Left and Right Myocardial Functionality Assessed by Two-Dimensional Speckle-Tracking Echocardiography in Cats with Restrictive Cardiomyopathy
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Standard Echocardiography
2.3. Two-Dimensional Speckle-Tracking Echocardiography
2.4. Reproducibility of 2D-STE Variables
2.5. Statistical Analysis
3. Results
3.1. Clinical Profiles and Standard Echocardiography
3.2. Two-Dimensional Speckle-Tracking Echocardiography
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Luis Fuentes, V.; Abbott, J.; Chetboul, V.; Côté, E.; Fox, P.R.; Häggström, J.; Kittleson, M.D.; Schober, K.; Stern, J.A. ACVIM consensus statement guidelines for the classification, diagnosis, and management of cardiomyopathies in cats. J. Vet. Intern. Med. 2020, 34, 1062–1077. [Google Scholar] [CrossRef]
- Fox, P.R.; Basso, C.; Thiene, G.; Maron, B.J. Spontaneously occurring restrictive nonhypertrophied cardiomyopathy in domestic cats: A new animal model of human disease. Cardiovasc. Pathol. 2014, 23, 28–34. [Google Scholar] [CrossRef]
- Kimura, Y.; Karakama, S.; Hirakawa, A.; Tsuchiaka, S.; Kobayashi, M.; Machida, N. Pathological Features and Pathogenesis of the Endomyocardial Form of Restrictive Cardiomyopathy in Cats. J. Comp. Pathol. 2016, 155, 190–198. [Google Scholar] [CrossRef]
- Amaki, M.; Savino, J.; Ain, D.L.; Sanz, J.; Pedrizzetti, G.; Kulkarni, H.; Narula, J.; Sengupta, P.P. Diagnostic concordance of echocardiography and cardiac magnetic resonance-based tissue tracking for differentiating constrictive pericarditis from restrictive cardiomyopathy. Circ. Cardiovasc. Imaging 2014, 7, 819–827. [Google Scholar] [CrossRef]
- Kusunose, K.; Dahiya, A.; Popović, Z.B.; Motoki, H.; Alraies, M.C.; Zurick, A.O.; Bolen, M.A.; Kwon, D.H.; Flamm, S.D.; Klein, A.L. Biventricular mechanics in constrictive pericarditis comparison with restrictive cardiomyopathy and impact of pericardiectomy. Circ. Cardiovasc. Imaging 2013, 6, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, P.P.; Krishnamoorthy, V.K.; Abhayaratna, W.P.; Korinek, J.; Belohlavek, M.; Sundt, T.M.; Chandrasekaran, K.; Mookadam, F.; Seward, J.B.; Tajik, A.J.; et al. Disparate Patterns of Left Ventricular Mechanics Differentiate Constrictive Pericarditis From Restrictive Cardiomyopathy. JACC Cardiovasc. Imaging 2008, 1, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, R.; Mochizuki, Y.; Yoshimatsu, H.; Niina, A.; Teshima, T.; Matsumoto, H.; Koyama, H. Layer-specific myocardial function in asymptomatic cats with obstructive hypertrophic cardiomyopathy assessed using 2-dimensional speckle-tracking echocardiography. J. Vet. Intern. Med. 2019, 33, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, R.; Mochizuki, Y.; Yoshimatsu, H.; Teshima, T.; Matsumoto, H.; Koyama, H. Determination of multidirectional myocardial deformations in cats with hypertrophic cardiomyopathy by using two-dimensional speckle-tracking echocardiography. J. Feline Med. Surg. 2017, 19, 1283–1289. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, R.; Mochizuki, Y.; Yoshimatsu, H.; Ohkusa, T.; Teshima, T.; Matsumoto, H.; Koyama, H. Myocardial torsional deformations in cats with hypertrophic cardiomyopathy using two-dimensional speckle-tracking echocardiography. J. Vet. Cardiol. 2016, 18, 350–357. [Google Scholar] [CrossRef]
- Locatelli, C.; Pradelli, D.; Campo, G.; Spalla, I.; Savarese, A.; Brambilla, P.G.; Bussadori, C. Survival and prognostic factors in cats with restrictive cardiomyopathy: A review of 90 cases. J. Feline Med. Surg. 2018, 20, 1138–1143. [Google Scholar] [CrossRef]
- Chetboul, V.; Passavin, P.; Trehiou-Sechi, E.; Gouni, V.; Poissonnier, C.; Pouchelon, J.L.; Desquilbet, L. Clinical, epidemiological and echocardiographic features and prognostic factors in cats with restrictive cardiomyopathy: A retrospective study of 92 cases (2001–2015). J. Vet. Intern. Med. 2019, 33, 1222–1231. [Google Scholar] [CrossRef]
