
2.4. Experimental Design
2.5. Sample Collection
2.6. Measurement of Cu Content in the Kidney
2.7. Biochemical Analysis
2.7.1. Serum Renal Parameters
2.7.2. Estimation of Serum Immunoglobulins
2.7.3. Oxidative Stress and Lipid Peroxidation Markers in Renal Tissues
2.8. Gene Expression Analysis of Cytokines and Apoptosis-Related Genes by Real-Time PCR
2.8.1. Total Extraction of RNA and Synthesis of cDNA
2.8.2. Quantitative Real-Time PCR
2.9. Renal Histopathological Assessment
2.10. Immunohistochemistry
2.11. Growth Parameter Measurements
2.12. Statistical Analysis
3. Results
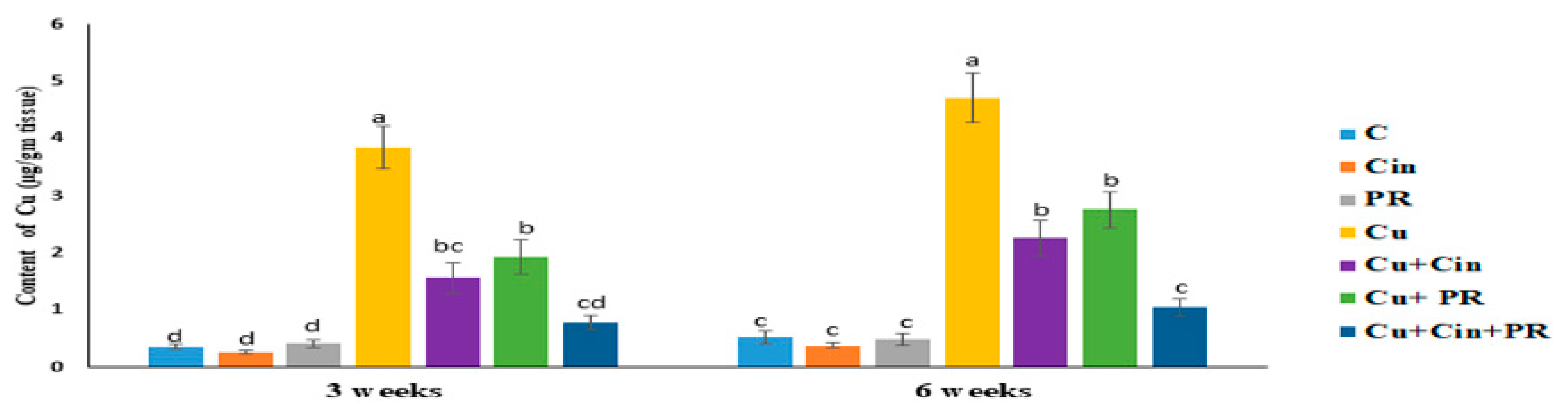
3.1. Cu Concentration in Renal Tissues
3.2. Serum Renal Injury Biomarkers
3.3. Serum Immunoglobulin Levels
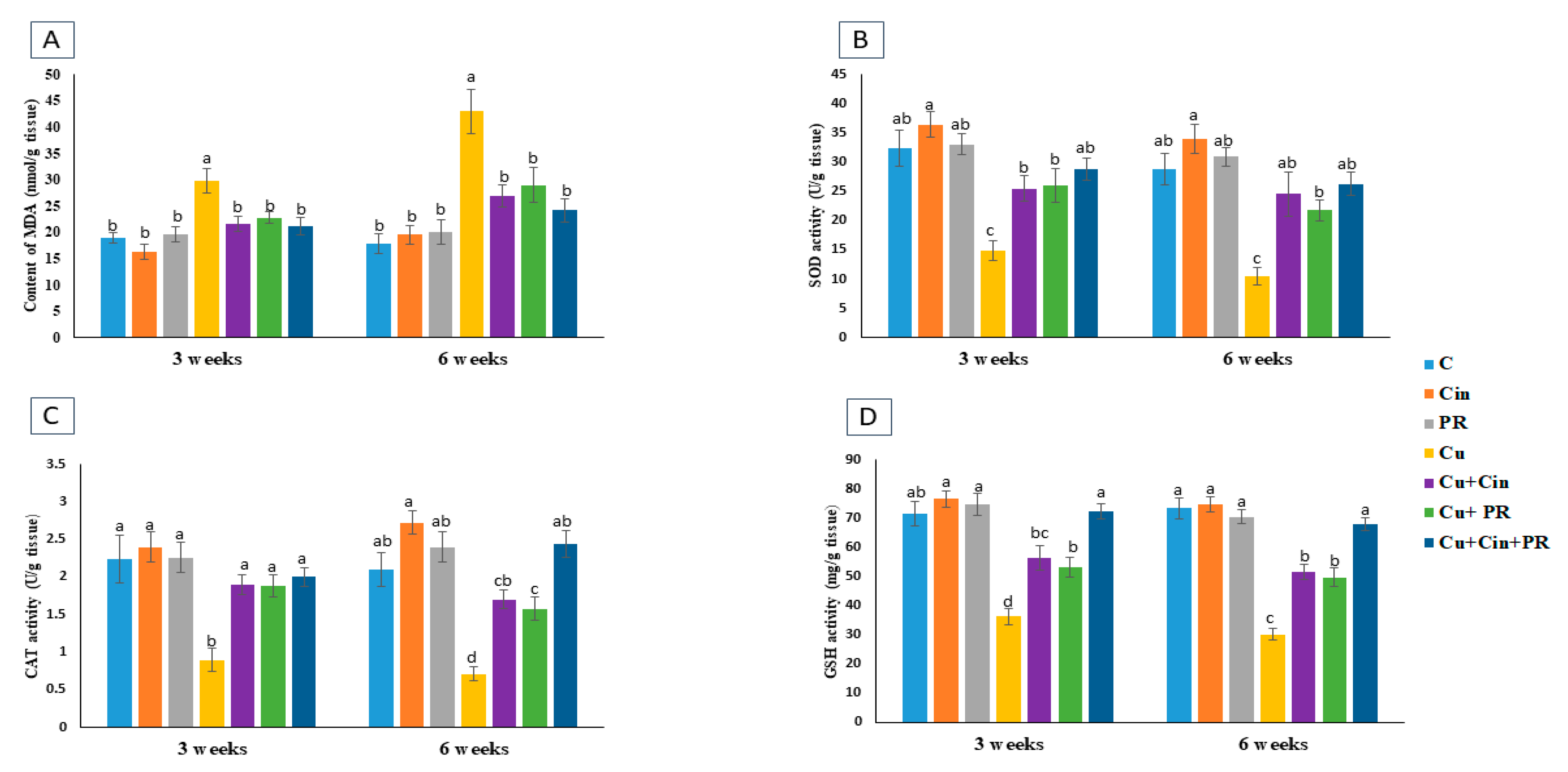
3.4. Oxidative Stress and Antioxidant Markers in Renal Tissues
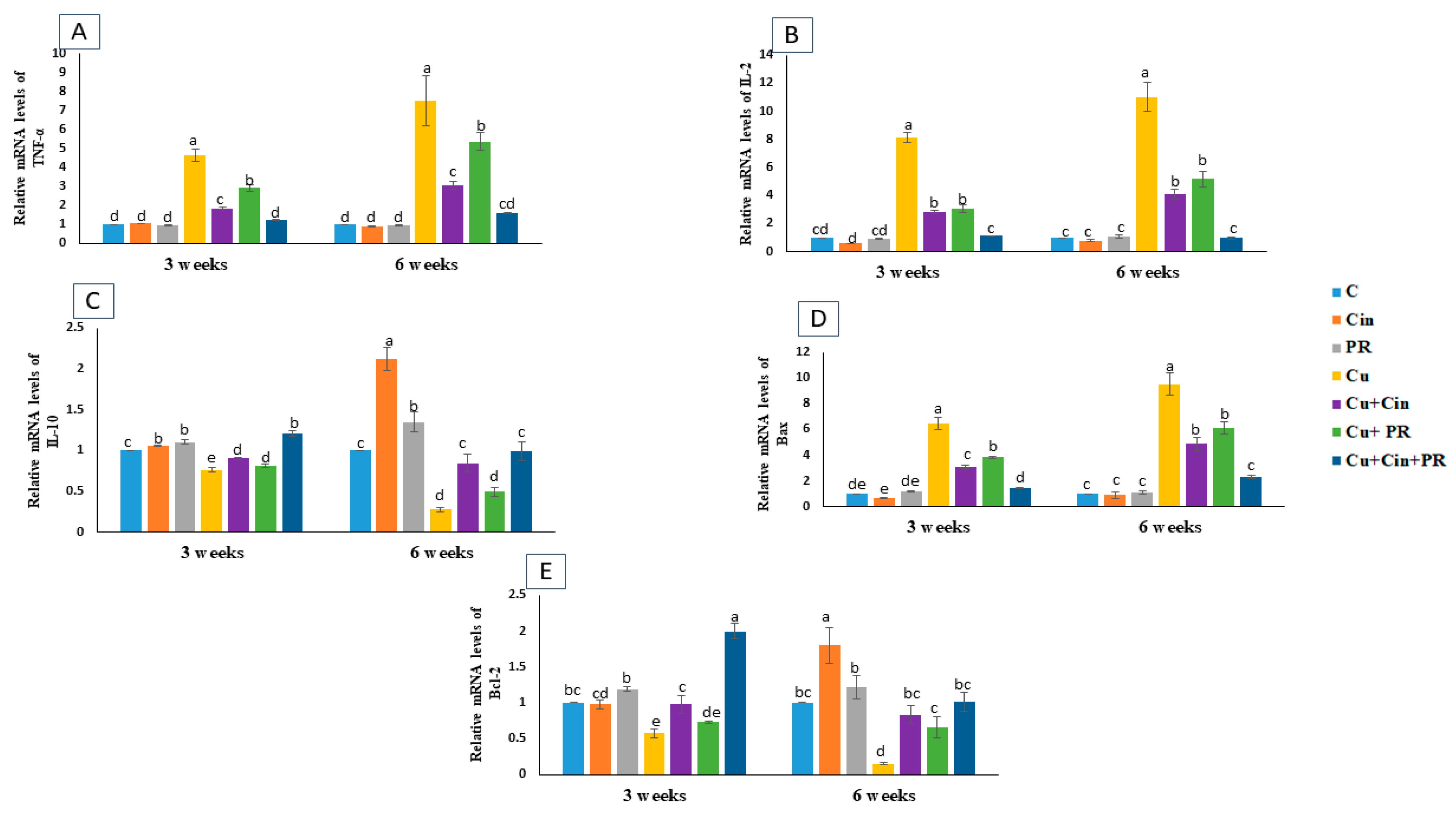
3.5. Expression of Cytokines and Apoptosis-Related Genes
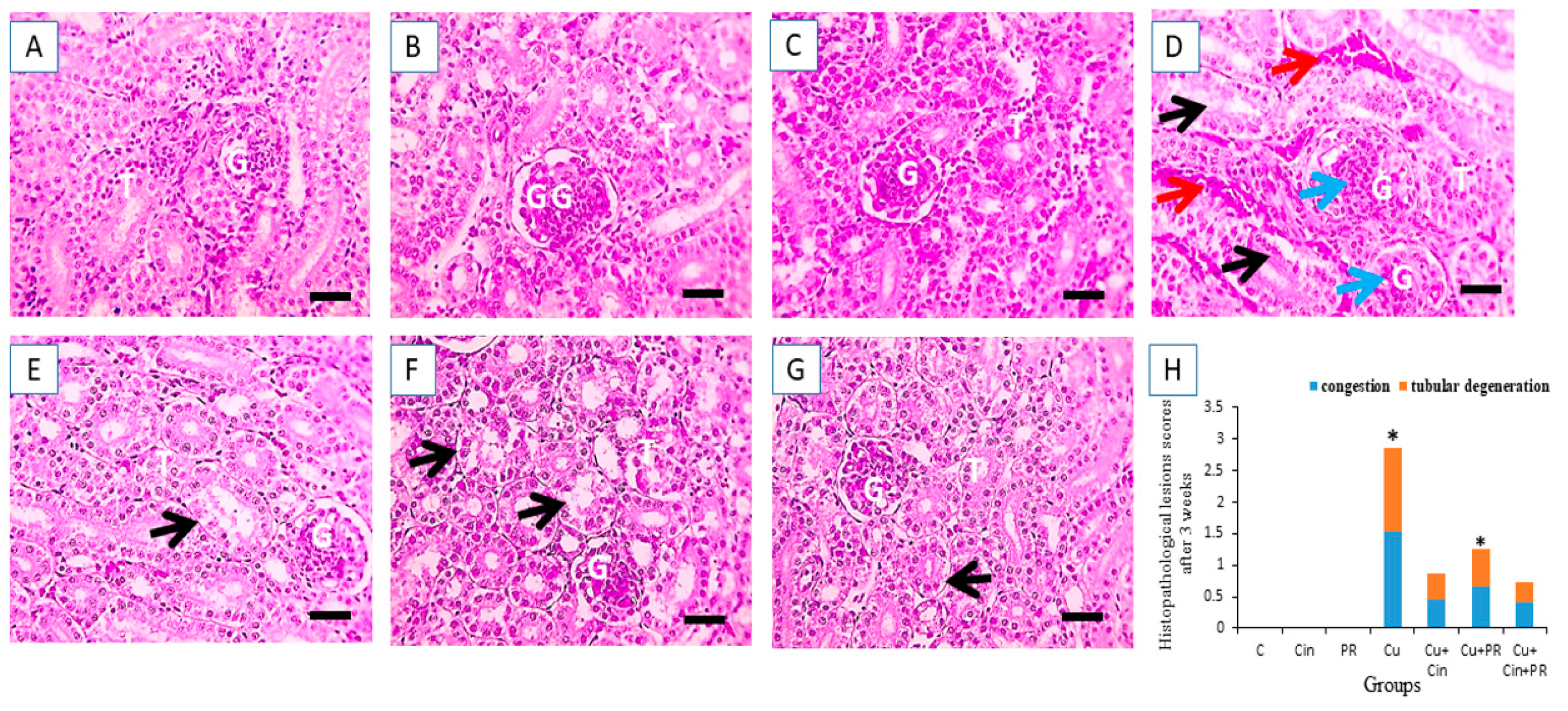
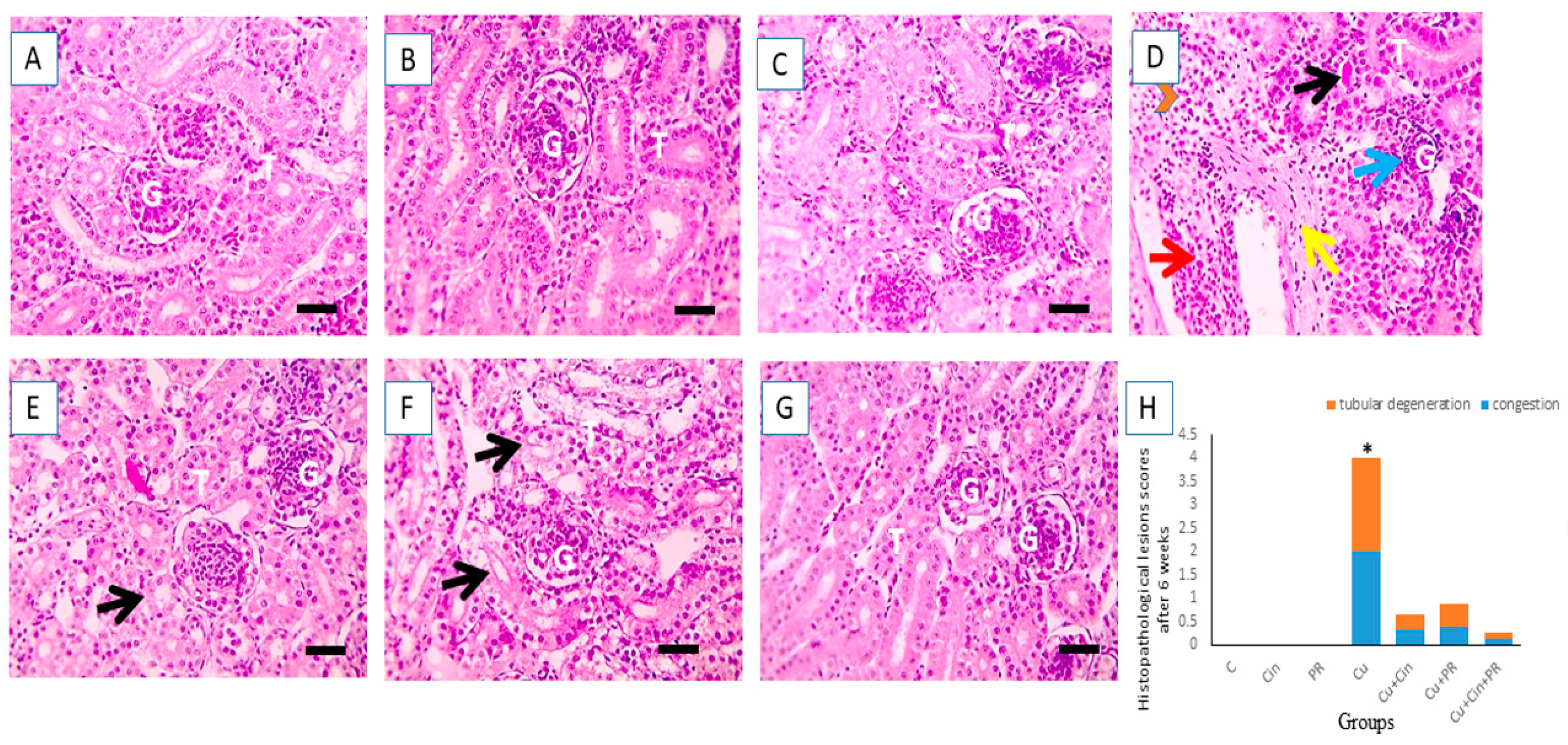
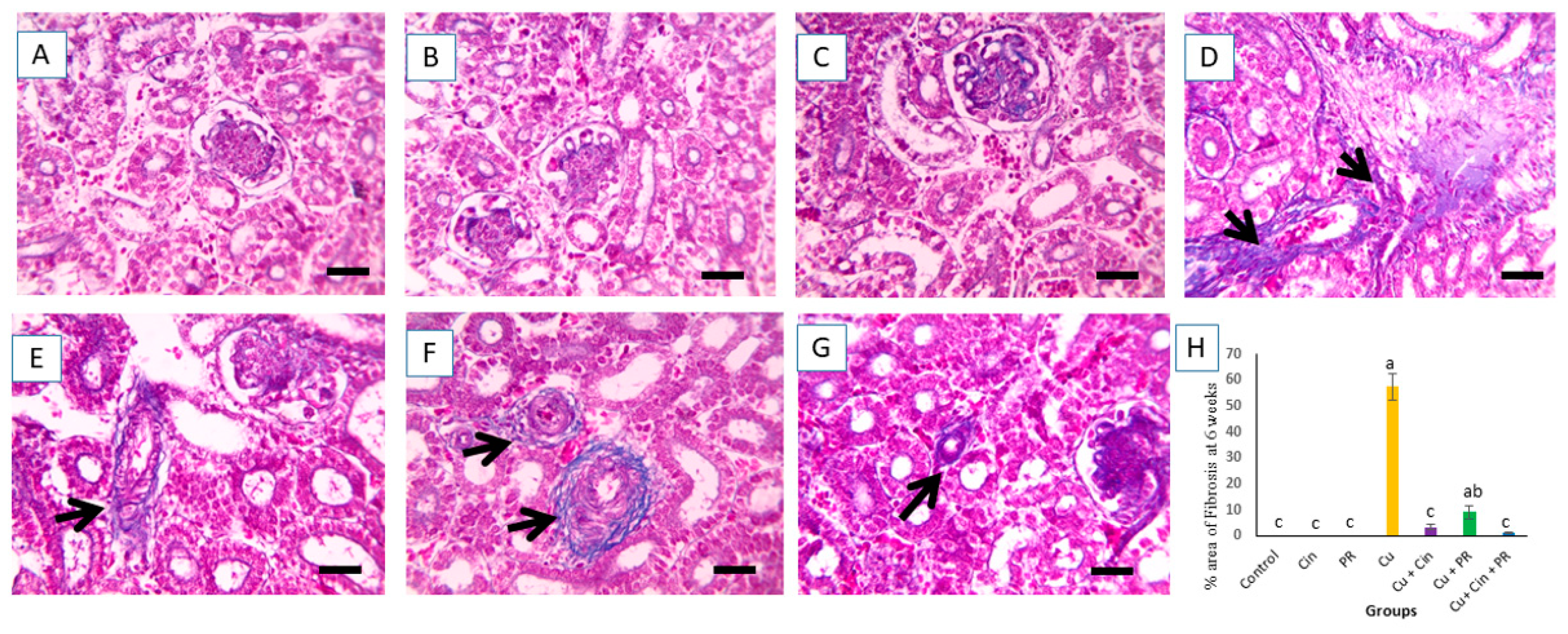
3.6. Histopathological Alterations
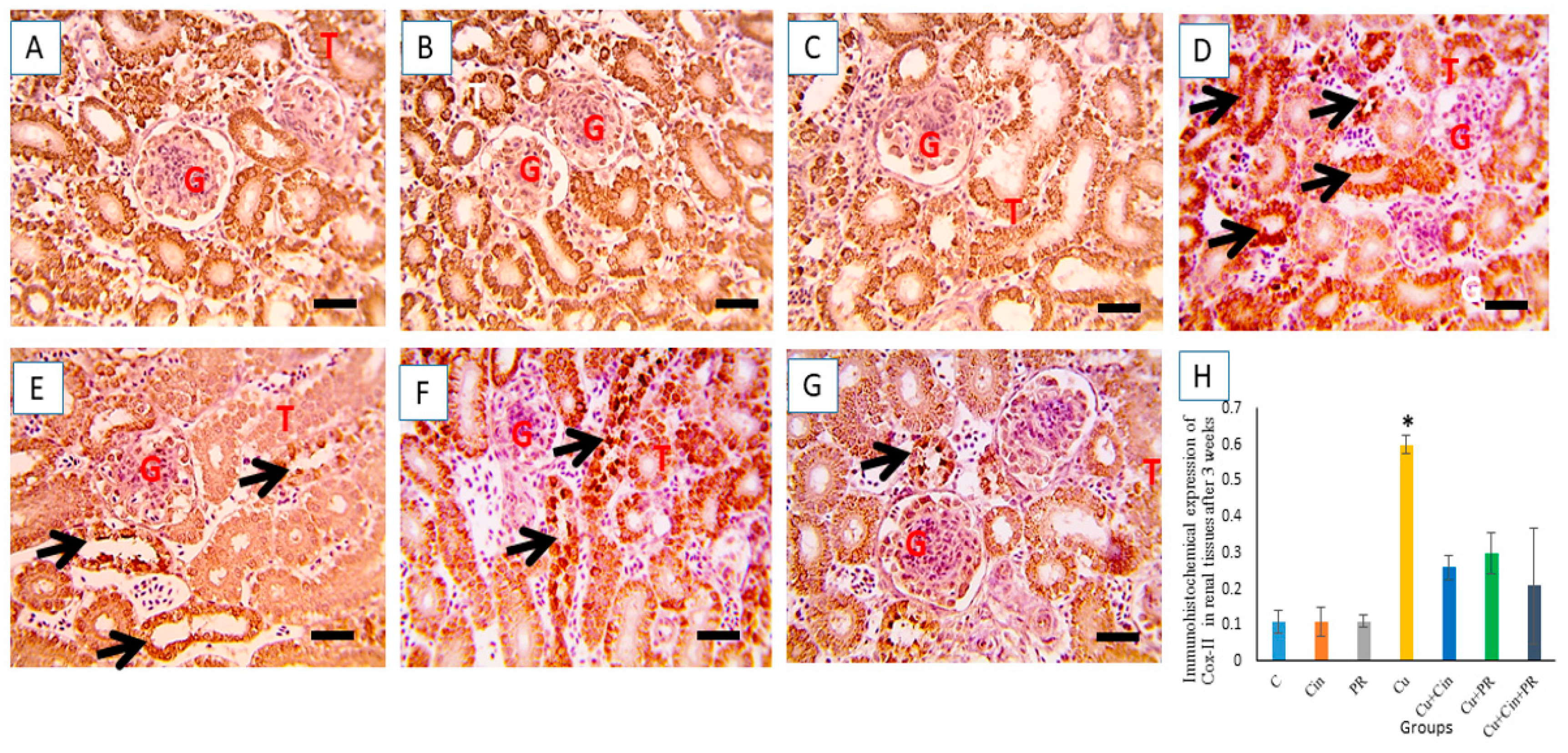
3.7. Immunohistochemical Findings
3.8. Survival Rate and Growth Performance
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Airede, A.K. Copper, zinc and superoxide dismutase activities in premature infants: A review. East Afr. Med. J. 1993, 70, 441–444. [Google Scholar]
- Durand, A.; Azzouzi, A.; Bourbon, M.-L.; Steunou, A.-S.; Liotenberg, S.; Maeshima, A.; Astier, C.; Argentini, M.; Saito, S.; Ouchane, S. c-Type Cytochrome Assembly Is a Key Target of Copper Toxicity within the Bacterial Periplasm. MBio 2015, 6, 5. [Google Scholar] [CrossRef]
- Gaetke, L.M.; Chow, C.K. Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology 2003, 189, 147–163. [Google Scholar] [CrossRef]
- Georgopoulos, P.G.; Roy, A.; Yonone-Lioy, M.J.; Opiekun, R.E.; Lioy, P.J. Copper: Environmental Dynamics and Human Exposure issues. Prepared for: The International Copper Association; Nu Horizon Enterprises Inc.: Cranford, NJ, USA, 2001; pp. 44–45. [Google Scholar]
- Ozcelik, D.; Ozaras, R.; Gurel, Z.; Uzun, H.; Aydin, S. Copper-Mediated Oxidative Stress in Rat Liver. Biol. Trace Element Res. 2003, 96, 209–216. [Google Scholar] [CrossRef]
- Liao, J.; Yang, F.; Chen, H.; Yu, W.; Han, Q.; Li, Y.; Hu, L.; Guo, J.; Pan, J.; Liang, Z.; et al. Effects of copper on oxidative stress and autophagy in hy-pothalamus of broilers. Ecotoxicol. Environ. Saf. 2019, 185, 109710. [Google Scholar] [CrossRef]
- Brewer, G.J. The risks of copper toxicity contributing to cognitive decline in the aging population and to Alzheimer’s disease. J. Am. Coll. Nutr. 2009, 28, 238–242. [Google Scholar] [CrossRef]
- Kim, J.; Oh, J.-M. Assessment of Trace Element Concentrations in Birds of Prey in Korea. Arch. Environ. Contam. Toxicol. 2015, 71, 26–34. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, H.; Shao, Y.; Liu, J.; Li, J.; Xing, M. Copper or/and arsenic induce oxidative stress-cascaded, nuclear factor kappa B-dependent inflammation and immune imbalance, trigging heat shock response in the kidney of chicken. Oncotarget 2017, 8, 98103–98116. [Google Scholar] [CrossRef][Green Version]
- Su, R.; Wang, R.; Guo, S.; Cao, H.; Pan, J.; Li, C.; Shi, D.; Tang, Z. In Vitro Effect of Copper Chloride Exposure on Reactive Oxygen Species Generation and Respiratory Chain Complex Activities of Mitochondria Isolated from Broiler Liver. Biol. Trace Element Res. 2011, 144, 668–677. [Google Scholar] [CrossRef]
