Appeasing Pheromones against Bovine Respiratory Complex and Modulation of Immune Transcript Expressions
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Facilities
2.2. Animals
2.3. Batches Constitution and Pheromone Administration
2.4. Pheromone Administration
2.5. Clinical Assessments
2.6. Activity and Behavioral Observations
2.7. Zootechnical Performances
2.8. Immune Gene Expression Analysis Using Reverse-Transcription Quantitative Polymerase Chain Reaction
2.9. Statistical Analysis
3. Results
3.1. Clinical Signs
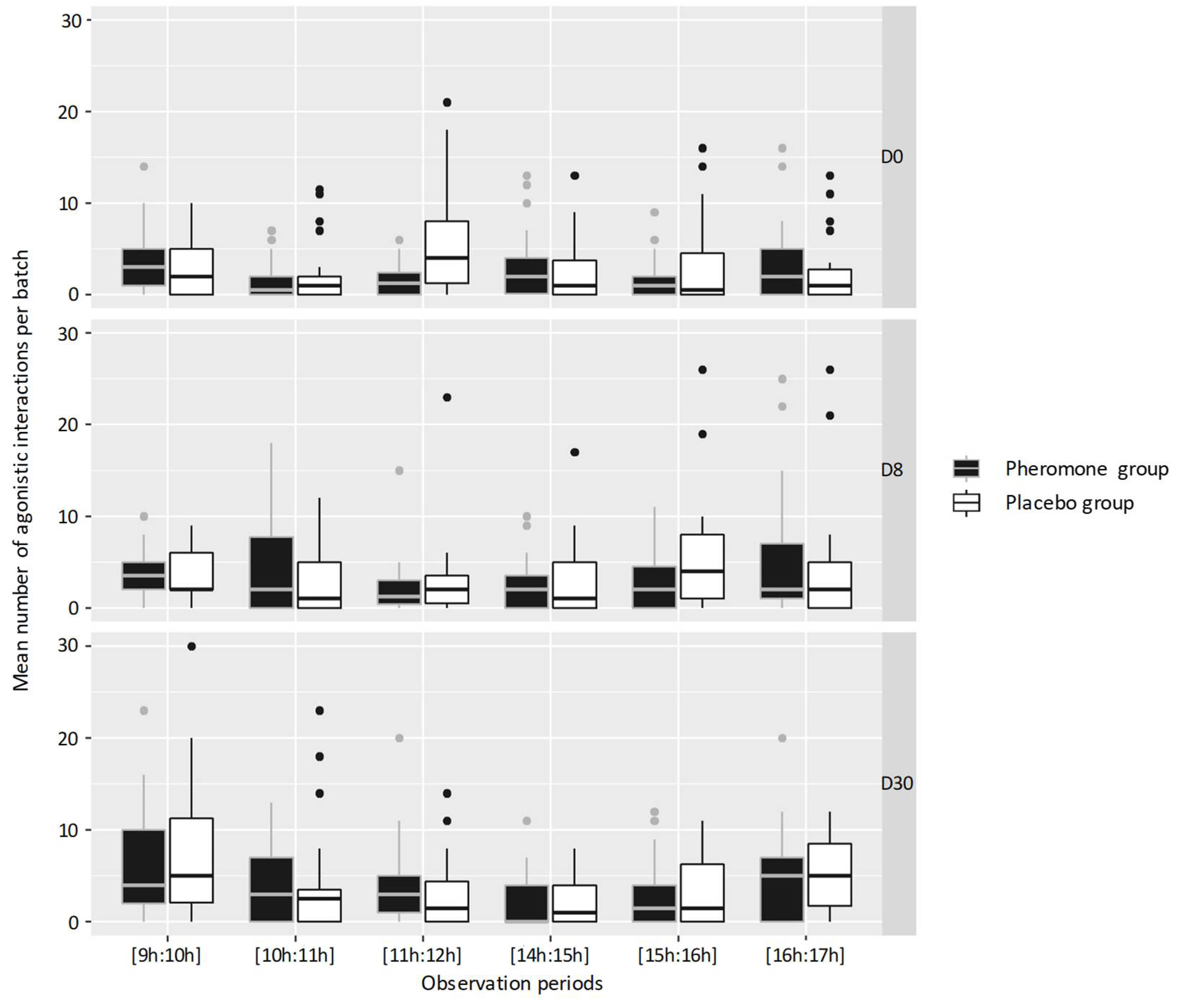
3.2. Activities and Behaviors
3.3. Average Daily Gain
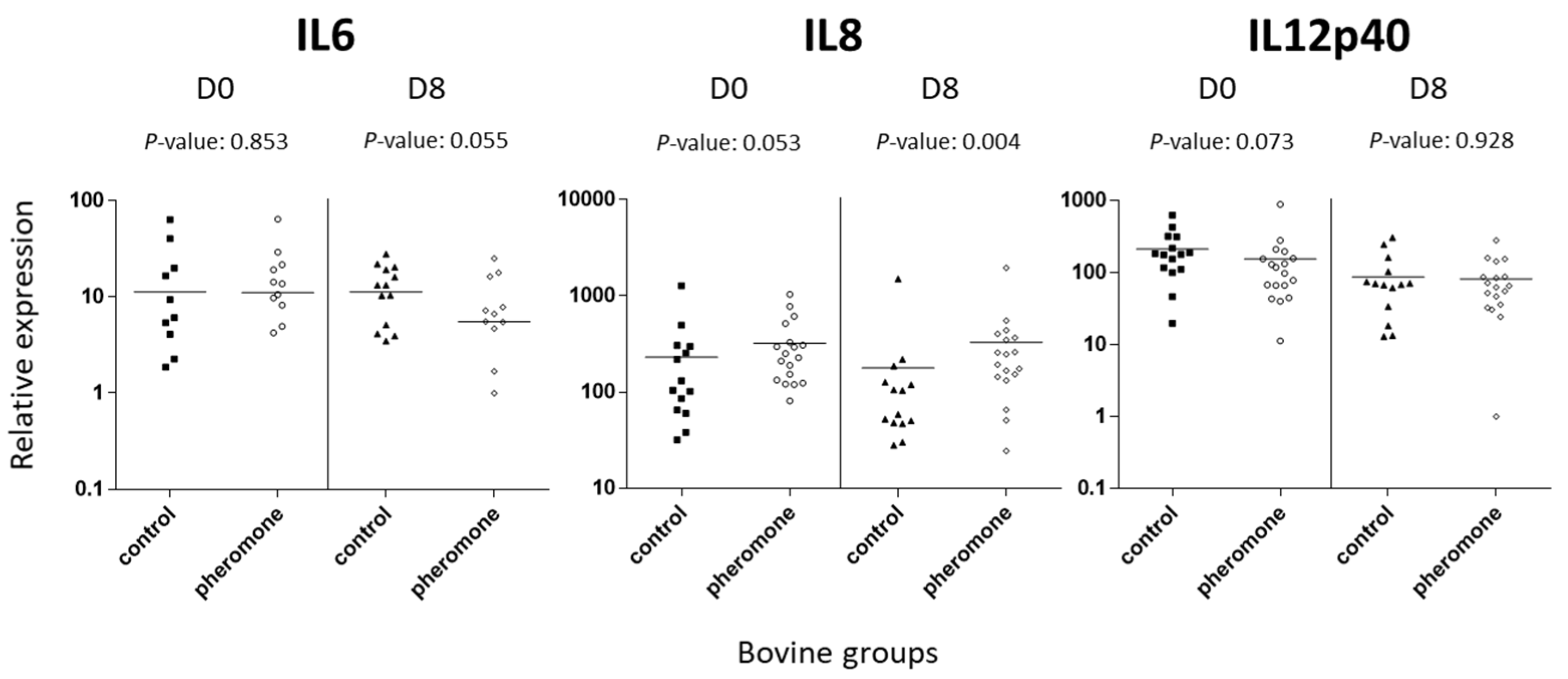
3.4. Assessment of Immune Transcript Expression by Quantitative Polymerase Chain Reaction
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Assié, S.; Seegers, H.; Makoschey, B.; Désiré-Bousquié, L.; Bareille, N. Exposure to Pathogens and Incidence of Respiratory Disease in Young Bulls on Their Arrival at Fattening Operations in France. Vet. Rec. 2009, 165, 195–199. [Google Scholar] [CrossRef]
- Bell, R.L.; Turkington, H.L.; Cosby, S.L. The Bacterial and Viral Agents of BRDC: Immune Evasion and Vaccine Developments. Vaccines 2021, 9, 337. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J.A. The Immunology of the Bovine Respiratory Disease Complex. Vet. Clin. N. Am. Food Anim. Pract. 2001, 17, 535–550. [Google Scholar] [CrossRef]
- Ellis, J.A. Update on Viral Pathogenesis in BRD. Anim. Health Res. Rev. 2009, 10, 149–153. [Google Scholar] [CrossRef]
- Cooke, R.F. Invited Paper: Nutritional and Management Considerations for Beef Cattle Experiencing Stress-Induced Inflammation. This article was based on a presentation at the ARPAS Symposium “Understanding Inflammation and Inflammatory Biomarkers to Improve Animal Performance” at the 2016 Joint Annual Meeting, July 19–23, 2016, Salt Lake City, Utah. Prof. Anim. Sci. 2017, 33, 1–11. [Google Scholar] [CrossRef]
- McMullen, C.; Orsel, K.; Alexander, T.W.; van der Meer, F.; Plastow, G.; Timsit, E. Comparison of the Nasopharyngeal Bacterial Microbiota of Beef Calves Raised without the Use of Antimicrobials between Healthy Calves and Those Diagnosed with Bovine Respiratory Disease. Vet. Microbiol. 2019, 231, 56–62. [Google Scholar] [CrossRef]
- Watts, J.L.; Sweeney, M.T. Antimicrobial Resistance in Bovine Respiratory Disease Pathogens: Measures, Trends, and Impact on Efficacy. Vet. Clin. N. Am. Food Anim. Pract. 2010, 26, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Stanford, K.; Zaheer, R.; Klima, C.; McAllister, T.; Peters, D.; Niu, Y.D.; Ralston, B. Antimicrobial Resistance in Members of the Bacterial Bovine Respiratory Disease Complex Isolated from Lung Tissue of Cattle Mortalities Managed with or without the Use of Antimicrobials. Microorganisms 2020, 8, 288. [Google Scholar] [CrossRef]
