Simple Summary
Heat stress due to high environmental temperature negatively influences animal productivity. Extensive studies have been carried out to evaluate the mechanisms of heat stress in chickens. It was shown that the expression level of heat-shock factors (HSFs) and heat-shock proteins (HSPs) were affected. Tissue-specific responses to the thermal challenge were also found in the heart, liver and muscle. Our study examined the changes in primary production parameters and four heat-shock factor and two heat-shock protein expression profiles in chicken gonads. In the first experiment, 24 h after hatching, 80 Transylvanian Naked Neck chickens were heat-treated at 38.5 °C ambient temperature with 60% humidity for 12 h. In this experiment, we studied the primary productivity parameters of matured chickens after the performed heat stress. In the second experiment, the heat treatment was the same, and we examined the expression pattern of heat-shock factors and heat-shock proteins in the control and treated gonads. We collected the samples immediately after the heat-treatment in case of half of the treated and control group. We found a significant difference in egg production, and increased expression level of HSP90 and HSF4 in heat-treated female gonads.
Abstract
Although numerous studies reported the effects of heat stress in chickens, it was not investigated in the Transylvanian Naked Neck breed. In our research, Transylvanian Naked Neck chickens, 24 h after hatching, were heat-treated at 38.5 °C for 12 h. We compared the control and heat-treated adult chickens’ productivity parameters following 12 weeks of heat-stress at 30 °C. We found that the heat-treated layers had significantly higher egg production in heat stress, but in cockerels, the sperm quality did not differ significantly between the two groups. To detect the effect of heat-treatment on a molecular level, the expression of two heat-shock proteins and four heat-shock factors were analysed in the gonads of control and heat-treated chickens. We found that the expression level of HSP90 and HSF4 increased significantly in heat-treated female chicken gonads. Still, in adult females, the expression of HSF2 and HSF3 were substantially lower compared to the control. In adult heat-treated males, the HSP70, HSF1 and HSF3 expression levels showed a significant increase in both gonads compared to the control. We think that the presented significant differences in egg production might be related to the increased expression level of HSP90 and HSF4 in heat-treated female gonads.
1. Introduction
One of the most important environmental factors is the ambient temperature and due to the recent changes in global climate the average temperature is rising. Agriculture is very sensitive to the climate variability and extreme weather [1]. An increasing number of articles were published in previous years about the effects of climate change in agriculture [2,3] and in animal husbandry [1,4,5,6]. Animal exposure to hot environments deleteriously affects their reproductive functions [7]. Chickens are homeothermic animals and their body temperature is maintained in the range of 41 to 42 °C [8]. Higher environmental temperature negatively impacts the feed intake, the reproductive function, the hatchability and the meat and egg production [9,10,11,12,13,14]. In chickens, the high ambient temperature affects their endocrine system, reproductive and egg-laying performance, too [15].
Many researchers examined the effect of thermal manipulation in chicken. Two types of thermal manipulation are known: during the incubation period or after hatching. According to Al-Rubika et al. [16], thermal manipulation during the late embryogenesis has not affected the hatchability, but the body weight of thermally manipulated embryos was higher than the control group. Walstra et al. [17] investigated the effect of temperature manipulation on the behaviour of layer chickens. The thermally manipulated embryos were incubated at 37.8 °C, but between the 14th and 18th embryonic day the embryos were exposed to 40 °C for 4 h. The manipulated chicks preferred the lower ambient temperature, but no effect of thermal manipulation on behaviour and performance were observed [17]. Rozenboim et al. (2007) [15] examined the heat stress at the 31st life week for 12 h per day in White Leghorn Laying hens. They checked the body temperature, egg production, egg weight, feed intake and plasma hormone levels. They found the egg production was significantly lower in the heat stressed group. The effect of thermal manipulation was not detected in case of body weight in broiler, but the manipulation induced the up-regulation of muscle grow factors and muscle marker genes [18]. Vinoth et al. [19] investigated the effect of thermal manipulation and thermal stress on HSP gene expression, DNA methylation in brain tissue of Naked Neck and Punjab Broiler-2 chicken breeds. In hot regions the Naked Neck gene is very common [20]. They found that the DNA methylation level was lower, and the gene expression was higher in case of heat stressed but not heat-treated chickens, compared with other experimental groups (non-heat-treated but heat stressed; heat-treated and not heat stressed; and heat-treated and stressed). Rajkumar and his colleagues [21] demonstrated, that the Naked Neck breeds have better growth performance in higher temperature than the normal siblings, and the Heterophil/Lymphocyte ratio was significantly lower, what is indicating that Naked Neck chickens were less stressed in higher temperature.
In case of heat stress, a lot of heat-shock proteins (HSPs) and heat-shock factors (HSFs) start to be expressed to protect the cells from the effect of heat stress [22,23]. According to their molecular weights six HSP families are known (small HSPs, HSP40, HSP60, HSP70, HSP90 and HSP100) [24]. HSP70 and HSP90 are highly conserved ATP-dependent molecular chaperons that are essential for the eucaryote systems in unstressed conditions [25]. Of the many HSPs, the HSP70 correlates the best with thermotolerance [26]. The HSP90 chaperone is present in bacteria and all of eucaryotes. HSP90 participates in collaboration with the HSP70 chaperon system in protein folding and activation [27,28,29]. Increased HSP70 expression was detected in Japanese quail’s myocardial tissue in case of isolation in darkness, loud noise and cold temperature [30]. These results denoted that the heat-shock proteins are expressing in different types of stress. Under stress condition in poultry most heat-shock factors are activated. In animals, four HSFs were known until 2018, when Saju and his colleagues found [31] the fifth HSF: HSF5 in Danio rerio. The HSF5 is essential for the spermatogenesis in zebrafish. Two isoforms of HSF1 was discovered in gonads and liver of Danio [32]. In chicken, the HSF1, HSF2 and HSF3 genes were isolated by cross-hybridization with mouse hsf1 cDNA probe [33]. HSF1 was mapped to the 2nd chromosome, the HSF2 to the 3rd, the HSF3 to the 4th and the HSF4 gene to the 11th chromosome in chicken [34]. Zhang and his colleagues [10] examined the effects of acute heat stress in two different Chinese chicken breeds. They found that with increasing heat treatment time, both HSF3 and HSP70 expression first decreased then showed a significant increase in both breeds. They found that the expression of HSF3 and HSP70 is species-specific and tissue-specific during heat treatment.