- Gülçin, I. Antioxidant activity of caffeic acid (3,4-dihydroxycinnamic acid). Toxicology 2006, 217, 213–220. [Google Scholar] [CrossRef]
- Hussain, Z.; Khan, J.A.; Arshad, A.; Asif, P.; Rashid, H.; Arshad, M. Protective effects of Cinnamomum zeylanicum L. (Darchini) in acetaminophen-induced oxidative stress, hepatotoxicity and nephrotoxicity in mouse model. Biomed. Pharmacother. 2019, 109, 2285–2292. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S. Medicinal plants and natural products in amelioration of arsenic toxicity: A short review. Pharm. Biol. 2016, 55, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S. The role of medicinal plants and natural products in melioration of cadmium toxicity. Orient. Pharm. Exp. Med. 2018, 18, 177–186. [Google Scholar] [CrossRef]
- Ranasinghe, P.; Pigera, S.; Premakumara, G.S.; Galappaththy, P.; Constantine, G.R.; Katulanda, P. Medicinal properties of ‘true’cinnamon (Cinnamomum zeylanicum): A systematic review. BMC Complement. Altern. Med. 2013, 13, 1–10. [Google Scholar] [CrossRef]
- Su, L.; Yin, J.J.; Charles, D.; Zhou, K.; Moore, J.; Yu, L.L. Total phenolic contents, chelating capacities, and radi-cal-scavenging properties of black peppercorn, nutmeg, rosehip, cinnamon and oregano leaf. Food Chem. 2007, 100, 990–997. [Google Scholar] [CrossRef]
- Morgan, A.M.; El-Ballal, S.S.; El-Bialy, B.E.; Borai, N.E. Studies on the potential protective effect of cinnamon against bisphenol A- and octylphenol-induced oxidative stress in male albino rats. Toxicol. Rep. 2014, 1, 92–101. [Google Scholar] [CrossRef]
- Rice-Evans, C.; Miller, N.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Yang, Y.-C.; Lee, H.-S.; Lee, S.H.; Clark, J.M.; Ahn, Y.-J. Ovicidal and adulticidal activities of Cinnamomum zeylanicum bark essential oil compounds and related compounds against Pediculus humanus capitis (Anoplura: Pediculicidae). Int. J. Parasitol. 2005, 35, 1595–1600. [Google Scholar] [CrossRef]
- Preuss, H.G.; Echard, B.; Polansky, M.M.; Anderson, R. Whole Cinnamon and Aqueous Extracts Ameliorate Sucrose-Induced Blood Pressure Elevations in Spontaneously Hypertensive Rats. J. Am. Coll. Nutr. 2006, 25, 144–150. [Google Scholar] [CrossRef]
- Babu, P.S.; Prabuseenivasan, S.; Ignacimuthu, S. Cinnamaldehyde—A potential antidiabetic agent. Phytomedicine 2007, 14, 15–22. [Google Scholar] [CrossRef]
- Carmo, E.S.; Lima, E.D.O.; Souza, E.L.D.; Sousa, F.B.D. Effect of cinnamomum zeylanicum blume essential oil on the growth and morphogenesis of some potentially pathogenic Aspergillus species. Braz. J. Microbiol. 2008, 39, 91–97. [Google Scholar] [CrossRef]
- Tung, Y.T.; Chua, M.T.; Wang, S.Y.; Chang, S.T. Anti-inflammation activities of essential oil and its constituents from in-digenous cinnamon (Cinnamomum osmophloeum) twigs. Bioresour. Technol. 2008, 99, 3908–3913. [Google Scholar] [CrossRef]
- Hafizur, R.M.; Hameed, A.; Shukrana, M.; Raza, S.A.; Chishti, S.; Kabir, N.; Siddiqui, R.A. Cinnamic acid exerts an-ti-diabetic activity by improving glucose tolerance in vivo and by stimulating insulin secretion in vitro. Phytomedicine 2015, 22, 297–300. [Google Scholar] [CrossRef]
