The Detection of Bovine Estrus by Lactoferrin Monoclonal Antibody
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Peptide Conjugation
2.3. Production of Rat Anti-bLF mAbs
2.4. Assesement of Rat Anti-bLF mAbs
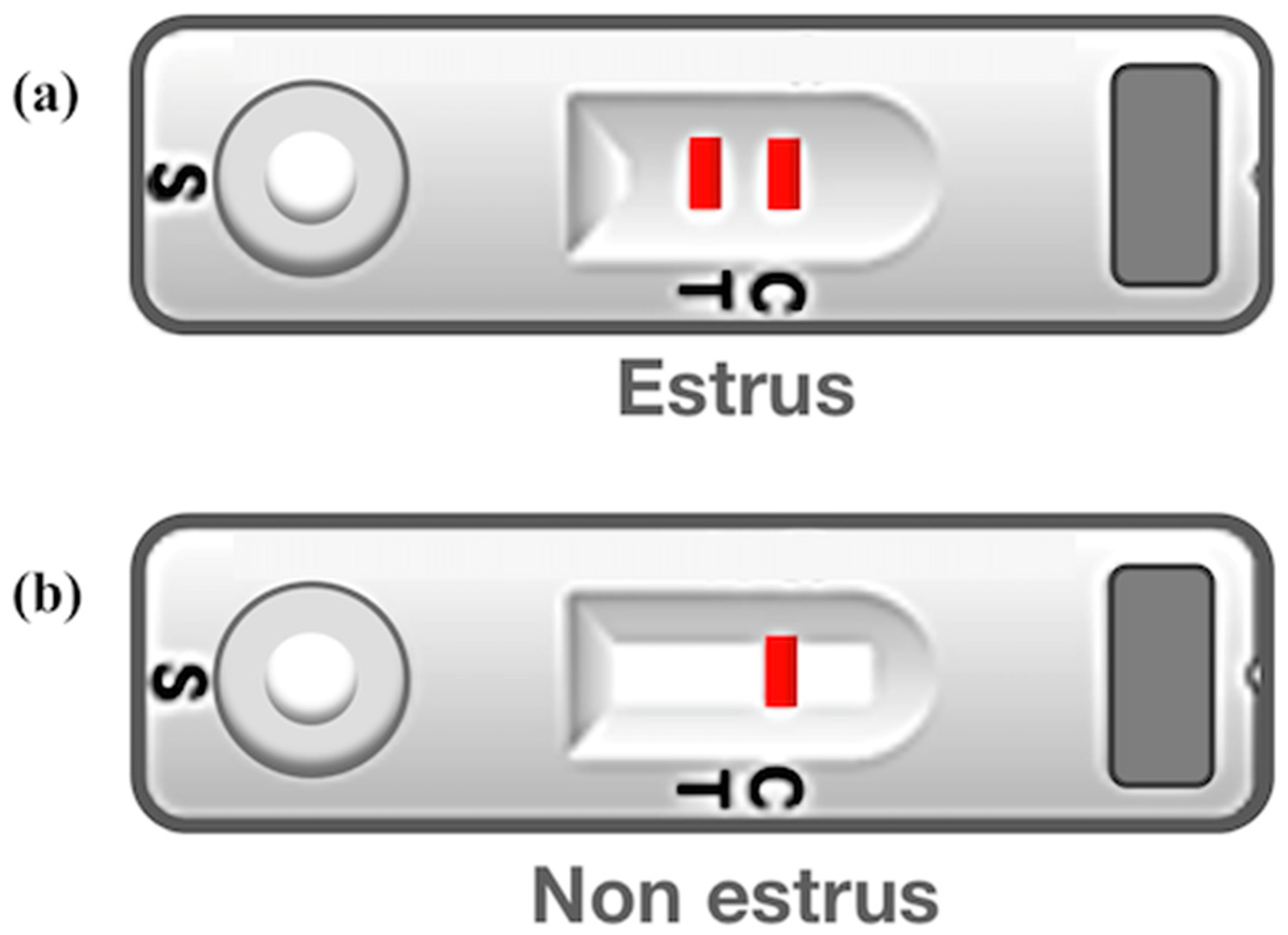
2.5. Evaluation of Heat Detection Kit