- Schober, K.E.; Savino, S.I.; Yildiz, V. Right ventricular involvement in feline hypertrophic cardiomyopathy. J. Vet. Cardiol. 2016, 18, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Visser, L.C.; Sloan, C.Q.; Stern, J.A. Echocardiographic Assessment of Right Ventricular Size and Function in Cats with Hypertrophic Cardiomyopathy. J. Vet. Intern. Med. 2017, 31, 668–677. [Google Scholar] [CrossRef]
- Abbott, J.A.; MacLean, H.N. Two-dimensional echocardiographic assessment of the feline left atrium. J. Vet. Intern. Med. 2006, 20, 111–119. [Google Scholar] [CrossRef]
- Häggström, J.; Andersson, Å.O.; Falk, T.; Nilsfors, L.; OIsson, U.; Kresken, J.G.; Höglund, K.; Rishniw, M.; Tidholm, A.; Ljungvall, I. Effect of Body Weight on Echocardiographic Measurements in 19,866 Pure-Bred Cats with or without Heart Disease. J. Vet. Intern. Med. 2016, 30, 1601–1611. [Google Scholar] [CrossRef] [PubMed]
- Vezzosi, T.; Schober, K.E. Doppler-derived echocardiographic evidence of pulmonary hypertension in cats with left-sided congestive heart failure. J. Vet. Cardiol. 2019, 23, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Koffas, H.; Dukes-McEwan, J.; Corcoran, B.M.; Moran, C.M.; French, A.; Sboros, V.; Simpson, K.; McDicken, W.N. Pulsed tissue Doppler imaging in normal cats and cats with hypertrophic cardiomyopathy. J. Vet. Intern. Med. 2006, 20, 65–77. [Google Scholar] [CrossRef]
- Suzuki, R.; Mochizuki, Y.; Yuchi, Y.; Yasumura, Y.; Saito, T.; Teshima, T.; Matsumoto, H.; Koyama, H. Assessment of myocardial function in obstructive hypertrophic cardiomyopathy cats with and without response to medical treatment by carvedilol. BMC Vet. Res. 2019, 15, 376. [Google Scholar] [CrossRef]
- Suzuki, R.; Mochizuki, Y.; Yoshimatsu, H.; Niina, A.; Teshima, T.; Matsumoto, H.; Koyama, H. Early detection of myocardial dysfunction using two-dimensional speckle tracking echocardiography in a young cat with hypertrophic cardiomyopathy. J. Feline Med. Surg. Open Rep. 2018, 4. [Google Scholar] [CrossRef]