- Carroll, J.A.; Forsberg, N.E. Influence of Stress and Nutrition on Cattle Immunity. Vet. Clin. N. Am. Food Anim. Pract. 2007, 23, 105–149. [Google Scholar] [CrossRef]
- Mounier, L.; Veissier, I.; Boissy, A. Behavior, Physiology, and Performance of Bulls Mixed at the Onset of Finishing to Form Uniform Body Weight Groups. J. Anim. Sci. 2005, 83, 1696–1704. [Google Scholar] [CrossRef]
- Aich, P.; Jalal, S.; Czuba, C.; Schatte, G.; Herzog, K.; Olson, D.J.H.; Ross, A.R.S.; Potter, A.A.; Babiuk, L.A.; Griebel, P. Comparative Approaches to the Investigation of Responses to Stress and Viral Infection in Cattle. OMICS 2007, 11, 413–434. [Google Scholar] [CrossRef]
- Hermann, G.; Tovar, C.A.; Beck, F.M.; Allen, C.; Sheridan, J.F. Restraint Stress Differentially Affects the Pathogenesis of an Experimental Influenza Viral Infection in Three Inbred Strains of Mice. J. Neuroimmunol. 1993, 47, 83–94. [Google Scholar] [CrossRef]
- Chirase, N.K.; Greene, L.W.; Purdy, C.W.; Loan, R.W.; Auvermann, B.W.; Parker, D.B.; Walborg, E.F.; Stevenson, D.E.; Xu, Y.; Klaunig, J.E. Effect of Transport Stress on Respiratory Disease, Serum Antioxidant Status, and Serum Concentrations of Lipid Peroxidation Biomarkers in Beef Cattle. Am. J. Vet. Res. 2004, 65, 860–864. [Google Scholar] [CrossRef]
- Mormede, P.; Soissons, J.; Bluthe, R.M.; Raoult, J.; Legarff, G.; Levieux, D.; Dantzer, R. Effect of Transportation on Blood Serum Composition, Disease Incidence, and Production Traits in Young Calves. Influence of the Journey Duration. Ann. Rech. Vet. 1982, 13, 369–384. [Google Scholar] [PubMed]
- Hickey, M.C.; Drennan, M.; Earley, B. The Effect of Abrupt Weaning of Suckler Calves on the Plasma Concentrations of Cortisol, Catecholamines, Leukocytes, Acute-Phase Proteins and in Vitro Interferon-Gamma Production. J. Anim. Sci. 2003, 81, 2847–2855. [Google Scholar] [CrossRef]
- Blecha, F.; Boyles, S.L.; Riley, J.G. Shipping Suppresses Lymphocyte Blastogenic Responses in Angus and Brahman X Angus Feeder Calves. J. Anim. Sci. 1984, 59, 576–583. [Google Scholar] [CrossRef]
- Sporer, K.R.B.; Xiao, L.; Tempelman, R.J.; Burton, J.L.; Earley, B.; Crowe, M.A. Transportation Stress Alters the Circulating Steroid Environment and Neutrophil Gene Expression in Beef Bulls. Vet. Immunol. Immunopathol. 2008, 121, 300–320. [Google Scholar] [CrossRef] [PubMed]