2.6. Statistical Analysis
3. Results
3.1. Prediction of Antigenic Peptides for bLF Amino Acid Sequence
3.2. Production of Rat Anti-bLF mAbs
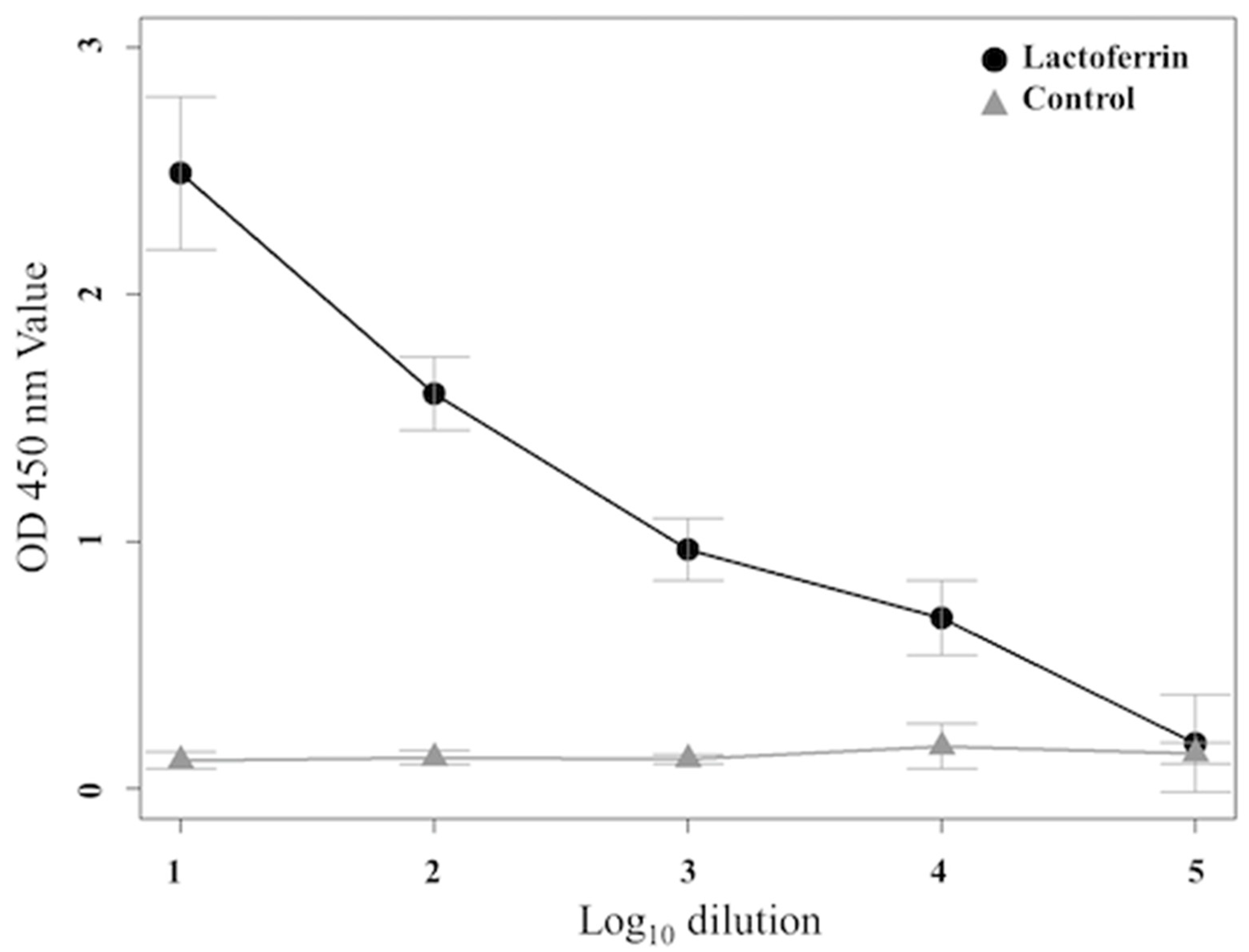
3.3. Determination of Binding Affinity by ELISA
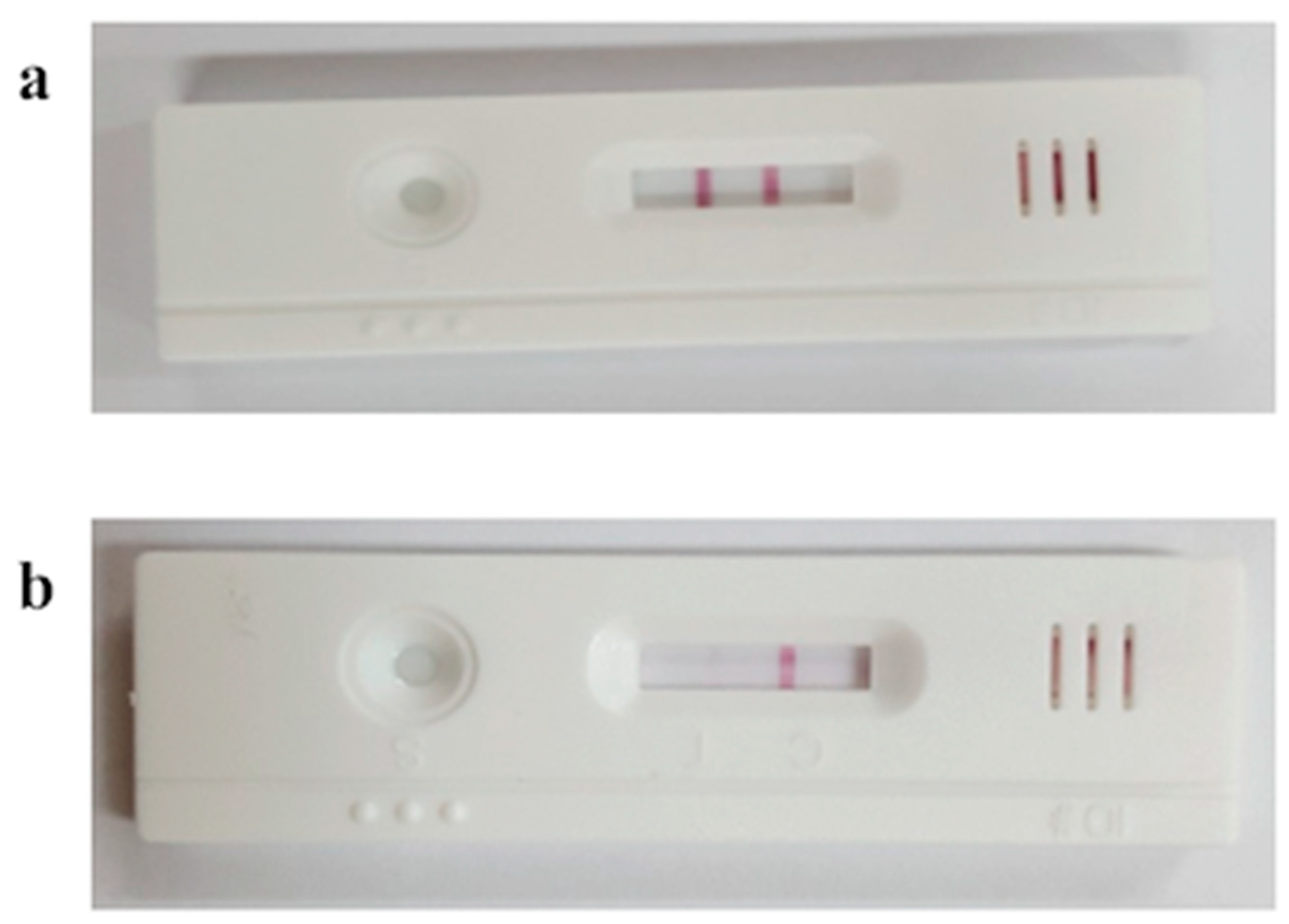
3.4. Estrous Discrimination Using Bovine Heat Detection Kit
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seo, M.D.H.I. Annual Report in Korea. Available online: http://www.dcic.co.kr/_uploadFiles/_annual_report/2019_main.pdf (accessed on 3 November 2020).