- Ozawa, K.; Funabashi, N.; Takaoka, H.; Kamata, T.; Kanaeda, A.; Saito, M.; Nomura, F.; Kobayashi, Y. Characteristic myocardial strain identified in hypertrophic cardiomyopathy subjects with preserved left ventricular ejection fraction using a novel multi-layer transthoracic echocardiography technique. Int. J. Cardiol. 2015, 184, 237–243. [Google Scholar] [CrossRef]
- Okada, K.; Yamada, S.; Iwano, H.; Nishino, H.; Nakabachi, M.; Yokoyama, S.; Abe, A.; Ichikawa, A.; Kaga, S.; Nishida, M.; et al. Myocardial shortening in 3 orthogonal directions and its transmural variation in patients with nonobstructive hypertrophic cardiomyopathy. Circ. J. 2015, 79, 2471–2479. [Google Scholar] [CrossRef] [PubMed]
- Riesen, S.C.; Schober, K.E.; Cervenec, R.M.; Bonagura, J.D. Comparison of the effects of ivabradine and atenolol on heart rate and echocardiographic variables of left heart function in healthy cats. J. Vet. Intern. Med. 2011, 25, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Hartlage, G.R.; Kim, J.H.; Strickland, P.T.; Cheng, A.C.; Ghasemzadeh, N.; Pernetz, M.A.; Clements, S.D.; Williams, B.R. 3rd. The prognostic value of standardized reference values for speckle-tracking global longitudinal strain in hypertrophic cardiomyopathy. Int. J. Cardiovasc. Imaging 2015, 31, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Reant, P.; Mirabel, M.; Lloyd, G.; Peyrou, J.; Lopez Ayala, J.M.; Dickie, S.; Bulluck, H.; Captur, G.; Rosmini, S.; Guttmann, O.; et al. Global longitudinal strain is associated with heart failure outcomes in hypertrophic cardiomyopathy. Heart 2016, 102, 741–747. [Google Scholar] [CrossRef]
- Wang, J.; Khoury, D.S.; Yue, Y.; Torre-Amione, G.; Nagueh, S.F. Preserved left ventricular twist and circumferential deformation, but depressed longitudinal and radial deformation in patients with diastolic heart failure. Eur. Heart J. 2007, 29, 1283–1289. [Google Scholar] [CrossRef]