- Masset, N.; Meurens, F.; Marie, M.; Lesage, P.; Lehébel, A.; Brisseau, N.; Assié, S. Effectiveness of Two Intranasal Vaccines for the Control of Bovine Respiratory Disease in Newborn Beef Calves: A Randomized Non-Inferiority Multicentre Field Trial. Vet. J. 2020, 263, 105532. [Google Scholar] [CrossRef]
- Temple, D.; Barthélémy, H.; Mainau, E.; Cozzi, A.; Amat, M.; Canozzi, M.E.; Pageat, P.; Manteca, X. Preliminary Findings on the Effect of the Pig Appeasing Pheromone in a Slow Releasing Block on the Welfare of Pigs at Weaning. Porcine Health Manag. 2016, 2, 13. [Google Scholar] [CrossRef]
- Taylor, K.; Mills, D.S. A Placebo-Controlled Study to Investigate the Effect of Dog Appeasing Pheromone and Other Environmental and Management Factors on the Reports of Disturbance and House Soiling during the Night in Recently Adopted Puppies (Canis familiaris). Appl. Anim. Behav. Sci. 2007, 105, 358–368. [Google Scholar] [CrossRef][Green Version]
- Falewee, C.; Gaultier, E.; Lafont, C.; Bougrat, L.; Pageat, P. Effect of a Synthetic Equine Maternal Pheromone during a Controlled Fear-Eliciting Situation. Appl. Anim. Behav. Sci. 2006, 101, 144–153. [Google Scholar] [CrossRef]
- Osella, M.C.; Cozzi, A.; Spegis, C.; Turille, G.; Barmaz, A.; Lecuelle, C.L.; Teruel, E.; Bienboire-Frosini, C.; Chabaud, C.; Bougrat, L.; et al. The Effects of a Synthetic Analogue of the Bovine Appeasing Pheromone on Milk Yield and Composition in Valdostana Dairy Cows during the Move from Winter Housing to Confined Lowland Pastures. J. Dairy Res. 2018, 85, 174–177. [Google Scholar] [CrossRef]
- Pageat, P. Appeasing Pheromones to Decrease Stress, Anxiety and Aggressiveness 1998. European Patent EP 0948,963 A1; U.S. Patent 6,054,481; U.S. Patent 6,077,867; U.S. Patent 6,169,113 B1; Japan Patent 2000-528279; Japan Patent 980,298, 21 January 1998. [Google Scholar]
- Angeli, B.; Cappellozza, B.; Moraes Vasconcelos, J.L.; Cooke, R.F. Administering an Appeasing Substance to Gir × Holstein Female Dairy Calves on Pre-Weaning Performance and Disease Incidence. Animals 2020, 10, 1961. [Google Scholar] [CrossRef]
- Cooke, R.F.; Millican, A.; Brandão, A.P.; Schumaher, T.F.; de Sousa, O.A.; Castro, T.; Farias, R.S.; Cappellozza, B.I. Short Communication: Administering an Appeasing Substance to Bos Indicus-Influenced Beef Cattle at Weaning and Feedlot Entry. Animal 2020, 14, 566–569. [Google Scholar] [CrossRef] [PubMed]