During heat stress different poultry breeds react differently. The broiler breeds are more sensitive than the local breeds. The results of heat tolerance indexes suggested that with age the local breeds easily overcome heat stress, while the other chickens become increasingly vulnerable. These results have been confirmed by the high mortality rate observed in the commercial stock under heat stress, while there was no mortality among the local chickens [35].
In our study we investigated the effect of heat treatment and heat stress on the egg production, sperm quality, heat-stress protein and heat-shock factor expression profile in the Transylvanian Naked Neck Hungarian chicken breed.
2. Materials and Methods
2.1. Animals
Eggs of Speckled Transylvanian Naked Neck (STN) chickens were from the National Centre for Biodiversity and Gene Conservation—Institute for Farm Animal Gene Conservation (NBGK-HGI), Gödöllő, Hungary. Hatching and heat treatment took place in the experimental hatchery, the comparative study under heat stress was done in the animal house of this institution.
2.2. Heat Treatment
The eggs were incubated in a MIDI F500S hatchery machine (Tárnok, Hungary) in the regular way. For the first 24 h after hatching, all 160 chicks were placed under an infrared lamp at 32 °C on absorbent paper litter with ad libitum starter feed and water. Then, 80 chicks were placed back to the hatcher for heat treatment. The temperature was set to 38.5 °C and the humidity was 60% for 12 h. Drinking water and feed were ad libitum inside. The control 80 chicks were kept at 32 °C. Then, in Experiment I the animals were kept and raised together with the control group, in Experiment II 15 treated and 15 control chicks were immediately sacrificed for RNA analysis.
2.3. Heat Stress
In Experiment I, 80–80 animals were in the treated and control groups. We randomly selected for the further reproductive biology examinations under heat stress 31–31 layers with 3–3 males in the 24th life week. Both of them were kept on a wood chips and zeolite mixture litter with ad libitum layer feed and water under 16-h lighting, with egg nests (10) and perches. Additionally, 10–10 roosters were placed in the same air space, in individual cages, for semen examinations, with ad libitum rooster feed and water. Ventilation was provided 10 min per hour. The average temperature in the pen at the height of the birds’ habitat was constantly 30 °C from 24th life week during 12 weeks for all treated and control animals.
2.4. Examination of Embryonic Abnormalities
In Experiment I, eggs were collected daily from the 24th life week and marked with group number and date and placed to the incubator once a week. Every week 20–20 eggs were applied for molecular biological examinations. The incubation was made the regular way (37.5 °C and 60% humidity). Eggs were candled on the 7th day of incubation. The ratio of fertile and infertile eggs was determined.
2.5. Semen Collection and Classification
Sperm-donor animals were selected on the basis of responsiveness to semen collection, followed by individual semen evaluation data. Semen collection was performed using Burrows and Quinn’s [36] dorso-abdominal massage technique twice a week for 3 months—from 23 weeks of age to 34 weeks of age—following a two-week training period. Semen was classified weekly for the following spermatological parameters:
- Volume (mL): determined with a pipette;
- Motility: determined by subjective estimation using a light microscope (Leica) on a scoring scale from 0 to 5 at 40× magnification. The test was always performed by the same experienced person;
- Concentration: determined with a spectrophotometer (Accucell IMV, France). At the beginning of the experiment, the instrument was calibrated. A concentration curve was established by comparing the spectrophotometer data of the samples in a dilution series with the concentrations determined using the Makler chamber;
- Type of morphological abnormalities and live/dead cell ratio: the study was performed using eosin–aniline blue vital staining [37].
2.6. Collection of Gonadal Tissues
In Experiment II, the chickens were euthanized by cervical dislocation after the treatment. The tissue samples were collected in sterile plastic dishes. Small pieces from each gonad were placed into RNAlaterTM Solution (Thermo Fisher Scientific, 145 Waltham, MA, USA). We collected thigh muscle samples from each chicken for DNA analysis. The samples were transferred into TRizolTM reagent after two days. The samples were incubated in TRizolTM (Thermo Fisher Scientific, 145 Waltham, MA, USA) for 10 min at room temperature than stored at −80 °C.
2.7. DNA Isolation and Sex Determination
The thigh muscle samples were digested using 0.1 % Proteinase-K Lysis Buffer solution and incubated at 55 °C for 3 hours. After the incubation, the Proteinase-K was inactivated at 99 °C for 10 min. Total DNA was extracted using the Phenol-chloroform DNA isolation protocol [19]. The isolated DNA was quantified by measuring the absorbance at 260 nm using a NanoDrop One Spectrophotometer (Thermo Fisher Scientific, 145 Waltham, MA, USA). The purity was assessed by determining the ratio of the absorbance at 260 and 280 nm.
The sex of treated and control chickens were determined using CHD1 (Chromosome Helicase DNA binding protein 1) primer set (Supplementary Table S2). The isolated DNA was diluted to 25 ng/µL for the PCR and gel electrophoresis. PCR was performed using MyTaq Red Mix (Thermo Fisher Scientific, 145 Waltham, MA, USA). A total of 13 µL of the reaction solution was used: 6.75 µL MyTaq Mix; 0.5 µL reverse CHD1 primer (10 µmol); 0.5 µL forward CHD1 primer (10 µmol); 4.25 µL sterile water; and finally, 1 µL DNA sample. The cycling parameters were 95 °C for 1 min. 28 cycles of 95 °C for 15 s followed 30 s at 48 °C and 72 °C for 10 sec. It was finally melting at 72 °C for 5 min. The PCR products were separated by electrophoresis, using 1.5 % agarose gel stained with ethidium bromide, at 100 V for 30 min. The DNA bands were visualized under UV illumination and photographed.