- Walsh, S.; Williams, E.; Evans, A. A review of the causes of poor fertility in high milk producing dairy cows. Anim. Reprod. Sci. 2011, 123, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Albarran-Portillo, B.; Pollott, G. The relationship between fertility and lactation characteristics in Holstein cows on United Kingdom commercial dairy farms. J. Dairy Sci. 2013, 96, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Zduńczyk, S.; Janowski, T.; Raś, M. Current Views on the Phenomenon of Silent Heat in Cows. Med. Weter. 2005, 61, 726–729. [Google Scholar]
- Roelofs, J.B.; van Erp-van der Kooij, E. Estrus Detection Tools and Their Applicability in Cattle: Recent and Perspectival Situation. Anim. Reprod. 2018, 12, 498–504. [Google Scholar]
- Yusuf, M.; Nakao, T.; Ranasinghe, R.B.K.; Gautam, G.; Long, S.T.; Yoshida, C.; Koike, K.; Hayashi, A. Reproductive performance of repeat breeders in dairy herds. Theriogenology 2010, 73, 1220–1229. [Google Scholar] [CrossRef]
- Garforth, C.; McKemey, K.; Rehman, T.; Tranter, R.; Cooke, R.; Park, J.; Dorward, P.; Yates, C. Farmers’ attitudes towards techniques for improving oestrus detection in dairy herds in South West England. Livest. Sci. 2006, 103, 158–168. [Google Scholar] [CrossRef]
- Esslemont, R.J.; Kossaibati, M.A. Culling in 50 dairy herds in England. Vet. Rec. 1997, 140, 36–39. [Google Scholar] [CrossRef]
- Senger, P. The Estrus Detection Problem: New Concepts, Technologies, and Possibilities. J. Dairy Sci. 1994, 77, 2745–2753. [Google Scholar] [CrossRef]
- Baek, K.S.; Lee, W.S.; Park, S.J.; Lim, H.J.; Son, J.K.; Kim, S.B.; Kwon, E.G.; Jung, Y.S.; Kim, K.H. The Accuracy Analysis and Applied Field Research of a Newly Developed Automatic Heat Detector in Dairy Cow. Reprod. Dev. Biol. 2011, 35, 395–398. [Google Scholar]
- Holman, A.; Thompson, J.; Routly, J.E.; Cameron, J.; Jones, D.N.; Grove-White, D.; Smith, R.; Dobson, H. Comparison of oestrus detection methods in dairy cattle. Vet. Rec. 2011, 169, 47. [Google Scholar] [CrossRef]
- Lee, W.; Park, M.; Kim, K.; Song, H.; Kim, K.; Lee, C.; Kim, N.; Park, J.; Yang, B.; Oh, K.; et al. Identification of lactoferrin and glutamate receptor-interacting protein 1 in bovine cervical mucus: A putative marker for oestrous detection. Reprod. Domest. Anim. 2016, 52, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Kolaskar, A.; Tongaonkar, P.C. A semi-empirical method for prediction of antigenic determinants on protein antigens. FEBS Lett. 1990, 276, 172–174. [Google Scholar] [CrossRef]
- Hopp, T.P.; Woods, K.R. Prediction of protein antigenic determinants from amino acid sequences. Proc. Natl. Acad. Sci. USA 1981, 78, 3824–3828. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Gomase, V.S. Identification of Antigenic Determinants, Solvent Accessibility and MHC Binders of Antigen NADH Dehydrogenase Subunit 5 from a Little Dragon from Medina (Dracunculusmedinensis). Drug Des. 2016, 5. [Google Scholar] [CrossRef]
- Chung, H.J.; Kim, G.W.; Park, J.G.; Yang, B.C.; Han, D.W.; Lee, M.S.; Lee, S.D.; Hong, S.K.; Chang, W.K. Composition, Kit and Method for Determining Optimal Insemination Time of Hanwoo. KR Patent No. 1014292250000, 9 May 2012. [Google Scholar]
- RStudio Team. RStudio: Integrated Development for R; RStudio, Inc.: Boston, MA, USA, 2019; Available online: http://www.rstudio.com/ (accessed on 12 October 2020).