- Colombo, E.A.; Cooke, R.F.; Brandão, A.P.; Wiegand, J.B.; Schubach, K.M.; Duff, G.C.; Gouvêa, V.N.; Cappellozza, B.I. Administering an Appeasing Substance to Optimize Performance and Health Responses in Feedlot Receiving Cattle. J. Anim. Sci. 2020, 98, 1–8. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Bougarn, S.; Cunha, P.; Gilbert, F.B.; Meurens, F.; Rainard, P. Technical Note: Validation of Candidate Reference Genes for Normalization of Quantitative PCR in Bovine Mammary Epithelial Cells Responding to Inflammatory Stimuli. J. Dairy Sci. 2011, 94, 2425–2430. [Google Scholar] [CrossRef]
- Shukla, S.K.; Shukla, S.; Chauhan, A.; Sarvjeet, N.; Khan, R.; Ahuja, A.; Singh, L.V.; Sharma, N.; Prakash, C.; Singh, A.V.; et al. Differential Gene Expression in Mycobacterium bovis Challenged Monocyte-Derived Macrophages of Cattle. Microb. Pathog. 2017, 113, 480–489. [Google Scholar] [CrossRef]
- Maeda, Y.; Ohtsuka, H.; Tomioka, M.; Oikawa, M. Effect of Progesterone on Th1/Th2/Th17 and Regulatory T Cell-Related Genes in Peripheral Blood Mononuclear Cells during Pregnancy in Cows. Vet. Res. Commun. 2013, 37, 43–49. [Google Scholar] [CrossRef]
- Martin, E.T.; Kuypers, J.; Wald, A.; Englund, J.A. Multiple versus Single Virus Respiratory Infections: Viral Load and Clinical Disease Severity in Hospitalized Children: Viral Coinfection in Children. Influenza Other Respir. Viruses 2012, 6, 71–77. [Google Scholar] [CrossRef]
- Whelehan, C.J.; Meade, K.G.; Eckersall, P.D.; Young, F.J.; O’Farrelly, C. Experimental Staphylococcus aureus Infection of the Mammary Gland Induces Region-Specific Changes in Innate Immune Gene Expression. Vet. Immunol. Immunopathol. 2011, 140, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, M.P.; Browne, J.A.; Doyle, M.B.; Fitzsimons, T.; McGill, K.; Gormley, E. IL-10 Suppression of IFN-γ Responses in Tuberculin-Stimulated Whole Blood from Mycobacterium bovis Infected Cattle. Vet. Immunol. Immunopathol. 2017, 189, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate Normalization of Real-Time Quantitative RT-PCR Data by Geometric Averaging of Multiple Internal Control Genes. Genome Biol. 2002, 3, RESEARCH0034. [Google Scholar] [CrossRef] [PubMed]
- McGill, J.L.; Sacco, R.E. The Immunology of Bovine Respiratory Disease. Vet. Clin. N. Am. Food Anim. Pract. 2020, 36, 333–348. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, S.R.; Carvalho, B.; González-Porta, M.; Rung, J.; Brazma, A.; Gustavo Gardinassi, L.; Ferreira, B.R.; Banin, T.M.; Veríssimo, C.J.; Katiki, L.M.; et al. Blood Transcriptome Profile Induced by an Efficacious Vaccine Formulated with Salivary Antigens from Cattle Ticks. NPJ Vaccines 2019, 4, 1–10. [Google Scholar] [CrossRef]