- Abdeen, A.; Abdelkader, A.; Abdo, M.; Wareth, G.; Aboubakr, M.; Aleya, L.; Abdel-Daim, M. Protective effect of cinnamon against acetaminophen-mediated cellular damage and apoptosis in renal tissue. Environ. Sci. Pollut. Res. 2019, 26, 240–249. [Google Scholar] [CrossRef]
- Dorri, M.; Hashemitabar, S.; Hosseinzadeh, H. Cinnamon (Cinnamomum zeylanicum) as an antidote or a protective agent against natural or chemical toxicities: A review. Drug Chem. Toxicol. 2018, 41, 338–351. [Google Scholar] [CrossRef] [PubMed]
- Elshopakey, G.E.; ElAzab, S.T. Cinnamon Aqueous Extract Attenuates Diclofenac Sodium and Oxytetracycline Mediated Hepato-Renal Toxicity and Modulates Oxidative Stress, Cell Apoptosis, and Inflammation in Male Albino Rats. Veter Sci. 2021, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- Fuller, R. Probiotics for farm animals. Probiotics Crit. Rev. 1999, 15, 15–22. [Google Scholar]
- Bhattacharya, S. The Role of Probiotics in the Amelioration of Cadmium Toxicity. Biol. Trace Element Res. 2020, 197, 440–444. [Google Scholar] [CrossRef]
- Afify, A.E.M.M.; Romeilah, R.M.; Sultan, S.I.; Hussein, M.M. Antioxidant activity and biological evaluations of probiotic bacteria strains. Int. J. Acad. Res. 2012, 4, 131–139. [Google Scholar] [CrossRef]
- Halttunen, T.; Collado, M.; El-Nezami, H.; Meriluoto, J.; Salminen, S. Combining strains of lactic acid bacteria may reduce their toxin and heavy metal removal efficiency from aqueous solution. Lett. Appl. Microbiol. 2007, 46, 160–165. [Google Scholar] [CrossRef]
- Mrvcic, J.; Stanzer, D.; Bacun-Druzina, V.; Stehlik-Tomas, V. Copper Binding by Lactic Acid Bacteria (LAB). Biosci. Microflora 2009, 28, 1–6. [Google Scholar] [CrossRef][Green Version]
- Zhai, Q.; Wang, G.; Zhao, J.; Liu, X.; Tian, F.; Zhang, H.; Chen, W. Protective Effects of Lactobacillus plantarum CCFM8610 against Acute Cadmium Toxicity in Mice. Appl. Environ. Microbiol. 2012, 79, 1508–1515. [Google Scholar] [CrossRef]
- Tabatabaei, S.M.; Badalzadeh, R.; Mohammadnezhad, G.R.; Balaei, R. Effects of Cinnamon extract on biochemical enzymes, TNF-α and NF-κB gene expression levels in liver of broiler chickens inoculated with Escherichia coli. Pesq. Vet. Bras. 2015, 35, 781–787. [Google Scholar] [CrossRef]
- Vantress, Broiler performance and nutrition supplement. Cobb500 Ark. USA 2015. Available online: http://sedima.com/wp-content/uploads/2017/07/Cobb-Performance-July-2015.pdf (accessed on 28 May 2021).
- NRC. Nutrient Requirements of Poultry; National Academy Press: Colombia, WA, USA, 1994. [Google Scholar]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists, 15th ed.; Association of Official Analytical Chemistry: Washington, DC, USA, 1990; Volume 2. [Google Scholar]
- Satoh, K. Estimation of lipid peroxides by thiobarbituric acid reactive substances (TBARS). Clin. Chim. Acta. 1978, 90, 37–43. [Google Scholar] [PubMed]
- Claiborne, A.L. Catalase Activity. CRC Handbook of Methods for Oxygen Radical Research; CRC Press: Boca Raton, FL, USA, 1986. [Google Scholar]
- Sun, Y.; Oberley, L.W.; Li, Y. A simple method for clinical assay of superoxide dismutase. Clin. Chem. 1988, 34, 497–500. [Google Scholar] [CrossRef]
- Beutler, E.; Duron, O.; Kelly, B.M. Improved method for the determination of blood glutathione. J. Lab. Clin. Med. 1963, 61, 882–888. [Google Scholar]