2.8. RNA Isolation, cDNA Writing, and Real-time qPCR
Total RNA extraction and purification from cells collected in the TRIzol Reagent was following the manufacturers’ protocol. The RNA was quantified by measuring the absorbance at 260 nm using a NanoDrop One Spectrophotometer (Thermo Fisher Scientific, 145 Waltham, MA, USA), and the purity was assessed by determining the ratio of the absorbance at 260 and 280 nm. Total RNA (15 μL) was reverse transcribed using a cDNA synthesis kit (High-Capacity cDNA Reverse Transcription Kit, Thermo Fisher Scientific, 145 Waltham, MA, USA). SYBR Green PCR master mix was applied for the qPCR as a double-stranded fluorescent DNA-specific dye according to the manufacturer’s instructions (Thermo Fisher Scientific, 145 Waltham, MA, USA). The primers used for real-time PCR are displayed in Supplementary Table S2. Amplification was carried out in a total volume of 15 μL containing Power SYBR Green PCR Master Mix (Thermo Fisher Scientific, 145 Waltham, MA, USA), forward and reverse primers (0.1 μg/μL), sterile water (Thermo Fisher Scientific, 145 Waltham, MA, USA) and 0.75 μL of cDNA. After an initial 10 min denaturation step at 95 °C, the reactions were cycled 40 times under the following parameters: 95 °C for 15 s, 60 °C for 40 s and 68°C for 20 s. Optical detection was carried out at 68 °C.
We tested the expression of two housekeeping genes (GAPDH and ß-Actin) [11]. We compared the average Ct values of GAPDH and ß-Actin in control and heat-treated left gonads. We decided to use GAPDH as we found lower Ct values in the case of GAPDH, and the standard deviation were higher using ß-Actin (Supplementary Figure S1A). There was no significant difference between the control and heat-treated samples (p = 0.523) comparing the average GAPDH Ct values. We used chicken embryonic fibroblast as a reference sample [11,38,39]. All reactions were performed in triplicate. Those qPCR measurements were used at the analysis where the reference sample (CEF) CT value on the plate has not significantly different from the average CEF CT value. From the collected gonads we performed qPCR to analyse the expression profile of two heat-shock protein (HSP70, HSP90) genes and four heat-stress factor (HSF1, HSF2, HSF3, HSF4) genes in male left and right gonads, and female genital ridges. To determine whether there is any difference in the expression profile of heat treated and control groups, we pooled the RNAs of the individual samples group by group. As we found differences in the expression pattern compared to the heat treated and control groups, we performed qPCR runs from the individual samples to prove, whether these differences are statistically different or not. The individual chickens were chosen by random sampling. We compared the Delta Ct values of pooled samples with the average values of individual RNA samples in different groups. The number of used samples is indicated in Supplementary Table S1B.
2.9. Statistical Analysis
To evaluate and analyse the collected data RStudio (1.0.136), R (R-3.2.2), GenEx (7.0) (MultiD Analyses AB, Göteborg, Sveden) and Excel (Microsoft Excel for Mac, 16.49 version) software were used. For the data obtained from the qPCR runs, expression changes of the target genes were calculated compared to the expression of the housekeeping gene with the standard 2^(−ΔΔCt) method, where Ct = cycle threshold; ΔCt = Ct (target gene)—Ct (housekeeping gene) and ΔΔCt = ΔCt (test sample)—ΔCt (control sample). The mean values in case of every group were compared using Welch’s t-tests. Prior testing, general assumptions of the t-test were checked, such as normality (Shapiro–Wilk’s test) and homogeneity of variances (Levene’s test). Furthermore, power analysis was made in case of every comparison to make sure the sample size was sufficient for testing. The categorical data was tested with Chi-squared tests. Significance levels were set as follows: * p < 0.05, ** p < 0.01, and *** p < 0.001.
3. Results
3.1. Analysis the Effect of Heat Stress on Reproductive Parameters of Heat-Treated Chickens
3.1.1. Spermatological Analysis in Roosters
The spermatological parameters of heat-treated (HTHS) and control (HS) Transylvanian Naked Neck roosters were examined under heat stress. In the spermatological analysis, ten heat-treated and ten control roosters were used. Four parameters (quantity, concentration, motility and live–dead ratio) of sperm was determined (Figure 1). The volume of the semen was measured by pipetting. We could not find significant difference between the two experimental groups (p = 0.5075). According to the sperm concentration, no significant difference was found between the treated and the control groups (p = 0.1077) nor in the motility rate between the two groups (p = 0.6972). Finally, in the live–dead sperm ratio, no significant difference was found (p = 0.8816) between the two experimental groups. In summary, we can conclude that the pre-heat treatment has no effect on sperm quantity and quality.

Figure 1.
In this figure the results of heat-treated (HTHS) and control (HS) groups under 12 weeks of heat stress on sperm parameters are summarized. Significant difference between the two groups was not found. (A): Volume of the semen. (B): Concentration of the sperm. (C): Motility of the sperm. (D): Sperm with normal morphology.
3.1.2. Examination the Egg Production and Fertilization Rate in Hens
In the females, two parameters, the egg production and the percent of unfertilized eggs were measured. The daily egg production of heat-treated chickens (HTHS) was significantly higher than the control (HS) hens in high environmental temperature (30 °C) (p = 0.00002) (Figure 2). Altogether, 1654 eggs were collected. The HTHS group produced 890 eggs, while in case of the HS group, we could collect 764 eggs. Based on candling at the 7th day of incubation in the case of HT group 55 eggs were discarded (6.18%), while in the HS group 42 eggs (5.42%) were discarded. The ratio of unfertilized eggs among the discarded ones was 10.91% in the treated and 42.86% in the control groups.

Figure 2.