- Scott, M.A.; Woolums, A.R.; Swiderski, C.E.; Perkins, A.D.; Nanduri, B.; Smith, D.R.; Karisch, B.B.; Epperson, W.B.; Blanton, J.R. Whole Blood Transcriptomic Analysis of Beef Cattle at Arrival Identifies Potential Predictive Molecules and Mechanisms That Indicate Animals That Naturally Resist Bovine Respiratory Disease. PLoS ONE 2020, 15, 7507. [Google Scholar] [CrossRef]
- Matsushima, K.; Morishita, K.; Yoshimura, T.; Lavu, S.; Kobayashi, Y.; Lew, W.; Appella, E.; Kung, H.F.; Leonard, E.J.; Oppenheim, J.J. Molecular Cloning of a Human Monocyte-Derived Neutrophil Chemotactic Factor (MDNCF) and the Induction of MDNCF MRNA by Interleukin 1 and Tumor Necrosis Factor. J. Exp. Med. 1988, 167, 1883–1893. [Google Scholar] [CrossRef]
- Yoshimura, T.; Matsushima, K.; Tanaka, S.; Robinson, E.A.; Appella, E.; Oppenheim, J.J.; Leonard, E.J. Purification of a Human Monocyte-Derived Neutrophil Chemotactic Factor That Has Peptide Sequence Similarity to Other Host Defense Cytokines. Proc. Natl. Acad. Sci. USA 1987, 84, 9233–9237. [Google Scholar] [CrossRef]
- Mukaida, N. Pathophysiological Roles of Interleukin-8/CXCL8 in Pulmonary Diseases. Am. J. Physiol. Lung Cell. Mol. Physiol. 2003, 284, L566–L577. [Google Scholar] [CrossRef]
- Mitchell, G.B.; Albright, B.N.; Caswell, J.L. Effect of Interleukin-8 and Granulocyte Colony-Stimulating Factor on Priming and Activation of Bovine Neutrophils. Infect. Immun. 2003, 71, 1643–1649. [Google Scholar] [CrossRef]
- Caswell, J.L.; Middleton, D.M.; Gordon, J.R. Production and Functional Characterization of Recombinant Bovine Interleukin-8 as a Specific Neutrophil Activator and Chemoattractant. Vet. Immunol. Immunopathol. 1999, 67, 327–340. [Google Scholar] [CrossRef]
- Caswell, J.L.; Middleton, D.M.; Sorden, S.D.; Gordon, J.R. Expression of the Neutrophil Chemoattractant Interleukin-8 in the Lesions of Bovine Pneumonic Pasteurellosis. Vet. Pathol. 1998, 35, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Wessely-Szponder, J. The Influence of TNFalpha and IL-8 on Secretory Action of Neutrophils Isolated from Heifers in the Course of Bovine Respiratory Disease. Acta Vet. Hung. 2008, 56, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Johansson, C.; Kirsebom, F.C.M. Neutrophils in respiratory viral infections. Mucosal Immunol. 2021. [Google Scholar] [CrossRef]
- Zinicola, M.; Bicalho, M.L.S.; Santin, T.; Marques, E.C.; Bisinotto, R.S.; Bicalho, R.C. Effects of Recombinant Bovine Interleukin-8 (RbIL-8) Treatment on Health, Metabolism, and Lactation Performance in Holstein Cattle II: Postpartum Uterine Health, Ketosis, and Milk Production. J. Dairy Sci. 2019, 102, 10316–10328. [Google Scholar] [CrossRef] [PubMed]