- Yuan, J.S.; Reed, A.; Chen, F.; Stewartjr, C.N. Statistical analysis of real-time PCR data. BMC Bioinform. 2006, 7, 85. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yan, F.; Hu, J.; Wu, Y.; Tucker, C.; Green, A.; Cheng, H. Immune Response of Laying Hens Exposed to 30 ppm Ammonia for 25 Weeks. Int. J. Poult. Sci. 2017, 16, 139–146. [Google Scholar] [CrossRef][Green Version]
- Kaiser, P.; Rothwell, L.; Galyov, E.E.; Barrow, P.A.; Burnside, J.; Wigley, P. Differential cytokine expression in avian cells in response to invasion by Salmonella typhimurium, Salmonella enteritidis and Salmonella gallinarumThe GenBank accession numbers for the sequences reported in this paper are AI982185 for chicken IL-6 cDNA and AJ250838 for the partial chicken IL-6 genomic sequence, respectively. Microbiology 2000, 146, 3217–3226. [Google Scholar]
- Samy, A.A.; El-Enbaawy, M.I.; El-Sanousi, A.A.; Abd El-Wanes, S.A.; Ammar, A.M.; Hikono, H.; Saito, T. In-vitro as-sessment of differential cytokine gene expression in response to infections with Egyptian classic and variant strains of highly pathogenic H5N1 avian influenza virus. Int. J. Vet. Sci. Med. 2015, 3, 1–8. [Google Scholar] [CrossRef][Green Version]
- Liu, J.; Zhao, H.; Wang, Y.; Shao, Y.; Li, J.; Xing, M. Alterations of antioxidant indexes and inflammatory cytokine expression aggravated hepatocellular apoptosis through mitochondrial and death receptor-dependent pathways in Gallus gallus ex-posed to arsenic and copper. Environ. Sci. Pollut. Res. 2018, 25, 15462–15473. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.M.; Guo, Y.M.; Yang, Y.; Wang, Z. Characterization of Fatty Acid Digestion of Beijing Fatty and Arbor Acres Chickens. Asian Australas. J. Anim. Sci. 2007, 20, 1222–1228. [Google Scholar] [CrossRef]
- Bancroft, J.D.; Layton, C. Connective and mesenchymal tissues with their stains. Bancroft’s Theory Pract. Histol. Tech. 2013, 187–214. [Google Scholar] [CrossRef]
- Gibson-Corley, K.N.; Olivier, A.K.; Meyerholz, D.K. Principles for Valid Histopathologic Scoring in Research. Veter Pathol. 2013, 50, 1007–1015. [Google Scholar] [CrossRef]
- Noreldin, A.E.; Sogabe, M.; Yamano, Y.; Uehara, M.; Mahdy, M.A.A.; Elnasharty, M.A.; Sayed-Ahmed, A.; Warita, K.; Hosaka, Y.Z. Spatial distribution of osteoblast activating peptide in the rat stomach. Acta Histochem. 2016, 118, 109–117. [Google Scholar] [CrossRef]
- Vis, A.N.; Kranse, R.; Nigg, A.L.; Van Der Kwast, T.H. Quantitative Analysis of the Decay of Immunoreactivity in Stored Prostate Needle Biopsy Sections. Am. J. Clin. Pathol. 2000, 113, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Wagner, D.D.; Furrow, R.D.; Bradley, B.D. Subchronic Toxicity of Monensin in Broiler Chickens. Veter Pathol. 1983, 20, 353–359. [Google Scholar] [CrossRef]
- Oldenquist, G.; Salem, M. Parenteral copper sulfate poisoning causing acute renal failure. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 1999, 14, 441–443. [Google Scholar] [CrossRef][Green Version]
- Dai, C.; Liu, Q.; Li, D.; Sharma, G.; Xiong, J.; Xiao, X. Molecular Insights of Copper Sulfate Exposure-Induced Nephrotoxicity: Involvement of Oxidative and Endoplasmic Reticulum Stress Pathways. Biomolecules 2020, 10, 1010. [Google Scholar] [CrossRef]
- Ogra, Y. Molecular Mechanisms Underlying Copper Homeostasis in Mammalian Cells. Nippon. Eiseigaku Zasshi Jpn. J. Hyg. 2014, 69, 136–145. [Google Scholar] [CrossRef][Green Version]
- Bresciani, G.; Da Cruz, I.B.M.; González-Gallego, J. Manganese Superoxide Dismutase and Oxidative Stress Modulation. Int. Rev. Cytol. 2015, 68, 87–130. [Google Scholar] [CrossRef]