Demonstration of the daily egg production and the ratio of fertilized eggs in heat-treated (HTHS) and control (HS) groups under heat stress. The egg collection began from the 24th life week but the data in the figure presents the egg number from the 27th life week because the egg production was stable from this time.
3.2. Comparison the Expression Profile of Heat-Shock Proteins and Heat-Shock Factors in Heat-Treated and Control Chicken Gonads
3.2.1. Comparison the Delta Ct Values
The comparison showed high similarity in the expression profile of the pooled samples to the average of individual sample values (Supplementary Figure S2). Comparing the Delta Ct values calculated from the individual samples, we found a significant difference between the chicks and adults (Figure 3(1A–6A)). A significant difference was determined between the Delta Ct values of HSP70 (p = 0.0289) and HSF3 (p = 0.0482) expressions in the heat-treated and control samples in case of adult male left gonads (Figure 3(1A) and Figure 3(5A)). In case of the right gonads, significant differences were found in HSP70 (p = 0.023), HSF1 (p = 0.0007) and HSF3 (p = 0.0013) (Figure 3(1B,3B); Figure 3(5B)) between the control chicks and control adults. Only the HSP70 showed a significant difference comparing the values of control and heat-treated right gonads in adults (p = 0.0136) (Figure 3(2A)).

Figure 3.
Delta Ct values of two heat-shock protein (HSP70 and HSP90) and four heat-shock factor HSF1, HSF2, HSF3, HSF4) genes were determined in male and female left gonads of (heat-treated) HT and (control) CTRL chicks and adults. In every case the GAPDH was the reference gene. (1): HSP70; (2): HSP90; (3): HSF1; (4): HSF2; (5): HSF3; (6): HSF4. (A): Male left gonads; (B): Male right gonads; (C): Female gonads. * p < 0.05, ** p < 0.01, *** p < 0.001.
In case of the female gonads, only HSF4 Delta Ct values defined a significant difference (p = 0.0016) between the control chicks and control adults (Figure 3(6C)). Significant differences were found between the control and heat-treated chicks in case of HSP90 (p = 0.0355) (Figure 3(2C)) and HSF4 (p = 0.0342) values (Figure 3(6C)). In other cases, we could not find significant differences between the groups.
The expression levels of 1: HSP70 (HSPA2), 2: HSP90 (HSP90AA1), 3: HSF1, 4: HSF2, 5: HSF3 and 6: HSF4 genes in chicks and adults, females-males, left and right gonads in case of HT and CTRL. A: Male left gonads, B: Male right gonads, C: Female gonads, HT: heat-treated, CTRL: control (* p < 0.05, ** p < 0.01 and *** p < 0.001).
3.2.2. Comparison the Relative Expression Profile
To get more detailed information whether there is any significant difference in relative expression of HSPs and HSFs in control and treated samples, we calculated the relative expression values using GenEx (7.0) software (MultiD Analyses AB, Göteborg, Sveden). The HSP90 (p = 0.0094) and HSF4 (p = 0.0387) expression was significantly higher in the heat-treated female chick gonads than in the control (Figure 4E). However, in the adult female gonads all of the HSPs and HSFs decreased compared to the control groups, the HSF2 (p = 0.0181) and the HSF3 (p = 0.0011) were significantly lower in treated samples. (Figure 4F). In the case of chicks, there was no significant difference between the male left and right gonads compared to the control group (Figure 4A,C). However, in the left gonads of adults, the HSP70 (p = 0.0002), HSF1 (p = 0.0013), HSF2 (p = 0.0217) and HSF3 (0.0014) relative expression levels showed a significant increase compared to the controls (Figure 4D). Analysing the male right gonads in adult samples we found that the expression of all HSPs and HSFs increased. The expression of HSP70 (p = 0.0052), HSF1 (p = 0.0333), HSF3 (p = 0.0332) and HSF4 (0.0498) showed a significant increase compared to the control (Figure 4B).
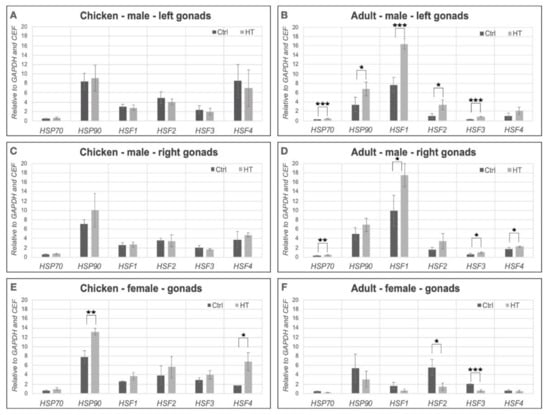
Figure 4.
The bar charts show the relative expression values in female and male chick and adult samples in control and heat-treated gonads relative to chicken embryonic fibroblast sample. The relative expression values of heat-shock protein (HSP70 and HSP90) and heat-shock factor (HSF1, HSF2, HSF3, HSF4) genes were determined in female and male gonads. GAPDH was chosen as reference gene. (A): Chicken mal left gonads. (B): Adult male left gonads. (C): Chicken male right gonads. (D): Adult male right gonads. (E): Chicken female gonads. (F): Adult female gonads. * p < 0.05, ** p < 0.01, *** p < 0.001.