| YB 1 | Age | Body Weight | Pheromone Group | Control Group | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Batches | YB | YB per Batches | Age | Body Weight | Batches | YB | YB per Batches | Age | Body Weight | |||||
| Fattening unit | 1 | 60 | 338 (±51) | 391 (±27) | 3 | 36 | 12 | 343 (±55) | 391 (±31) | 2 | 24 | 12 | 335 (±48) | 390 (±21) |
| 2 | 90 | 324 (±43) | 377 (±22) | 4 | 60 | 15 | 319 (±45) | 377 (±25) | 2 | 30 | 15 | 328 (±37) | 378 (±14) | |
| 3 | 60 | 338 (±47) | 371 (±25) | 2 | 30 | 15 | 339 (±52) | 382 (±26) | 2 | 30 | 15 | 337 (±39) | 361 (±18) | |
| 4 | 55 | 261 (±49) | 323 (±20) | 5 | 33 | 3 × 6 1 × 5 1 × 10 | 246 (±52) | 316 (±15) | 3 | 22 | 1 × 10 2 × 6 | 284 (±37) | 336 (±17) | |
| Total | 265 | 320 (±54) | 370 (±32) | 14 | 159 | 312 (±58) | 372 (±36) | 9 | 106 | 325 (±45) | 368 (±26) | |||
| Activity, Behavior and Stereotypy | Description | ||
|---|---|---|---|
| Activity | Ruminating | Chewing regurgitated boluses of feed | |
| Eating feed at the feeding trough | Eating and masticating at the feeding trough | ||
| Lying | Lying down in any resting position | ||
| Standing idling | Standing | ||
| Behavior | Agonistic | Fighting | Engaging in headbutts |
| Escaping | One young bull escaping from another hostile young bull | ||
| Threatening | One young bull has a hostile behavior but no contact is made | ||
| Non-agonistic | Chin-resting | One young bull places its chin on another young bull | |
| Grooming | One young bull licks another | ||
| Sniffing | Sniffing another young bull | ||
| Social rubbing | Rubbing another young bull | ||
| Stereotypy | Licking | Licking any equipment | |
| Rubbing | Rubbing repetitively own body against any equipment | ||
| Tongue-rolling | Twisting and twirling the tongue, either inside or outside the open mouth, for at least 5 s | ||
| Primer Abbreviation and Full Names | Primer Sequences: Sense (S) and Anti-Sense (AS) | Amplicon Sizes (bp) | Annealing Temperatures (°C) | Accession Number or References | |
|---|---|---|---|---|---|
| REFERENCE GENES | ACTB | S: ACGGGCAGGTCATCACCATC | 166 | 67 | 28 |
| Beta actin | AS: AGCACCGTGTTGGCGTAGAG | ||||
| GADPH | S: GGCATCGTGGAGGGACTTATG | 186 | 62 | 28 | |
| Glyceraldehyde-3-phosphate dehydrogenase | AS: GCCAGTGAGCTTCCCGTTGAG | ||||
| CYTOKINES | IL12p40 | S: CACCAGCAGCTTCTTCATCA | 105 | 60 | 33 |
| Interleukin 12 subunit p40 | AS: TACTCCCAGCTGACCTCCAC | ||||