- Dransfield, M.; Nebel, R.; Pearson, R.; Warnick, L. Timing of Insemination for Dairy Cows Identified in Estrus by a Radiotelemetric Estrus Detection System. J. Dairy Sci. 1998, 81, 1874–1882. [Google Scholar] [CrossRef]
- Trimberger, G.W. Breeding Efficiency in Dairy Cattle from Artificial Insemination at Various Intervals before and after Ovulation. Biology 1948, 153, 1–26. [Google Scholar]
- Silper, B.; Madureira, A.; Kaur, M.; Burnett, T.; Cerri, R. Short communication: Comparison of estrus characteristics in Holstein heifers by 2 activity monitoring systems. J. Dairy Sci. 2015, 98, 3158–3165. [Google Scholar] [CrossRef]
- Reith, S.; Hoy, S. Review: Behavioral signs of estrus and the potential of fully automated systems for detection of estrus in dairy cattle. Animal 2018, 12, 398–407. [Google Scholar] [CrossRef]
- Zduńczyk, S.; Janowski, T.; Raś, A.; Barański, W. Accuracy of Ultrasonography and Rectal Palpation in the Diagnosis of Silent Heat in Cows Compared to Plasma Progesterone Concentration. Bull. Vet. Inst. Pulawy 2009, 53, 407–410. [Google Scholar]
- Oltenacu, P.A.; Hultgren, J.; Algers, B. Associations between use of electric cow-trainers and clinical diseases, reproductive performance and culling in Swedish dairy cattle. Prev. Vet. Med. 1998, 37, 77–90. [Google Scholar] [CrossRef]
- Zduńczyk, S.; Mwaanga, E.S.; Małecki-Tepicht, J.; Barański, W.; Janowski, T. Plasma Progesterone Levels and Clinical Findings in Dairy Cows with Post-Partum Anoestrus. Bull. Vet. Inst. Pulawy 2002, 46, 79–86. [Google Scholar]
- Platz, S.; Ahrens, F.; Bendel, J.; Meyer, H.; Erhard, M. What Happens with Cow Behavior When Replacing Concrete Slatted Floor by Rubber Coating: A Case Study. J. Dairy Sci. 2008, 91, 999–1004. [Google Scholar] [CrossRef]