- Pineda, J.; Herrera, A.; Antonio, M.T. Comparison between hepatic and renal effects in rats treated with arsenic and/or an-tioxidants during gestation and lactation. J. Trace Elem. Med. Biol. 2013, 27, 236–241. [Google Scholar] [CrossRef]
- Zhu, X.; Zhu, L.; Lang, Y.; Chen, Y. Oxidative stress and growth inhibition in the freshwater fish Carassius auratus in-duced by chronic exposure to sublethal fullerene aggregates. Environ. Toxicol. Chem. An. Inter. J. 2008, 27, 1979–1985. [Google Scholar] [CrossRef] [PubMed]
- Milić, M.; Zunec, S.; Micek, V.; Kashuba, V.; Mikolic, A.; Tariba Lovakovic, B.; Želježić, D. Oxidative stress, cholinester-ase activity, and DNA damage in the liver, whole blood, and plasma of Wistar rats following a 28-day exposure to glypho-sate. Arch. Occup. Hyg. Toxicol. 2018, 69, 154–168. [Google Scholar]
- Baş, H.; Kalender, S.; Pandir, D. In vitro effects of quercetin on oxidative stress mediated in human erythrocytes by benzoic acid and citric acid. Folia Biol. 2014, 62, 57–64. [Google Scholar] [CrossRef]
- Liu, H.; Guo, H.; Jian, Z.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Copper Induces Oxidative Stress and Apoptosis in the Mouse Liver. Oxidative Med. Cell. Longev. 2020, 2020, 1–20. [Google Scholar] [CrossRef]
- Surh, Y.J.; Chun, K.S.; Cha, H.H.; Han, S.S.; Keum, Y.S.; Park, K.K.; Lee, S.S. Molecular mechanisms underlying chemo-preventive activities of anti-inflammatory phytochemicals: Down-regulation of COX-2 and iNOS through suppression of NF-κB activation. Mutat. Res. Fundam. Mol. Mech. Mutagenesis 2001, 480, 243–268. [Google Scholar] [CrossRef]
- Haddad, J.J. Oxygen-sensitive pro-inflammatory cytokines, apoptosis signaling and redox-responsive transcription factors in development and pathophysiology. Cytokines Cell Mol. Ther. 2002, 7, 1–14. [Google Scholar] [CrossRef]
- Anderson, M.T.; Staal, F.J.; Gitler, C.; Herzenberg, L.A. Separation of oxidant-initiated and redox-regulated steps in the NF-kappa B signal transduction pathway. Proc. Natl. Acad. Sci. USA 1994, 91, 11527–11531. [Google Scholar] [CrossRef] [PubMed]
- Flohé, L.; Brigelius-Flohé, R.; Saliou, C.; Traber, M.; Packer, L. Redox Regulation of NF-kappa B Activation. Free Radic. Biol. Med. 1997, 22, 1115–1126. [Google Scholar] [CrossRef]
- Levine, B.; Sinha, S.C.; Kroemer, G. Bcl-2 family members: Dual regulators of apoptosis and autophagy. Autophagy 2008, 4, 600–606. [Google Scholar] [CrossRef]
- Deng, H.; Kuang, P.; Cui, H.; Chen, L.; Fang, J.; Zuo, Z.; Deng, J.; Wang, L.; Zhao, L. Sodium fluoride induces apoptosis in cultured splenic lymphocytes from mice. Oncotarget 2016, 7, 67880. [Google Scholar] [CrossRef]
- Aghvami, M.; Ebrahimi, F.; Zarei, M.H.; Salimi, A.; Jaktaji, R.P.; Pourahmad, J. Matrine induction of ROS mediated apopto-sis in human ALL B-lymphocytes via mitochondrial targeting. Asian Pac. J. Cancer Prev. APJCP 2018, 19, 555. [Google Scholar] [PubMed]
- Kawakami, M.; Inagawa, R.; Hosokawa, T.; Saito, T.; Kurasaki, M. Mechanism of apoptosis induced by copper in PC12 cells. Food Chem. Toxicol. 2008, 46, 2157–2164. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-L.; Wong, Y.-S.; Xu, G.; Chan, J. Selenium-enriched Spirulina protects INS-1E pancreatic beta cells from human islet amyloid polypeptide-induced apoptosis through suppression of ROS-mediated mitochondrial dysfunction and PI3/AKT pathway. Eur. J. Nutr. 2015, 54, 509–522. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, W.A.; Ahad, A.; Ahsan, H. The mystery of BCL2 family: Bcl-2 proteins and apoptosis: An update. Arch. Toxicol. 2015, 89, 289–317. [Google Scholar] [CrossRef]