4. Discussion
El-Tarabany [40] reported the impact of high temperature humidity index in Japanese quail. They found that the control groups had significantly greater fertility and hatchability than the heat stressed group. It was published that in broiler chickens the genetically lean breed is more resistant to the higher ambient temperature, than the fat counterpart [20]. Laine and her colleagues [41] studied the effect of higher temperature in the hypothalamic–pituitary–gonadal–liver axis of Great Tit (Parus major). They found that the zona pellucida glycoprotein 4 (ZP4) is differently expressed before and after the onset of egg-laying [41]. On the other hand, we could not detect difference in spermatological parameters between the heat-treated and control groups under heat-stress (Figure 1). Analysing the female reproductive performance, we found significantly higher egg production and fertility rate (Figure 2). However, Végi and her colleagues found that the spermatological parameters decline after heat-treatment in Cobb chicken hybrid [42]. It was published that the Naked Neck chickens show better body temperature regulation and higher radiation rates from the naked neck than the covered neck breeds if they are kept at 35 °C [43].The reason why we did not find any difference among the spermatological parameters might be the effect of the Transylvanian Naked Neck breed
Mezquita et al. [44] investigated the HSP70 expression in adult chicken testes. They found that the HSP70 was highly expressed in the left and right testes at higher temperatures (44 or 46 °C). However, at normal internal temperature the HSP70 is not expressed in the left gonad but it is present in the regressed (right) gonad [44]. We could detect low HSP70 expression level, but we found significantly higher HSP70 expression at adult age in roosters both in left and right gonads when they were heat treated. Interestingly, we found that HSF3 expression level increased parallel with HSP70 expression. Zhang and his colleagues [10] found that the level of HSP70 declined in the heart 6 hours after the heat stress, but the HSF3 expression remained high. Tarkhan and his colleagues [45] examined the HSP70 and HSF3 expression levels in cold stress. They found decreased expression levels in the liver in case of both genes.
In AA Broiler breed (from China) the HSP90 mRNA level increased in the liver, heart and kidney after 2 hours of high temperature. The HSP90 expressed in the endothelium cells and the blood vessel walls, which influences the regulation of the blood flow [46]. Hao and Gu examined the expression of HSP90 on pectoralis major in broiler breed after acute heat stress. They found that the HSP90 expression is positively correlate with corticosterone and superoxide dismutase, but negatively correlate with the pH in pectoralis major [47].
The gonads are the place where gametes are produced, so it appears useful to compare the impact of stress on both the tissues and the cells that result from it. In order to start the mechanistic studies, it is first necessary to identify the actors who intervene and the first screening that was done made it possible to find actors who are sensitive. This is the first step to launch mechanistic approaches.
We found high HSP90 expression in chickens compared to the HSP70 expression level. We could observe significant differences only in case of female chicks between the control and heat-treated HSP90 expression level.
It was reported that numerous transcripts in the testes expressed differentially between the heat-stressed broiler-type and layer-type chickens [48]. We found in the left gonads of the adult heat-treated males that the HSP70, HSF1, HSF2 and HSF3 relative expression levels showed significant increase compared to the controls. Whether these expression patterns associate with the heat-tolerance require a further investigation. It was found that after 2 hours of heat treatment the expression of HSP27, HSP90 and HSP70 increased in a Taiwanese country chicken rooster, but the mRNA of CDH5, CIRBP, SLA and NTF3 were downregulated in the testes [48]. Wang et al. [49] published that in the heat-stressed chicken testes the proteins that involve in autophagy and the major HSPs (HSP90α, HSPA5, HSPA8) were upregulated but the proteins that negatively regulate apoptosis were downregulated. In the future, we plan to check the expression level of these factors in our heat-treated samples, too.
Furukawa et al. [50] shows that the HSF1 is a very important regulator in the ovarian differentiation of Medaka. They made an HSF1 knock-out animal and found that HSF1 protects the female germ cells under heat stress. We could detect significantly higher HSF1 expression in heat-treated roosters, in both left and right gonads, compared to the control, but there was no significant difference in the level of HSF1 in treated and control females.
HSF2 is very important in the development of brain and reproductive organs, but the fundamental rule is not identified yet [51]. In HSF2 knock-out B Lymphocyte cells they found that the KO line was more sensitive to the heat stress than the wild type [52]. We found higher HSF2 expression in heat-treated gonads in adult roosters, but significantly lower expression in adult females.
The mutation of HSF4 gene may cause a congenital or senile cataract in human. We found significantly higher HSF4 expression in heat-treated female chicks parallel with high HSP90 expression. In case of males, we could not detect difference in the expression profile between the heat-treated and the control ones. According to these findings, we propose that the increased HSP90 and HSF4 levels could eliminate somehow the effect of heat stress, but further analysis is needed to find the molecular pathways responsible for this effect.
5. Conclusions
The average global temperature has increased over the century. Heat-shock proteins and heat-shock factors play an essential role in normal cellular physiology and protection against different stressors, including heat stress. In chickens, HSP and HSF levels are increased in almost all the tissues in response to heat stress. This increased HSP level protects cellular proteins from heat-stress induced damage. We found that the post-hatch (24 h after hatching) heat manipulation had an influence mainly on the female reproductive parameters, while in adult animals, we found significant difference in heat-stress protein and heat-shock factor expressions in both genders. These are indeed the first hypotheses and only future experiments of genetic modifications of these actors will be able to validate these hypotheses. With the difficulty of generating animals in a reasonable time scale.
The search for genetic variants (SNP for example) more particularly in these genes would also be an approach to be pursued in order to try to correlate the character with the impact of the genes. Our findings show a significant effect on egg production but not on the sperm quality after post-hatch heat treatment. The egg production is more complex, longer and energy intense process than spermiogenesis and that could be one of the reasons why we could not find any difference in the sperm quality between the control and heat-stressed group. The found significant differences might be related to the increased expression level of HSP90 and HSF4 in heat-treated female chicks.