- Gangwar, P.; Branton, C.; Evans, D. Reproductive and Physiological Responses of Holstein Heifers to Controlled and Natural Climatic Conditions. J. Dairy Sci. 1965, 48, 222–227. [Google Scholar] [CrossRef]
- Choi, S.-H.; Jin, H.-J. Effects of Optimal Heat Detection Kit on Fertility after Artificial Insemination (AI) in Hanwoo (Korean Native cattle). J. Anim. Reprod. Biotechnol. 2017, 32, 153–157. [Google Scholar] [CrossRef]
- Lee, M.-S.; Rahman, S.; Kwon, W.-S.; Chung, H.-J.; Yang, B.-S.; Pang, M.-G. Efficacy of four synchronization protocols on the estrus behavior and conception in native Korean cattle (Hanwoo). Theriogenology 2013, 80, 855–861. [Google Scholar] [CrossRef] [PubMed]
- Pentecost, B.; Teng, C. Lactotransferrin is the major estrogen inducible protein of mouse uterine secretions. J. Biol. Chem. 1987, 262, 10134–10139. [Google Scholar] [CrossRef]
- Teng, C.T. Regulation of Lactoferrin Gene Expression by Estrogen and Epidermal Growth Factor: Molecular Mechanism. Cell Biochem. Biophys. 1999, 31, 49–64. [Google Scholar] [CrossRef]
- Teng, C.T. Lactoferrin gene expression and regulation: An overview. Biochem. Cell Biol. 2002, 80, 7–16. [Google Scholar] [CrossRef]
- González-Chávez, S.A.; Arévalo-Gallegos, S.; Rascón-Cruz, Q. Lactoferrin: Structure, function and applications. Int. J. Antimicrob. Agents 2009, 33, 301.e1–301.e8. [Google Scholar] [CrossRef]

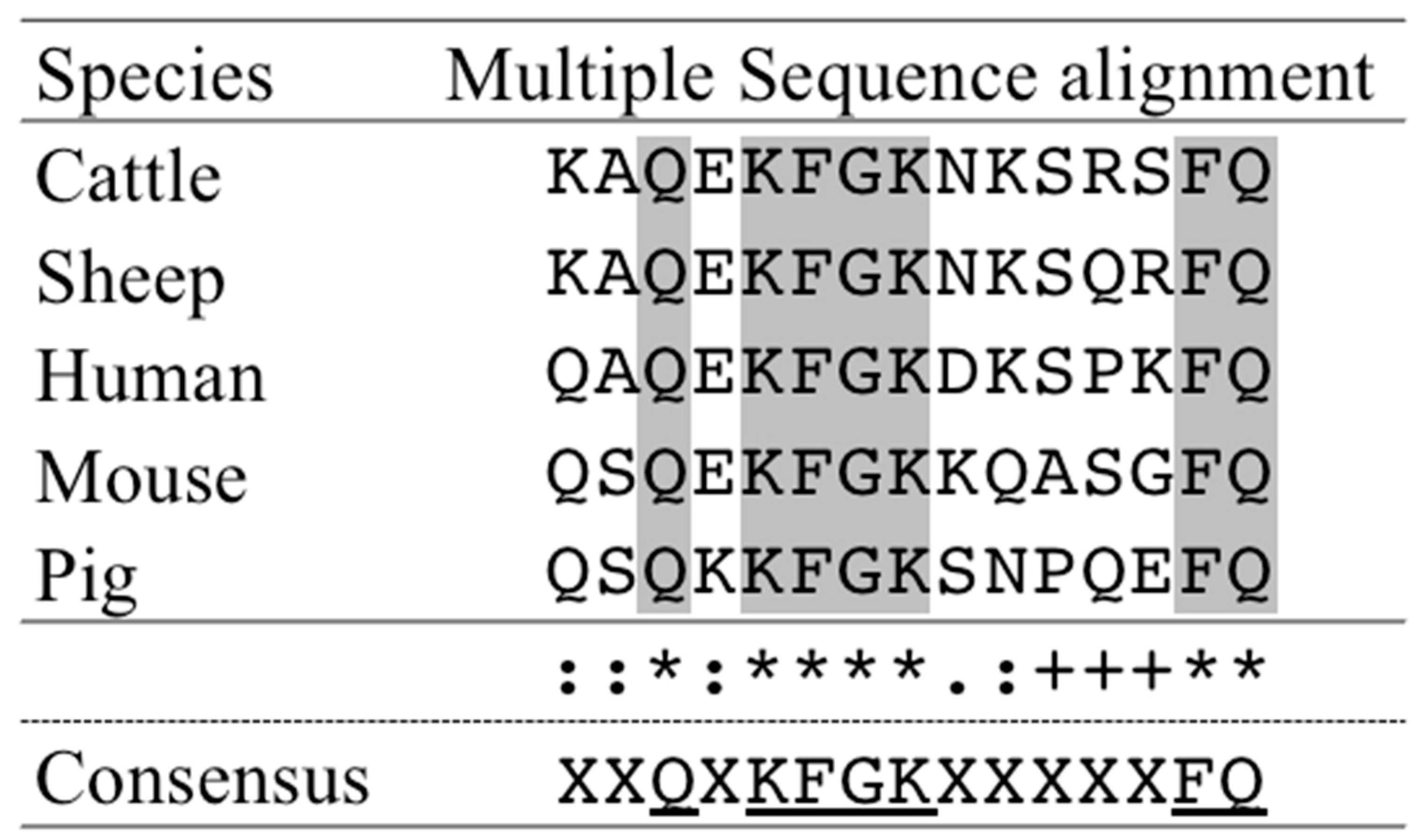
| Prediction # | Location | Amino Acid Sequence |
|---|---|---|
| 1 | 64–77 amino acid | CIRAIAEKKADAVT |
| 2 | 234–247 amino acid | FENLPEKADRDQYE |
| 3 | 292–306 amino acid | KAQEKFGKNKSRSFQ |
| 4 | 348–363 amino acid | KNLRETAEEVKARYTR |
| 5 | 431–444 amino acid | AENRKSSKHSSLDC |