- Brenner, C.; Cadiou, H.; Vieira, H.L.; Zamzami, N.; Marzo, I.; Xie, Z.; Leber, B.; Andrews, D.; Duclohier, H.; Reed, J.C.; et al. Bcl-2 and Bax regulate the channel activ-ity of the mitochondrial adenine nucleotide translocator. Oncogene 2000, 19, 329–333. [Google Scholar] [CrossRef]
- Yang, F.; Cao, H.; Su, R.; Guo, J.; Li, C.; Pan, J.; Tang, Z. Liver mitochondrial dysfunction and electron transport chain defect induced by high dietary copper in broilers. Poult. Sci. 2017, 96, 3298–3304. [Google Scholar] [CrossRef]
- Yang, F.; Liao, J.; Pei, R.; Yu, W.; Han, Q.; Li, Y.; Guo, J.; Hu, L.; Pan, J.; Tang, Z. Autophagy attenuates copper-induced mitochondrial dysfunc-tion by regulating oxidative stress in chicken hepatocytes. Chemosphere 2018, 204, 36–43. [Google Scholar] [CrossRef]
- Saporito-Magriñá, C.; Musacco-Sebio, R.; Acosta, J.M.; Bajicoff, S.; Paredes-Fleitas, P.; Boveris, A.; Repetto, M.G. Rat liver mitochondrial dysfunction by addition of copper(II) or iron(III) ions. J. Inorg. Biochem. 2017, 166, 5–11. [Google Scholar] [CrossRef]
- Sakr, S.A.; Albarakai, A.Y. Effect of cinnamon on cypermethrin-induced nephrotoxicity in albino rats. Int. J. Adv. Res. 2014, 2, 578–586. [Google Scholar]
- Shobana, S.; Naidu, K.A. Antioxidant activity of selected Indian spices. Prostaglandins Leukot. Essent. Fat. Acids 2000, 62, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Eidi, A.; Mortazavi, P.; Bazargan, M.; Zaringhalam, J. Hepatoprotective activity of cinnamon ethanolic extract against CCI4-induced liver injury in rats. EXCLI J. 2012, 11, 495–507. [Google Scholar] [PubMed]
- Rao, P.V.; Gan, S.H. Cinnamon: A Multifaceted Medicinal Plant. Evidence-Based Complement. Altern. Med. 2014, 2014, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Niphade, S.R.; Asad, M.; Chandrakala, G.K.; Toppo, E.; Deshmukh, P. Immunomodulatory activity of Cinnamomum zeylanicum bark. Pharm. Boil. 2009, 47, 1168–1173. [Google Scholar] [CrossRef]
- Sengul, E.; Gelen, S.U.; Yıldırım, S.; Çelebi, F.; Çınar, A. Probiotic bacteria attenuates cisplatin-induced nephrotoxicity through modulation of oxidative stress, inflammation and apoptosis in rats. Asian Pac. J. Trop. Biomed. 2019, 9, 116. [Google Scholar] [CrossRef]
- Guven, A.; Gulmez, M. The Effect of Kefir on the Activities of GSH-Px, GST, CAT, GSH and LPO Levels in Carbon Tetrachloride-Induced Mice Tissues. J. Veter Med. Ser. B 2003, 50, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Du, R.; Wang, L.; Zhang, H. The antioxidative effects of probiotic Lactobacillus casei Zhang on the hyper-lipidemic rats. Eur. Food Res. Technol. 2010, 231, 151–158. [Google Scholar] [CrossRef]
- Kullisaar, T.; Songisepp, E.; Aunapuu, M.; Kilk, K.; Arend, A.; Mikelsaar, M.; Rehema, A.; Zilmer, M. Complete glutathione system in probiotic Lactobacillus fermentum ME. Appl. Biochem. Microbiol. 2010, 46, 481–486. [Google Scholar] [CrossRef]
- Mikelsaar, M.; Zilmer, M. Lactobacillus fermentum ME-3–an antimicrobial and antioxidative probiotic. Microb. Ecol. Health Dis. 2009, 21, 1–27. [Google Scholar]