- Mizuguchi, Y.; Oishi, Y.; Miyoshi, H.; Iuchi, A.; Nagase, N.; Oki, T. Concentric left ventricular hypertrophy brings deterioration of systolic longitudinal, circumferential, and radial myocardial deformation in hypertensive patients with preserved left ventricular pump function. J. Cardiol. 2010, 55, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, R.; Matsumoto, H.; Teshima, T.; Koyama, H. Clinical assessment of systolic myocardial deformations in dogs with chronic mitral valve insufficiency using two-dimensional speckle-tracking echocardiography. J. Vet. Cardiol. 2013, 15, 41–49. [Google Scholar] [CrossRef]
- Mizuguchi, Y.; Oishi, Y.; Miyoshi, H.; Iuchi, A.; Nagase, N.; Oki, T. The functional role of longitudinal, circumferential, and radial myocardial deformation for regulating the early impairment of left ventricular contraction and relaxation in patients with cardiovascular risk factors: A study with two-dimensional strain imaging. J. Am. Soc. Echocardiogr. 2008, 21, 1138–1144. [Google Scholar]
- Vitarelli, A.; Terzano, C. Do we have two hearts? New insights in right ventricular function supported by myocardial imaging echocardiography. Hear. Fail. Rev. 2010, 15, 39–61. [Google Scholar] [CrossRef]
- Caivano, D.; Rishniw, M.; Birettoni, F.; Petrescu, V.F.; Porciello, F. Transverse right ventricle strain and strain rate assessed by 2-dimensional speckle tracking echocardiography in dogs with pulmonary hypertension. Vet. Sci. 2020, 7, 19. [Google Scholar] [CrossRef] [PubMed]
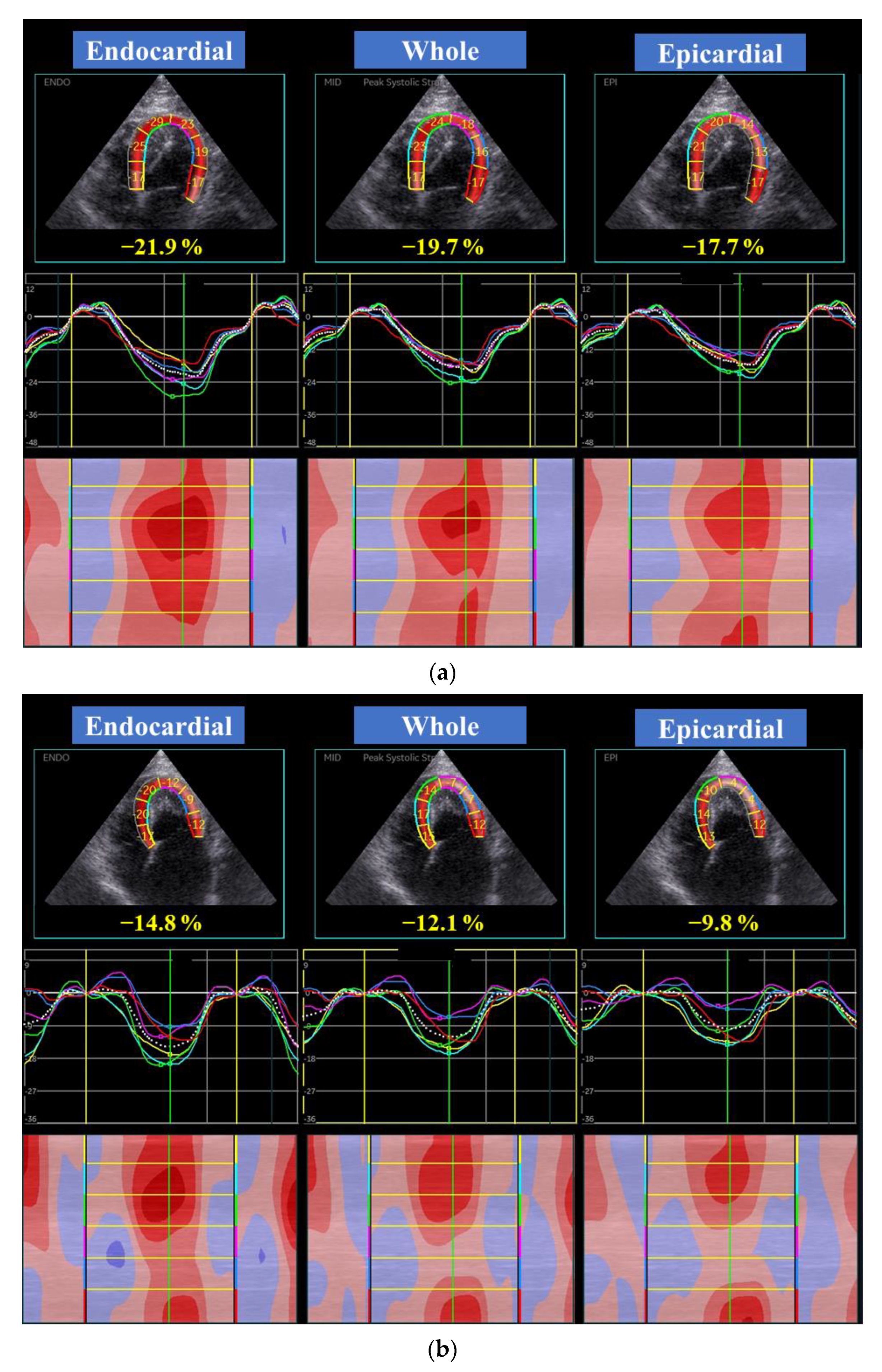
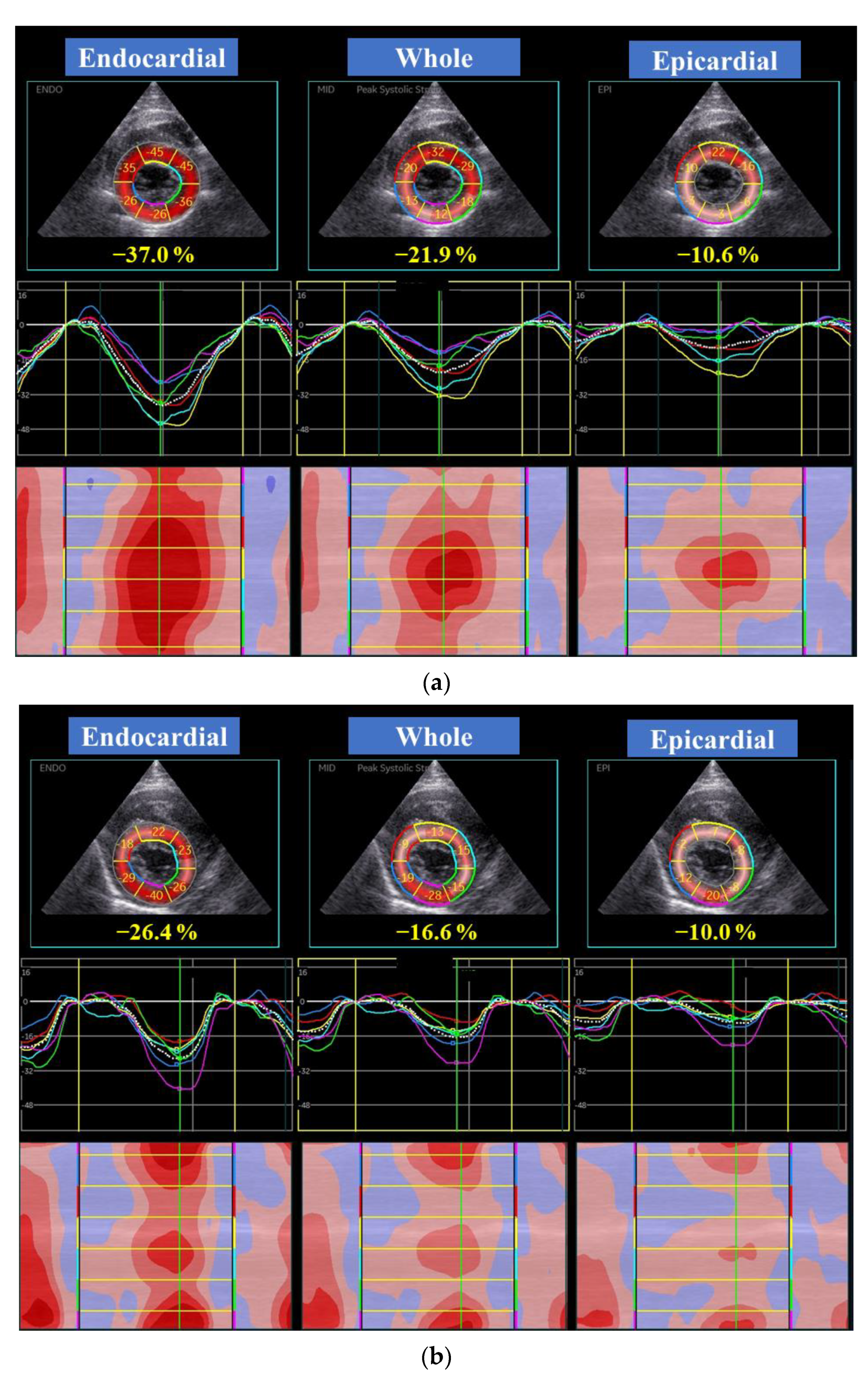
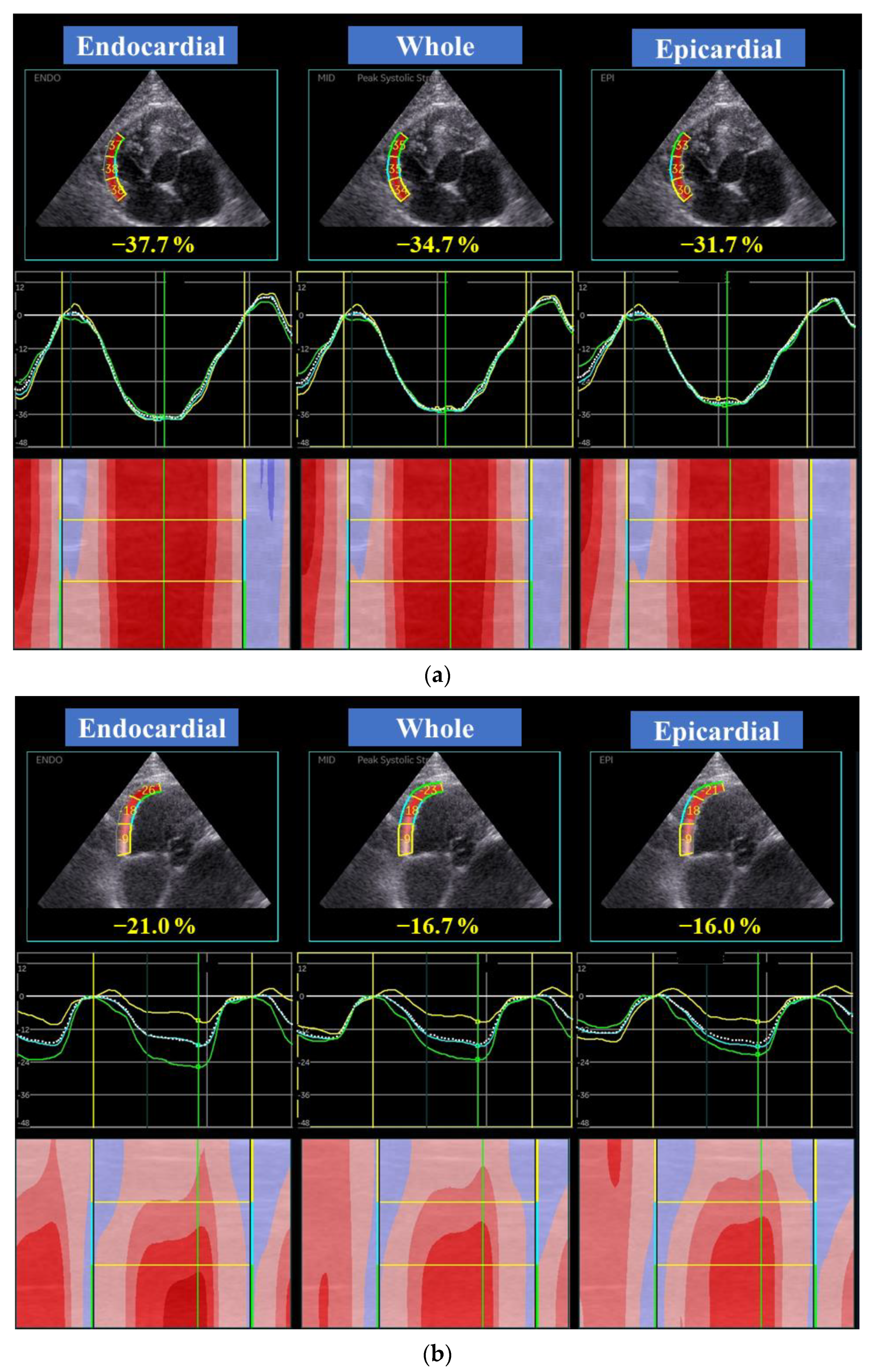

| EMF-RCM | n | Control | n | p | |||
|---|---|---|---|---|---|---|---|
| Age (month) | 82.5 | (41.5, 164.3) | 10 | 69.0 | (25.0, 140.0) | 15 | 0.08 |
| Body weight (kg) | 4.7 | (4.0, 5.2) | 10 | 4.2 | (3.5, 4.5) | 15 | 0.16 |
| Heart rate (bpm) | 218 | (167, 240) | 10 | 203 | (181, 225) | 15 | 0.96 |
| Systolic blood pressure (mmHg) | 145 | (121, 160) | 10 | 140 | (125, 149) | 15 | 0.66 |
| Gender (male/female) | 6/4 | 8/7 | 1.00 | ||||
| LA/Ao | 2.6 | (2.1, 3.2) | 10 | 1.3 | (1.2, 1.4) | 15 | <0.01 |
| IVSd (mm) | 4.0 | (2.7, 5.1) | 10 | 3.8 | (3.4, 4.1) | 15 | 0.71 |
| LVIDd (mm) | 16.8 | (15.1, 17.6) | 10 | 14.0 | (13.5, 15.5) | 15 | <0.01 |