| 6 | 574–588 amino acid | ESTADWAKNLNREDF |
| 7 | 649–664 amino acid | CLFKSETKNLLFNDNT |
| Rat Serum | Control | 1:10 | 1:102 | 1:103 | 1:104 | 1:105 |
|---|---|---|---|---|---|---|
| 1 | 0.17 ± 0.07 | 2.55 ± 0.04 | 1.88 ± 0.06 | 0.94 ± 0.10 | 0.64 ± 0.09 | 0.21 ± 0.06 |
| 2 | 0.17 ± 0.04 | 2.79 ± 0.07 | 2.00 ± 0.07 | 1.12 ± 0.06 | 0.74 ± 0.07 | 0.28 ± 0.06 |
| 3 | 0.19 ± 0.08 | 3.01 ± 0.14 | 1.67 ± 0.09 | 0.96 ± 0.08 | 0.67 ± 0.11 | 0.22 ± 0.07 |
| 4 | 0.14 ± 0.07 | 2.31 ± 0.06 | 1.52 ± 0.06 | 0.84 ± 0.08 | 0.60 ± 0.08 | 0.19 ± 0.05 |
| Binding Affinity (Mean ± SE) | Control | Clone 1 | Clone 2 | Clone 3 | Clone 4 |
|---|---|---|---|---|---|
| mAb-bLF | 0.12 ± 0.02 | 0.86 ± 0.11 | 2.28 ± 0.07 | 2.07 ± 0.18 | 2.49 ± 0.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Lee, S.; Park, Y.; Lee, S.; Ha, S.; Han, M.; Jang, G.; Park, M.; Kim, K.; Chung, H. The Detection of Bovine Estrus by Lactoferrin Monoclonal Antibody. Animals 2021, 11, 1582. https://doi.org/10.3390/ani11061582
Lee J, Lee S, Park Y, Lee S, Ha S, Han M, Jang G, Park M, Kim K, Chung H. The Detection of Bovine Estrus by Lactoferrin Monoclonal Antibody. Animals. 2021; 11(6):1582. https://doi.org/10.3390/ani11061582
Chicago/Turabian StyleLee, Jihwan, Suhyun Lee, Younbae Park, Seokhyun Lee, Seungmin Ha, Manhye Han, Gulwon Jang, Myunghum Park, Kyungwoon Kim, and Hakjae Chung. 2021. "The Detection of Bovine Estrus by Lactoferrin Monoclonal Antibody" Animals 11, no. 6: 1582. https://doi.org/10.3390/ani11061582
APA StyleLee, J., Lee, S., Park, Y., Lee, S., Ha, S., Han, M., Jang, G., Park, M., Kim, K., & Chung, H. (2021). The Detection of Bovine Estrus by Lactoferrin Monoclonal Antibody. Animals, 11(6), 1582. https://doi.org/10.3390/ani11061582