- Zoghi, A.; Khosravi-Darani, K.; Sohrabvandi, S. Surface Binding of Toxins and Heavy Metals by Probiotics. Mini Reviews Med. Chem. 2014, 14, 84–98. [Google Scholar] [CrossRef] [PubMed]
- Jawhara, S.; Habib, K.; Maggiotto, F.; Pignede, G.; Vandekerckove, P.; Maes, E.; Fontaine, T.; Guerardel, Y.; Poulain, D. Modulation of intestinal in-flammation by yeasts and cell wall extracts: Strain dependence and unexpected anti-inflammatory role of glucan fractions. PLoS ONE 2012, 7, e40648. [Google Scholar] [CrossRef]
- Ekperigin, H.E.; Vohra, P. Influence of dietary excess methionine on the relationship between dietary copper and the con-centration of copper and iron in organs of broiler chicks. J. Nutr. 1981, 111, 1630–1640. [Google Scholar] [CrossRef] [PubMed]
- Mehring, A.L.; Brumbaugh, J.H.; Sutherland, A.J.; Titus, H.W. The tolerance of growing chickens for dietary cop-per. Poult. Sci. 1960, 39, 713–719. [Google Scholar] [CrossRef]
- Yang, C.; Kennes, Y.M.; Lepp, D.; Yin, X.; Wang, Q.; Yu, H.; Yang, C.; Gong, J.; Diarra, M.S. Effects of encapsulated cinnamaldehyde and citral on the performance and cecal microbiota of broilers vaccinated or not vaccinated against coccidiosis. Poult. Sci. 2020, 99, 936–948. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Williams, B.A.; Kwakkel, R.P.; Li, H.S.; Li, X.P.; Luo, J.Y.; Li, W.K.; Verstegen, M.W.A. Effects of mushroom and herb polysaccharides, as alternatives for an antibiotic, on the cecal microbial ecosystem in broiler chickens. Poult. Sci. 2004, 83, 175–182. [Google Scholar] [CrossRef]

| Ingredients, % | Starter (1 to 10 Days) | Grower (11 to 22 Days) | Finisher (23 to 42 Days) |
|---|---|---|---|
| Yellow corn grain | 58.00 | 62.80 | 63.60 |
| Soybean meal, 47.5% | 34.46 | 27.40 | 25.00 |
| Corn gluten, 60% | 1.5 | 21.80 | 3.00 |
| Wheat bran | -- | 1.00 | 1.40 |
| Soybean oil | 1.75 | 2.00 | 3.30 |
| Calcium carbonate | 1.10 | 1.00 | 1.00 |
| Dicalciumphosphate | 1.90 | 1.70 | 1.50 |
| Common salt | 0.30 | 0.30 | 0.30 |
| Premix * | 0.30 | 0.30 | 0.30 |
| DL–Methionine, 98% | 0.18 | 0.19 | 0.11 |
| Lysine, Hcl, 78% | 0.16 | 0.16 | 0.14 |
| Antimycotoxin ** | 0.10 | 0.10 | 0.10 |
| Sodium bicarbonate | 0.25 | 0.25 | 0.25 |
| Analyzed Chemical Composition | |||
| ME ***, Kcal/Kg | 3020.56 | 3090.13 | 3180.70 |
| CP, % | 22.08 | 20.01 | 19.04 |
| EE, % | 4.25 | 4.63 | 5.92 |
| CF, % | 1.64 | 2.62 | 2.60 |
| Ca, % | 1.03 | 0.93 | 0.87 |
| Available P, % | 0.48 | 0.44 | 0.40 |
| Lysine, % | 1.35 | 1.16 | 1.10 |
| Methionin, % | 0.53 | 0.53 | 0.45 |
| Target Gene | Forward Primer (5′–3′) | Reverse Primer (5′–3′) | References |
|---|---|---|---|
| TNF-α | CCCCTACCCTGTCCCACAA | ACTGCGGAGGGTTCATTCC | [43] |
| IL-2 | TTGGAAAATATCAAGAACAAGATTCATC | TCCCAGGTAACACTGCAGAGTTT | [44] |
| IL-10 | CATGCTGCTGGGCCTGAA | CGTCTCCTTGATCTGCTTGATG | [45] |
| Bax | TCCATTCAGGTTCTCTTGACC | GCCAAACATCCAAACACAGA | [46] |
| Bcl-2 | ATCGTCGCCTTCTTCGAGTT | ATCCCATCCTCCGTTGTTCT | [46] |
| ß. actin | CCACCGCAAATGCTTCTAAAC | AAGACTGCTGCTGACACCTTC | [47] |
| Experimental Groups | 3 Weeks | 6 Weeks | ||||
|---|---|---|---|---|---|---|
| Creatinine (mg/dL) | Urea (mg/dL) | Uric Acid (mg/dL) | Creatinine (mg/dL) | Urea (mg/dL) | Uric Acid (mg/dL) | |
| C | 0.25 ± 0.03 c | 2.21 ± 0.29 b | 4.68 ± 0.61 c | 0.28 ± 0.04 d | 2.26 ± 0.37 d | 5.10 ± 0.49 cd |
| Cin | 0.20 ± 0.02 c | 1.83 ± 0.34 b | 4.16 ± 0.32 c | 0.23 ± 0.03 d | 2.06 ± 0.25 d | 4.32 ± 0.26 d |