| LVFWd (mm) | 4.1 | (3.5, 5.1) | 10 | 4.2 | (3.6, 4.3) | 15 | 0.80 |
| FS (%) | 34.0 | (26.4, 40.3) | 10 | 49.1 | (40.8, 53.1) | 15 | <0.01 |
| E-wave velocity (cm/s) | 0.82 | (0.79, 0.85) | 9 | 0.60 | (0.57, 0.73) | 12 | <0.01 |
| A-wave velocity (m/s) | 0.30 | (0.24, 0.39) | 9 | 0.69 | (0.57, 0.73) | 12 | <0.01 |
| E/A ratio | 2.9 | (2.5, 3.4) | 9 | 0.9 | (0.8, 1.2) | 12 | <0.01 |
| MV s’ (cm/s) | 5.2 | (4.6, 5.5) | 8 | 8.0 | (6.9, 10.5) | 12 | 0.02 |
| MV e’ (cm/s) | 5.8 | (4.9, 6.1) | 8 | 7.0 | (5.1, 7.6) | 12 | 0.10 |
| MV E/e’ | 14.1 | (13.0, 16.2) | 8 | 9.4 | (8.2, 12.3) | 12 | <0.01 |
| Right atrial diameter (mm) | 11.2 | (10.1, 13.9) | 10 | 9.1 | (8.3, 10.7) | 15 | 0.02 |
| RVIDd (mm) | 8.8 | (7.8, 9.1) | 10 | 7.1 | (6.2, 7.6) | 15 | 0.047 |
| RVFWd (mm) | 2.3 | (2.2, 2.7) | 10 | 1.9 | (1.7, 2.1) | 15 | <0.01 |
| TAPSE (mm) | 6.1 | (5.0, 8.4) | 10 | 9.1 | (7.6, 10.9) | 15 | <0.01 |
| RV FAC (%) | 53.3 | (39.2, 60.0) | 10 | 57.0 | (51.6, 63.0) | 15 | 0.12 |
| RV s’ (cm/s) | 7.4 | (6.4, 8.8) | 10 | 10.5 | (8.4, 12.2) | 14 | 0.07 |
| RV e’ (cm/s) | 10.7 | (7.1, 15.7) | 10 | 9.4 | (6.7, 11.0) | 14 | 0.64 |
| PV AT/ET | 0.33 | (0.22, 0.45) | 10 | 0.50 | (0.43, 0.53) | 15 | 0.01 |
| Intra-Observer | Inter-Observer | |||
|---|---|---|---|---|
| CV (%) | ICC | CV (%) | ICC | |
| Global LV systolic longitudinal strain | ||||
| Whole layer | 2.2 | 0.98 * | 1.8 | 0.98 * |
| Endocardial layer | 1.3 | 0.99 * | 3.1 | 0.95 * |
| Epicardial layer | 2.6 | 0.97 * | 3.6 | 0.94 * |
| Global LV systolic longitudinal strain rate | 4.0 | 0.94 * | 5.4 | 0.90 * |
| Global LV early-diastolic longitudinal strain rate | 7.5 | 0.91 * | 5.1 | 0.96 * |
| Global LV late-diastolic longitudinal strain rate | 7.4 | 0.99 * | 14.4 | 0.97 * |
| Global LV systolic circumferential strain | ||||
| Whole layer | 4.6 | 0.92 * | 6.6 | 0.91 * |
| Endocardial layer | 6.5 | 0.95 * | 5.8 | 0.96 * |
| Epicardial layer | 9.2 | −0.16 | 10.7 | 0.03 |
| Global LV systolic circumferential strain rate | 5.2 | 0.85 * | 5.7 | 0.84 * |