| IL4 | S: GCCACACGTGCTTGAACAAA | 63 | 60 | 30 | |
| Interleukin 4 | AS: TCTCAACAGCTTGGCAAGCA | ||||
| IL6 | S: TAAGCGCATGGTCGACAAAA | 150 | 60 | 32 | |
| Interleukin 6 | AS: TTGAACCCAGATTGGAAGCAT | ||||
| IL8 (CXCL8) | S: AGAACTTCGATGCCAATGCAT | 150 | 60 | NM_173925 | |
| Interleukin 8 | AS: GGGTTTAGGCAGACCTCGTTT | ||||
| IL17A | S: TCGTTAACCGGAGCACAAACT | 120 | 60 | 32 | |
| Interleukin 17A | AS: TGGCCTCCCAGATCACAGA | ||||
| IL10 | S: AGAACCACGGGCCTGACA | 121 | 60 | 32 | |
| Interleukin 10 | AS: ACCGCCTTGCTCTTGTTTTC | ||||
| TGFß | S: TGCTTCAGCTCCACAGAAAAGA | 116 | 60 | 32 | |
| Transforming growth factor ß | AS: AGGCAGAAATTGGCGTGGT | ||||
| IFNɣ | S: TTGAATGGCAGCTCTGAGAAAC | 150 | 60 | 32 | |
| Interferon ɣ | AS: TCTCTTCCGCTTTCTGAGGTTAGA | ||||
| CHEMOKINES | CXCL6 | S: GAGAGCTGCGTTGTGTGTGT | 107 | 60 | 29 |
| Chemokine (C-X-C motif) ligand 6 | AS: ACTTCCACCTTGGAGCACTG | ||||
| CCL20 | S: TTCGACTGCTGTCTCCGATA | 172 | 62 | 28 | |
| Chemokine (C-C motif) ligand 20 | AS: GCACAACTTGTTTCACCCACT | ||||
| TRANSCRIPTION FACTORS | FOXP3 | S: TGGTGCAATCTCTGGAGCAA | 116 | 60 | 30 |
| Forkhead box P3 | AS: GTCAGATGATGCCGCAGATG | ||||
| GATA-3 | S: CCAGACCAGAAACCGAAAAA | 234 | 62 | 31 | |
| Trans-acting T-cell-specific transcription factor GATA-3 | AS: ACCATACTGGAAGGGTGGTG | ||||
| RORɣ (RORC gene) | S: ACAGCCCTCGTCCTCATCAATGCC | 145 | 60 | 30 | |
| RAR-related orphan receptor gamma | AS: TGGGTGGCAGCTTTGCCAGGATA | ||||
| TBX21 | S: CGAGGACTATATACTGCCGC | 133 | 61 | 31 | |
| AS: CAAGACCACGTCCACATACA | |||||
| Variables and Levels | Odds Ratio | p-Value | |||
|---|---|---|---|---|---|
| Estimate | 95% Confidence Interval | ||||
| Lower Bound | Upper BOUND | ||||
| Exposure to pheromone | Control | Reference | |||
| Pheromone | 0.48 | −0.20 | 1.10 | 0.84 | |
| Day of clinical examination | D8 | Reference | |||
| D30 | 0.19 | −0.07 | 0.45 | 0.08 | |
| Pheromone × Day of clinical examination | 8.20 | 2.32 | 31.90 | 0.001 | |
| Variables and Levels | Mean ADG 1 | p-Value | |||
|---|---|---|---|---|---|
| Estimate | 95% Confidence Interval | ||||
| Lower Bound | Upper Bound | ||||
| Intercept | 1.498 | ||||
| Exposure to pheromone | Control | 1.48 | 1.33 | 1.67 | 0.98 |
| Pheromone | 1.48 | 1.33 | 1.67 | ||
| Messenger RNAs | Levels of Expression (Controls) | D0 | D8 | ||||
|---|---|---|---|---|---|---|---|
| mRNA Relative Expressions ± SEM | Pheromone vs. Control | mRNA Relative Expressions ± SEM | Pheromone vs. Control | ||||
| Control (N = 15) | Pheromone (N = 18) | Control (N = 15) | Pheromone (N = 18) | ||||