| PR | 0.23 ± 0.02 c | 2.15 ± 0.19 b | 4.84 ± 0.42 bc | 0.25 ± 0.04 d | 2.62 ± 0.31 cd | 4.76 ± 0.37 cd |
| Cu | 1.38 ± 0.21 a | 5.94 ± 0.85 a | 10.32 ± 0.69 a | 3.3 ± 0.37 a | 9.44 ± 0.72 a | 16.08 ± 1.47 a |
| Cu + Cin | 0.59 ± 0.07 bc | 3.48 ± 0.64 b | 6.1 ± 0.78 bc | 1.42 ± 0.19 bc | 4.56 ± 0.44 bc | 8.40 ± 0.73 bc |
| Cu + PR | 0.67 ± 0.08 b | 3.74 ± 0.33 b | 7.48 ± 0.75 b | 1.92 ± 0.27 b | 6.76 ± 0.69 b | 11.06 ± 1.28 b |
| Cu + Cin + PR | 0.36 ± 0.05 bc | 2.96 ± 0.42 b | 5.04 ± 0.60 bc | 0.84 ± 0.14 cd | 3.44 ± 0.54 cd | 6.82 ± 0.67 cd |
| Experimental Groups | 3 Weeks | 6 Weeks | ||
|---|---|---|---|---|
| IgM (mg/dL) | IgG (mg/dL) | IgM (mg/dL) | IgG (mg/dL) | |
| C | 11.74 ± 0.93 ab | 1.38 ± 0.22 ab | 13.62 ± 1.43 a | 1.78 ± 0.16 ab |
| Cin | 14.02 ± 1.75 a | 1.60 ± 0.18 ab | 15.24 ± 1.82 a | 2.40 ± 0.27 a |
| PR | 12.56 ±0.89 ab | 2.00 ± 0.26 a | 13.88 ± 1.16 a | 1.88 ± 0.25 b |
| Cu | 5.36 ± 0.68 c | 0.44 ± 0.10 c | 4.30 ± 0.52 b | 0.37 ± 0.05 d |
| Cu + Cin | 10.34 ± 0.76 ab | 0.88 ± 0.09 bc | 10.84 ± 1.50 a | 1.26 ± 0.15 bc |
| Cu + PR | 8.74 ± 0.84 bc | 1.06 ± 0.17 bc | 9.62 ± 0.65 a | 0.89 ± 0.13 cd |
| Cu + Cin + PR | 10.56 ± 1.00 ab | 1.24 ± 0.16 ab | 12.06 ± 0.94 a | 1.39 ± 0.19 b |
| Parameters | IW(g/Bird) | FBW (g/Bird) | BWG (g/Bird) | FI (g/Bird) | FCR | Survival % |
|---|---|---|---|---|---|---|
| C | 42.38 ± 0.17 | 2608 ± 30.23 a | 2565.62 ± 30.27 a | 4234 ± 28.39 a | 1.65 ± 0.02 cd | 100 ± 0.00 a |
| Cin | 42.38 ± 0.11 | 2602 ± 50.06 a | 2559.62 ± 49.99a | 4157 ± 62.96 a | 1.63 ± 0.02 d | 100 ± 0.00 a |
| PR | 42.42 ± 0.10 | 2718 ± 66.17 a | 2676.18 ± 66.10 a | 4203 ±55.54 a | 1.57 ± 0.02 d | 100 ±0.00 a |
| Cu | 42.34 ± 0.12 | 2046 ± 36.96 d | 2003.66 ± 36.92 d | 3756 ± 31.40 c | 1.88 ± 0.03 a | 66.60 ± 0.00 b |
| Cu + Cin | 42.30 ± 0.11 | 2167 ± 26.00 cd | 2124.70 ± 26.01 cd | 3712 ± 27.17c | 1.75 ± 0.02 b | 86.64 ± 8.18 a |
| Cu + PR | 42.34 ± 0.12 | 2191 ± 47.68 c | 2148.66 ± 47.75 c | 3811 ± 59.13 c | 1.78 ± 0.04 b | 79.96 ± 8.18 a |
| Cu + Cin + PR | 42.26 ± 0.14 | 2359 ± 41.18 b | 2316.74 ± 41.22 b | 3987 ± 95.27 b | 1.72 ± 0.04 bc | 93.32 ± 6.68 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elazab, S.T.; Elshater, N.S.; Kishaway, A.T.Y.; EI-Emam, H.A. Cinnamon Extract and Probiotic Supplementation Alleviate Copper-Induced Nephrotoxicity via Modulating Oxidative Stress, Inflammation, and Apoptosis in Broiler Chickens. Animals 2021, 11, 1609. https://doi.org/10.3390/ani11061609
Elazab ST, Elshater NS, Kishaway ATY, EI-Emam HA. Cinnamon Extract and Probiotic Supplementation Alleviate Copper-Induced Nephrotoxicity via Modulating Oxidative Stress, Inflammation, and Apoptosis in Broiler Chickens. Animals. 2021; 11(6):1609. https://doi.org/10.3390/ani11061609
Chicago/Turabian StyleElazab, Sara T., Nahla S. Elshater, Asmaa T. Y. Kishaway, and Huda A. EI-Emam. 2021. "Cinnamon Extract and Probiotic Supplementation Alleviate Copper-Induced Nephrotoxicity via Modulating Oxidative Stress, Inflammation, and Apoptosis in Broiler Chickens" Animals 11, no. 6: 1609. https://doi.org/10.3390/ani11061609
APA StyleElazab, S. T., Elshater, N. S., Kishaway, A. T. Y., & EI-Emam, H. A. (2021). Cinnamon Extract and Probiotic Supplementation Alleviate Copper-Induced Nephrotoxicity via Modulating Oxidative Stress, Inflammation, and Apoptosis in Broiler Chickens. Animals, 11(6), 1609. https://doi.org/10.3390/ani11061609