Supplementary Materials
The following information are available online at https://www.mdpi.com/article/10.3390/ani11061575/s1. Table S1: A.: The number of chickens in Experiment I. The egg production and sperm analysis experiments in case of heat treated and heat stressed (HTHS) and the control which was only heat stressed (HS) group. B: Summarized the pooled (B1) and individual (B2) heat-treated (HT) and control (Ctrl) RNA samples from Experiment II. Table S2: Information about the used primer pairs and target genes. In case of heat-shock markers (a) the applied primers were chose from the article of Xie et al. [11]. For sex determination, (b) the applied CHD1 primer-pair was chosen from the paper of Lee et al. [53]. In every case during the real-time PCR examination the GAPDH and ß ACTIN were the housekeeping genes. Figure S1: A.: The figure presents the expression of two housekeeping genes (GAPDH and ß-Actin). We compared the average Ct values of GAPDH and ß-Actin in control and heat-treated samples. We found lower Ct values in the case of GAPDH, and the standard deviation were higher using ß-Actin. B.: There was no significant difference between the control and heat-treated samples (p = 0.523) comparing the average GAPDH Ct values. We used chicken embryonic fibroblast as the reference sample. Figure S2: This figure represents the similarity between the individual and pooled delta Ct values measured in female gonads. A: Average values and standard deviations of individual samples are demonstrated. B: Delta Ct values measured in pooled samples. (Ch-Ctrl: Chick-control; Ch-HT: Chick-heat-treated; A-Ctrl: Adult-control; A-HT: Adult-heat-treated). References [11,53] are cited in the Supplementary Materials.
Author Contributions
The experiments designed by E.G. and K.L. The experiments were performed by R.T., N.T.S., B.L., K.B., K.L., B.V. and J.B. Statistical analysis was done by B.L. and E.G. The paper was written by R.T. and E.G. and was revised E.P.V., K.L., B.V. and B.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by VEKOP-2.3.2-16-2016-00012, TUDFO/51757-1/2019-ITM, TKP2020-NKA-24 and 2019-2.1.11-TÉT-2019-00036.
Institutional Review Board Statement
All applied methods in NBGK-HGI were approved by the Directorate of Food Safety and Animal Health of the Government Office of Pest County (License number: PE/EA197-4/2016) and by the Institutional Ethical Review Board.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We thank the animal keepers of National Centre for Biodiversity and Gene Conservation for the egg collection and the housing of poultry.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Babinszky, L.; Halas, V.; Verstegen, M.W. Impacts of Climate Change on Animal Production and Quality of Animal Food Products. In Climate Change Socioeconomic Effects; IntechOpen: London, UK, 2011. [Google Scholar] [CrossRef]
- Araya, A.; Prasad, P.; Zambreski, Z.; Gowda, P.; Ciampitti, I.; Assefa, Y.; Girma, A. Spatial analysis of the impact of climate change factors and adaptation strategies on productivity of wheat in Ethiopia. Sci. Total. Environ. 2020, 731, 139094. [Google Scholar] [CrossRef] [PubMed]
- Grünig, M.; Mazzi, D.; Calanca, P.; Karger, D.N.; Pellissier, L. Crop and forest pest metawebs shift towards increased linkage and suitability overlap under climate change. Commun. Biol. 2020, 3, 1–10. [Google Scholar] [CrossRef]
- Wolfenson, D.; Lew, B.; Thatcher, W.; Graber, Y.; Meidan, R. Seasonal and acute heat stress effects on steroid production by dominant follicles in cows. Anim. Reprod. Sci. 1997, 47, 9–19. [Google Scholar] [CrossRef]
- Rath, P.; Behura, N.; Sahoo, S.; Panda, P.; Mandal, K.; Panigrahi, P. Amelioration of Heat Stress for Poultry Welfare: A Strategic Approach. Int. J. Livest. Res. 2015, 5, 1–9. [Google Scholar] [CrossRef]
- Mashaly, M.M.; Hendricks, G.L.; Kalama, M.A.; Gehad, A.E.; Abbas, A.O.; Patterson, P.H. Effect of Heat Stress on Production Parameters and Immune Responses of Commercial Laying Hens. Poult. Sci. 2004, 83, 889–894. [Google Scholar] [CrossRef]
- Cheng, C.-Y.; Tu, W.-L.; Wang, S.-H.; Tang, P.-C.; Chen, C.-F.; Chen, H.-H.; Lee, Y.-P.; Chen, S.-E.; Huang, S.-Y. Annotation of Differential Gene Expression in Small Yellow Follicles of a Broiler-Type Strain of Taiwan Country Chickens in Response to Acute Heat Stress. PLoS ONE 2015, 10, e0143418. [Google Scholar] [CrossRef]
- Murugesan, S.; Ullengala, R.; Amirthalingam, V. Heat shock protein and thermal stress in chicken. In Heat Shock Proteins in Veterinary Medicine and Sciences; Springer Science and Business Media LLC: Berlin, Germany, 2018; pp. 179–193. ISBN 9783319733777. [Google Scholar]
- Xie, J.; Tang, L.; Lu, L.; Zhang, L.; Lin, X.; Liu, H.-C.; Odle, J.; Luo, X. Effects of acute and chronic heat stress on plasma metabolites, hormones and oxidant status in restrictedly fed broiler breeders. Poult. Sci. 2015, 94, 1635–1644. [Google Scholar] [CrossRef]
- Zhang, W.W.; Kong, L.N.; Zhang, X.Q.; Luo, Q.B. Alteration of HSF3 and HSP70 mRNA expression in the tissues of two chicken breeds during acute heat stress. Genet. Mol. Res. 2014, 13, 9787–9794. [Google Scholar] [CrossRef]