| Global LV early-diastolic circumferential strain rate | 7.8 | 0.38 | 10.5 | −0.41 |
| Global LV late-diastolic circumferential strain rate | 23.9 | 0.38 | 9.4 | 0.97 * |
| Global RV systolic longitudinal strain | ||||
| Whole layer | 3.7 | 0.98 * | 5.4 | 0.98 * |
| Endocardial layer | 3.3 | 0.99 * | 5.1 | 0.98 * |
| Epicardial layer | 3.8 | 0.98 * | 5.8 | 0.97 * |
| Global RV systolic longitudinal strain rate | 16.4 | 0.40 | 12.3 | 0.80 |
| Global RV early-diastolic longitudinal strain rate | 13.4 | 0.58 | 13.5 | 0.78 * |
| Global RV late-diastolic longitudinal strain rate | 20.3 | 0.92 * | 9.2 | 0.95 * |
| EMF-RCM | n | Control | n | p | |||
|---|---|---|---|---|---|---|---|
| Global LV longitudinal strain (%) | |||||||
| Whole layer | 13.4 | (11.4, 20.8) | 10 | 20.8 | (18.3, 22.6) | 15 | 0.04 |
| Endocardial layer | 16.3 | (13.6, 23.6) | 10 | 23.8 | (21.9, 25.3) | 15 | 0.08 |
| Epicardial layer | 11.8 | (9.2, 17.3) | 10 | 18.5 | (16.1, 19.8) | 15 | <0.01 |
| Endo/Epi | 1.43 | (1.34, 1.55) | 10 | 1.36 | (1.2, 1.4) | 15 | 0.03 |
| Global LV longitudinal strain rate (%/s) | 2.8 | (1.8, 3.0) | 10 | 2.9 | (2.3, 3.5) | 15 | 0.10 |
| Global LV circumferential strain (%) | |||||||
| Whole layer | 17.4 | (15.4, 21.3) | 10 | 19.1 | (17.3, 23.9) | 15 | 0.09 |
| Endocardial layer | 27.7 | (25.4, 32.6) | 10 | 38.7 | (32.7, 48.3) | 15 | <0.01 |
| Epicardial layer | 10.4 | (8.5, 12.3) | 10 | 7.3 | (6.2, 9.3) | 15 | 0.14 |
| Endo/Epi | 2.73 | (2.08, 4.06) | 10 | 4.77 | (4.12, 6.50) | 15 | <0.01 |
| Global LV circumferential strain rate (%/s) | 2.7 | (2.2, 3.0) | 10 | 2.8 | (2.5, 3.2) | 15 | 0.14 |
| Global RV longitudinal strain (%) | |||||||
| Whole layer | 22.9 | (16.4, 31.9) | 10 | 31.6 | (27.8, 39.1) | 15 | 0.07 |
| Endocardial layer | 24.9 | (19.0, 34.2) | 10 | 34.8 | (29.6, 42.3) | 15 | 0.10 |
| Epicardial layer | 21.3 | (14.0, 29.8) | 10 | 29.0 | (26.0, 36.4) | 15 | 0.045 |
| Endo/Epi | 1.21 | (1.13, 1.30) | 10 | 1.15 | (1.13, 1.18) | 15 | 0.03 |