| CCL20 | low | 13.87 ± 4.13 | 20.72 ± 4.84 | ns | 12.38 ± 1.79 | 18.24 ± 3.26 | ns |
| CXCL6 | moderate | 5.56 ± 1.64 | 14.77 ± 6.74 | ns | 5.91 ± 1.32 | 8.84 ± 2.18 | ns |
| FOXP3 | moderate | 6.3 ± 0.9 | 5.85 ± 0.77 | ns | 4.86 ± 0.71 | 4.39 ± 0.71 | ns |
| GATA-3 | high | 6.32 ± 0.92 | 8.15 ± 1.26 | ns | 4.72 ± 0.51 | 4.7 ± 0.62 | ns |
| IFNɣ | low | 18.44 ± 4.49 | 45.86 ± 16.27 | ns | 17.93 ± 3.92 | 37.83 ± 11.35 | ns |
| IL10 | moderate | 4.15 ± 0.70 | 4.57 ± 1.11 | ns | 2.68 ± 0.51 | 2.76 ± 0.25 | ns |
| IL12 p40 | moderate | 213.23 ± 40.59 | 155.05 ± 47.51 | 0.073 | 87.49 ± 22.68 | 82.28 ± 15.69 | ns |
| IL17A | low | 22.06 ± 10.57 | 34.94 ± 15.77 | ns | 57.53 ± 30.78 | 43.67 ± 14.51 | ns |
| IL4 | low | 6.28 ± 4.00 | 0.81 ± 0.45 | ns | 1.13 ± 0.71 | 3.34 ± 2.13 | ns |
| IL6 | low | 11.32 ± 4.68 | 11.15 ± 3.88 | ns | 11.33 ± 2.21 | 5.55 ± 1.78 | 0.055 |
| IL8 | moderate | 231.45 ± 82.35 | 321.85 ± 62.56 | 0.053 | 178.25 ± 95.58 | 330.61 ± 104.61 | 0.004 ** |
| RORɣ | moderate | 6.25 ± 0.86 | 5.32 ± 0.46 | ns | 4.86 ± 0.64 | 5.03 ± 0.64 | ns |
| TBX21 | moderate | 8.51 ± 1.47 | 9.33 ± 1.66 | ns | 4.78 ± 0.74 | 3.8 ± 0.60 | ns |
| TGFß | high | 3.36 ± 0.38 | 3.12 ± 0.32 | ns | 2.78 ± 0.37 | 3.32 ± 0.18 | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hervet, C.; Boullier, J.; Guiadeur, M.; Michel, L.; Brun-Lafleur, L.; Aupiais, A.; Zhu, J.; Mounaix, B.; Meurens, F.; Renois, F.; et al. Appeasing Pheromones against Bovine Respiratory Complex and Modulation of Immune Transcript Expressions. Animals 2021, 11, 1545. https://doi.org/10.3390/ani11061545
Hervet C, Boullier J, Guiadeur M, Michel L, Brun-Lafleur L, Aupiais A, Zhu J, Mounaix B, Meurens F, Renois F, et al. Appeasing Pheromones against Bovine Respiratory Complex and Modulation of Immune Transcript Expressions. Animals. 2021; 11(6):1545. https://doi.org/10.3390/ani11061545
Chicago/Turabian StyleHervet, Caroline, Justine Boullier, Marlène Guiadeur, Léa Michel, Laure Brun-Lafleur, Anne Aupiais, Jianzhong Zhu, Béatrice Mounaix, François Meurens, Fanny Renois, and et al. 2021. "Appeasing Pheromones against Bovine Respiratory Complex and Modulation of Immune Transcript Expressions" Animals 11, no. 6: 1545. https://doi.org/10.3390/ani11061545
APA StyleHervet, C., Boullier, J., Guiadeur, M., Michel, L., Brun-Lafleur, L., Aupiais, A., Zhu, J., Mounaix, B., Meurens, F., Renois, F., & Assié, S. (2021). Appeasing Pheromones against Bovine Respiratory Complex and Modulation of Immune Transcript Expressions. Animals, 11(6), 1545. https://doi.org/10.3390/ani11061545