- Xie, J.; Tang, L.; Lu, L.; Zhang, L.; Xi, L.; Liu, H.-C.; Odle, J.; Luo, X. Differential Expression of Heat Shock Transcription Factors and Heat Shock Proteins after Acute and Chronic Heat Stress in Laying Chickens (Gallus gallus). PLoS ONE 2014, 9, e102204. [Google Scholar] [CrossRef]
- Cedraz, H.; Gromboni, J.G.G.; Garcia, A.A.P.; Filho, R.V.F.; Souza, T.M.; De Oliveira, E.R.; De Oliveira, E.B.; Nascimento, C.S.D.; Meneghetti, C.; Wenceslau, A.A. Heat stress induces expression of HSP genes in genetically divergent chickens. PLoS ONE 2017, 12, e0186083. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Saelao, P.; Chanthavixay, K.; Gallardo, R.; Bunn, D.; Lamont, S.J.; Dekkers, J.M.; Kelly, T.; Zhou, H. Physiological responses to heat stress in two genetically distinct chicken inbred lines. Poult. Sci. 2018, 97, 770–780. [Google Scholar] [CrossRef] [PubMed]
- Akbarian, A.; Michiels, J.; DeGroote, J.; Majdeddin, M.; Golian, A.; De Smet, S. Association between heat stress and oxidative stress in poultry; mitochondrial dysfunction and dietary interventions with phytochemicals. J. Anim. Sci. Biotechnol. 2016, 7, 1–14. [Google Scholar] [CrossRef]
- Rozenboim, I.; Tako, E.; Gal-Garber, O.; Proudman, J.A.; Uni, Z. The Effect of Heat Stress on Ovarian Function of Laying Hens. Poult. Sci. 2007, 86, 1760–1765. [Google Scholar] [CrossRef] [PubMed]
- Al-Rukibat, R.K.; Al-Zghoul, M.B.; Hananeh, W.M.; Al-Natour, M.Q.; Abu-Basha, E.A. Thermal manipulation during late embryogenesis: Effect on body weight and temperature, thyroid hormones, and differential white blood cell counts in broiler chickens. Poult. Sci. 2017, 96, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Walstra, I.; Napel, J.T.; Kemp, B.; Brand, H.V.D. Temperature manipulation during layer chick embryogenesis. Poult. Sci. 2010, 89, 1502–1508. [Google Scholar] [CrossRef]
- Al-Zghoul, M.B.; El-Bahr, S.M. Thermal manipulation of the broilers embryos: Expression of muscle markers genes and weights of body and internal organs during embryonic and post-hatch days. BMC Veter- Res. 2019, 15, 166. [Google Scholar] [CrossRef]
- Vinoth, A.; Thirunalasundari, T.; Shanmugam, M.; Uthrakumar, A.; Suji, S.; Rajkumar, U. Evaluation of DNA methylation and mRNA expression of heat shock proteins in thermal manipulated chicken. Cell Stress Chaperon. 2017, 23, 235–252. [Google Scholar] [CrossRef] [PubMed]
- Geraert, P.A.; Guillaumin, S.; Leclercq, B. Are genetically lean broilers more resistant to hot climate? Br. Poult. Sci. 1993, 34, 643–653. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, U.; Reddy, M.R.; Rao, S.V.R.; Radhika, K.; Shanmugam, M. Evaluation of Growth, Carcass, Immune Response and Stress Parameters in Naked Neck Chicken and Their Normal Siblings under Tropical Winter and Summer Temperatures. Asian-Australasian J. Anim. Sci. 2011, 24, 509–516. [Google Scholar] [CrossRef]
- Goel, A.; Ncho, C.M.; Choi, Y.-H. Regulation of gene expression in chickens by heat stress. J. Anim. Sci. Biotechnol. 2021, 12, 1–13. [Google Scholar] [CrossRef]
- Perini, F.; Cendron, F.; Rovelli, G.; Castellini, C.; Cassandro, M.; Lasagna, E. Emerging Genetic Tools to Investigate Molecular Pathways Related to Heat Stress in Chickens: A Review. Animals 2020, 11, 46. [Google Scholar] [CrossRef]
- Feder, M.E.; Hofmann, G.E. Heat-shock proteins, molecular chaperons, and the stress response: Evolutionary and Ecological Physiology. Annu. Rev. Physiol. 1999, 61, 243–282. [Google Scholar] [CrossRef]
- Genest, O.; Wickner, S.; Doyle, S.M. Hsp90 and Hsp70 chaperones: Collaborators in protein remodeling. J. Biol. Chem. 2019, 294, 2109–2120. [Google Scholar] [CrossRef]
- Li, G.C.; Mak, J.Y. Re-induction of hsp70 synthesis: An assay for thermotolerance. Int. J. Hyperth. 2009, 25, 249–257. [Google Scholar] [CrossRef]
- Johnson, J.L. Evolution and function of diverse Hsp90 homologs and cochaperone proteins. Biochim. Biophys. Acta (BBA) Bioenerg. 2012, 1823, 607–613. [Google Scholar] [CrossRef]
- Stankiewicz, M.; Mayer, M.P. The universe of Hsp90. Biomol. Concepts 2012, 3, 79–97. [Google Scholar] [CrossRef]
- Taipale, M.; Jarosz, D.F.; Lindquist, S. HSP90 at the hub of protein homeostasis: Emerging mechanistic insights. Nat. Rev. Mol. Cell Biol. 2010, 11, 515–528. [Google Scholar] [CrossRef] [PubMed]
- Hoekstra, K.A.; Iwama, G.K.; Nichols, C.R.; Godin, D.V.; Cheng, K.M. Increased Heat Shock Protein Expression after Stress in Japanese Quail. Stress 1998, 2, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Saju, J.M.; Hossain, M.S.; Liew, W.C.; Pradhan, A.; Thevasagayam, N.M.; Tan, L.S.E.; Anand, A.; Olsson, P.-E.; Orbán, L. Heat Shock Factor 5 Is Essential for Spermatogenesis in Zebrafish. Cell Rep. 2018, 25, 3252–3261. [Google Scholar] [CrossRef] [PubMed]
- Rabergh, C.; Airaksinen, S.; Soitamo, A.; Bjorklund, H.; Johansson, T.; Nikinmaa, M.; Sistonen, L. Tissue-specific expression of zebrafish (Danio rerio) heat shock factor 1 mRNAs in response to heat stress. J. Exp. Biol. 2000, 203, 1817–1824. [Google Scholar] [CrossRef]