| Global RV longitudinal strain rate (%/s) | 4.0 | (3.7, 5.5) | 10 | 5.5 | (4.8, 6.7) | 15 | 0.17 |
| EMF-RCM | n | Control | n | p | |||
|---|---|---|---|---|---|---|---|
| Early-diastolic LV longitudinal strain rate (%/s) | 4.1 | (2.6, 4.3) | 9 | 5.2 | (4.1, 5.8) | 8 | <0.01 |
| Late-diastolic LV longitudinal strain rate (%/s) | 1.3 | (0.3, 2.1) | 9 | 2.3 | (1.5, 4.1) | 8 | 0.049 |
| Early-diastolic LV circumferential strain rate (%/s) | 3.7 | (2.8, 4.7) | 8 | 3.4 | (3.0, 4.0) | 15 | 0.45 |
| Late-diastolic LV circumferential strain rate (%/s) | 0.9 | (0.2, 1.9) | 8 | 2.7 | (1.7, 3.4) | 15 | <0.01 |
| Early-diastolic RV longitudinal strain rate (%/s) | 5.5 | (4.4, 6.3) | 10 | 5.6 | (4.8, 6.7) | 13 | 0.29 |
| Late-diastolic RV longitudinal strain rate (%/s) | 1.9 | (1.1, 5.2) | 10 | 4.6 | (3.8, 6.6) | 13 | 0.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suzuki, R.; Yuchi, Y.; Kanno, H.; Teshima, T.; Matsumoto, H.; Koyama, H. Left and Right Myocardial Functionality Assessed by Two-Dimensional Speckle-Tracking Echocardiography in Cats with Restrictive Cardiomyopathy. Animals 2021, 11, 1578. https://doi.org/10.3390/ani11061578
Suzuki R, Yuchi Y, Kanno H, Teshima T, Matsumoto H, Koyama H. Left and Right Myocardial Functionality Assessed by Two-Dimensional Speckle-Tracking Echocardiography in Cats with Restrictive Cardiomyopathy. Animals. 2021; 11(6):1578. https://doi.org/10.3390/ani11061578
Chicago/Turabian StyleSuzuki, Ryohei, Yunosuke Yuchi, Haruka Kanno, Takahiro Teshima, Hirotaka Matsumoto, and Hidekazu Koyama. 2021. "Left and Right Myocardial Functionality Assessed by Two-Dimensional Speckle-Tracking Echocardiography in Cats with Restrictive Cardiomyopathy" Animals 11, no. 6: 1578. https://doi.org/10.3390/ani11061578
APA StyleSuzuki, R., Yuchi, Y., Kanno, H., Teshima, T., Matsumoto, H., & Koyama, H. (2021). Left and Right Myocardial Functionality Assessed by Two-Dimensional Speckle-Tracking Echocardiography in Cats with Restrictive Cardiomyopathy. Animals, 11(6), 1578. https://doi.org/10.3390/ani11061578