- Nakai, A.; I Morimoto, R. Characterization of a novel chicken heat shock transcription factor, heat shock factor 3, suggests a new regulatory pathway. Mol. Cell. Biol. 1993, 13, 1983–1997. [Google Scholar] [CrossRef]
- Fujimoto, M.; Nakai, A. The heat shock factor family and adaptation to proteotoxic stress. FEBS J. 2010, 277, 4112–4125. [Google Scholar] [CrossRef] [PubMed]
- Melesse, A.; Maak, S.; Schmidt, R.; Von Lengerken, G. Effect of long-term heat stress on some performance traits and plasma enzyme activities in Naked-neck chickens and their F1 crosses with commercial layer breeds. Livest. Sci. 2011, 141, 227–231. [Google Scholar] [CrossRef]
- Burrows, W.; Quinn, J. The Collection of Spermatozoa from the Domestic Fowl and Turkey. Poult. Sci. 1937, 16, 19–24. [Google Scholar] [CrossRef]
- Váradi, É.; Drobnyák, Á.; Végi, B.; Liptói, K.; Kiss, C.; Barna, J. Cryopreservation of gander semen in cryovials – Comparative study. Acta Vet. Hung. 2019, 67, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Borowska, D.; Rothwell, L.; Bailey, R.; Watson, K.; Kaiser, P. Identification of stable reference genes for quantitative PCR in cells derived from chicken lymphoid organs. Vet. Immunol. Immunopathol. 2016, 170, 20–24. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, Y.-Y.; Huang, Y.-Q.; Fan, Q.; Lu, X.-T.; Wang, C.-K. Selection of housekeeping genes for quantitative gene expression analysis in yellow-feathered broilers. Ital. J. Anim. Sci. 2017, 17, 540–546. [Google Scholar] [CrossRef]
- El-Tarabany, M.S. Effect of thermal stress on fertility and egg quality of Japanese quail. J. Therm. Biol. 2016, 61, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Laine, V.N.; Verhagen, I.; Mateman, A.C.; Pijl, A.; Williams, T.D.; Gienapp, P.; Van Oers, K.; Visser, M.E. Exploration of tissue-specific gene expression patterns underlying timing of breeding in contrasting temperature environments in a song bird. BMC Genom. 2019, 20, 1–16. [Google Scholar] [CrossRef]
- Végi, B.; Váradi, É.; Szabó, Zs.; Ferencziné, Sz.Zs.; Molnár Kőrösiné, M.A.; Barna, J. A hőkezelés hatása hímivarú baromfifélék spermatológiai mutatóira. AWETH 2008, 4, 401–408. (In Hungarian) [Google Scholar]
- Yahav, S.; Luger, D.; Cahaner, A.; Dotan, M.; Rusal, M.; Hurwitz, S. Thermoregulation in naked neck chickens subjected to different ambient temperatures. Br. Poult. Sci. 1998, 39, 133–138. [Google Scholar] [CrossRef]
- Mezquita, J.; Mezquita, B.; Durfort, M.; Mezquita, C. Constitutive and heat-shock induced expression of Hsp70 mRNA during chicken testicular development and regression. J. Cell. Biochem. 2001, 82, 480–490. [Google Scholar] [CrossRef] [PubMed]
- Tarkhan, A.H.; Saleh, K.M.M.; Al-Zghoul, M.B. HSF3 and Hsp70 Expression during Post-Hatch Cold Stress in Broiler Chickens Subjected to Embryonic Thermal Manipulation. Vet. Sci. 2020, 7, 49. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Yu, J.; Bao, E. Expression of heat shock protein 90 (Hsp90) and transcription of its corresponding mRNA in broilers exposed to high temperature. Br. Poult. Sci. 2009, 50, 504–511. [Google Scholar] [CrossRef]
- Hao, Y.; Gu, X.H. Effects of heat shock protein 90 expression on pectoralis major oxidation in broilers exposed to acute heat stress. Poult. Sci. 2014, 93, 2709–2717. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-H.; Cheng, C.-Y.; Tang, P.-C.; Chen, C.-F.; Chen, H.-H.; Lee, Y.-P.; Huang, S.-Y. Acute Heat Stress Induces Differential Gene Expressions in the Testes of a Broiler-Type Strain of Taiwan Country Chickens. PLoS ONE 2015, 10, e0125816. [Google Scholar] [CrossRef]
- Wang, S.-H.; Cheng, C.-Y.; Chen, C.-J.; Chan, H.-L.; Chen, H.-H.; Tang, P.-C.; Chen, C.-F.; Lee, Y.-P.; Huang, S.-Y. Acute Heat Stress Changes Protein Expression in the Testes of a Broiler-Type Strain of Taiwan Country Chickens. Anim. Biotechnol. 2019, 30, 129–145. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, F.; Hamasaki, S.; Hara, S.; Uchimura, T.; Shiraishi, E.; Osafune, N.; Takagi, H.; Yazawa, T.; Kamei, Y.; Kitano, T. Heat shock factor 1 protects germ cell proliferation during early ovarian differentiation in medaka. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Shinkawa, T.; Tan, K.; Fujimoto, M.; Hayashida, N.; Yamamoto, K.; Takaki, E.; Takii, R.; Prakasam, R.; Inouye, S.; Mezger, V.; et al. Heat shock factor 2 is required for maintaining proteostasis against febrile-range thermal stress and polyglutamine aggregation. Mol. Biol. Cell 2011, 22, 3571–3583. [Google Scholar] [CrossRef] [PubMed]
- Joutsen, J.; Da Silva, A.J.; Luoto, J.C.; Budzynski, M.A.; Nylund, A.S.; de Thonel, A.; Concordet, J.-P.; Mezger, V.; Sabéran-Djoneidi, D.; Henriksson, E.; et al. Heat Shock Factor 2 Protects against Proteotoxicity by Maintaining Cell-Cell Adhesion. Cell Rep. 2020, 30, 583–597. [Google Scholar] [CrossRef]
- Lee, J.C.-I.; Tsai, L.-C.; Hwa, P.-Y.; Chan, C.-L.; Huang, A.; Chin, S.-C.; Wang, L.-C.; Lin, J.-T.; Linacre, A.; Hsieh, H.-M. A novel strategy for avian species and gender identification using the CHD gene. Mol. Cell. Probes 2010, 24, 27–31. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).