Aquaporins Are Differentially Regulated in Canine Cryptorchid Efferent Ductules and Epididymis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Tissue Collection
2.2. Immunohistochemistry
2.3. Western Blotting
2.4. Real-Time RT-PCR and Data Processing
2.5. Statistical Analysis
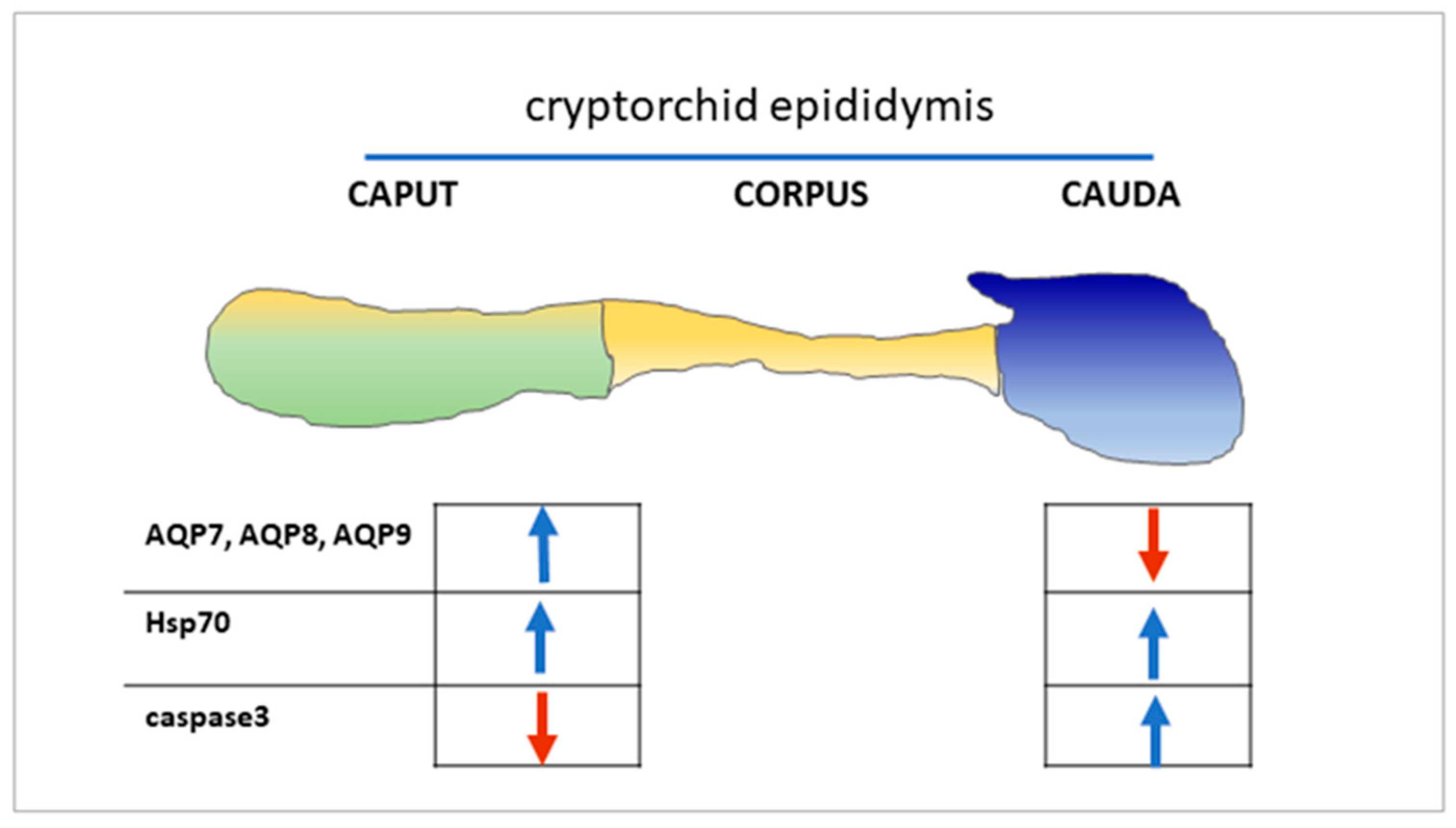
3. Results
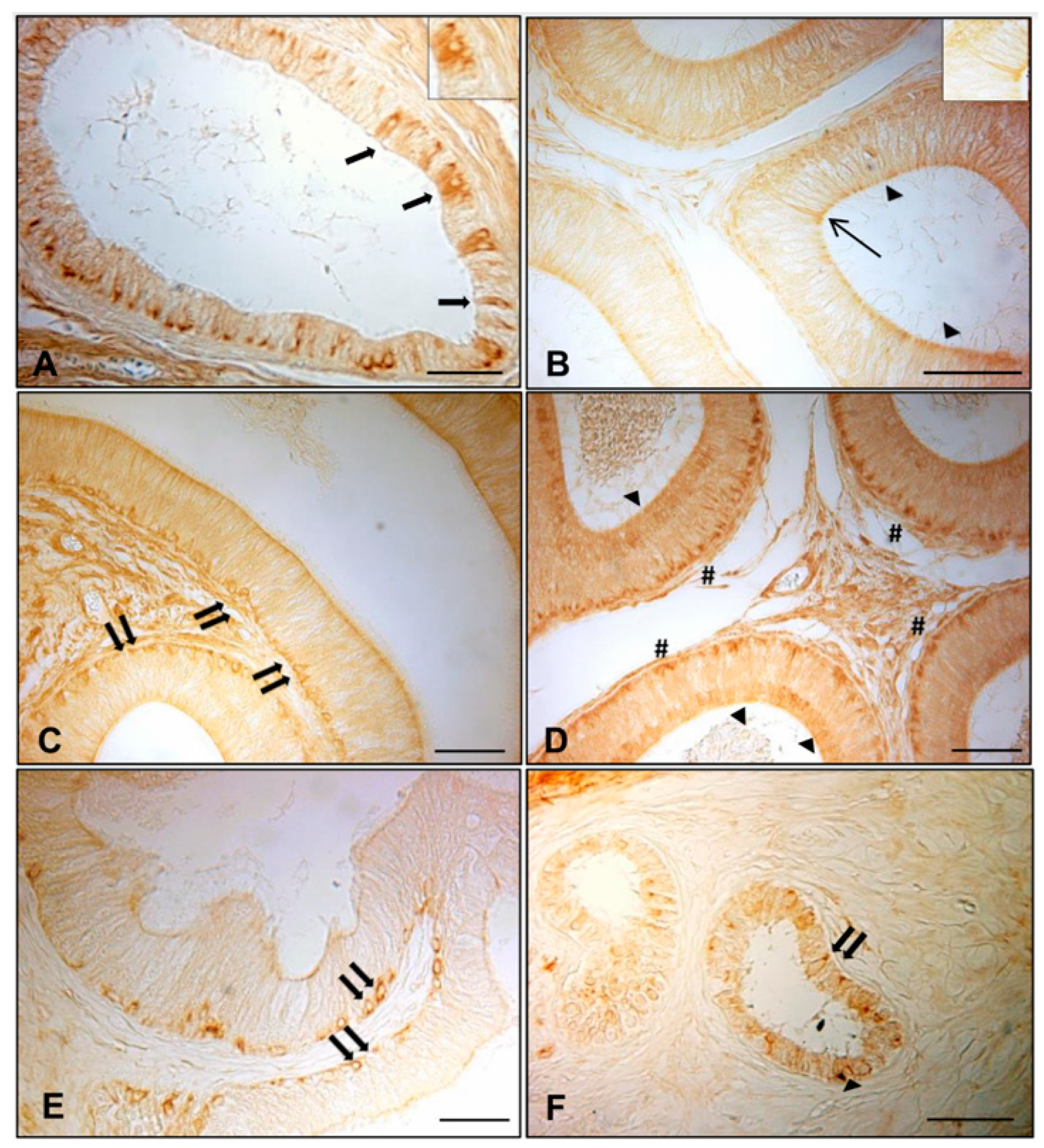
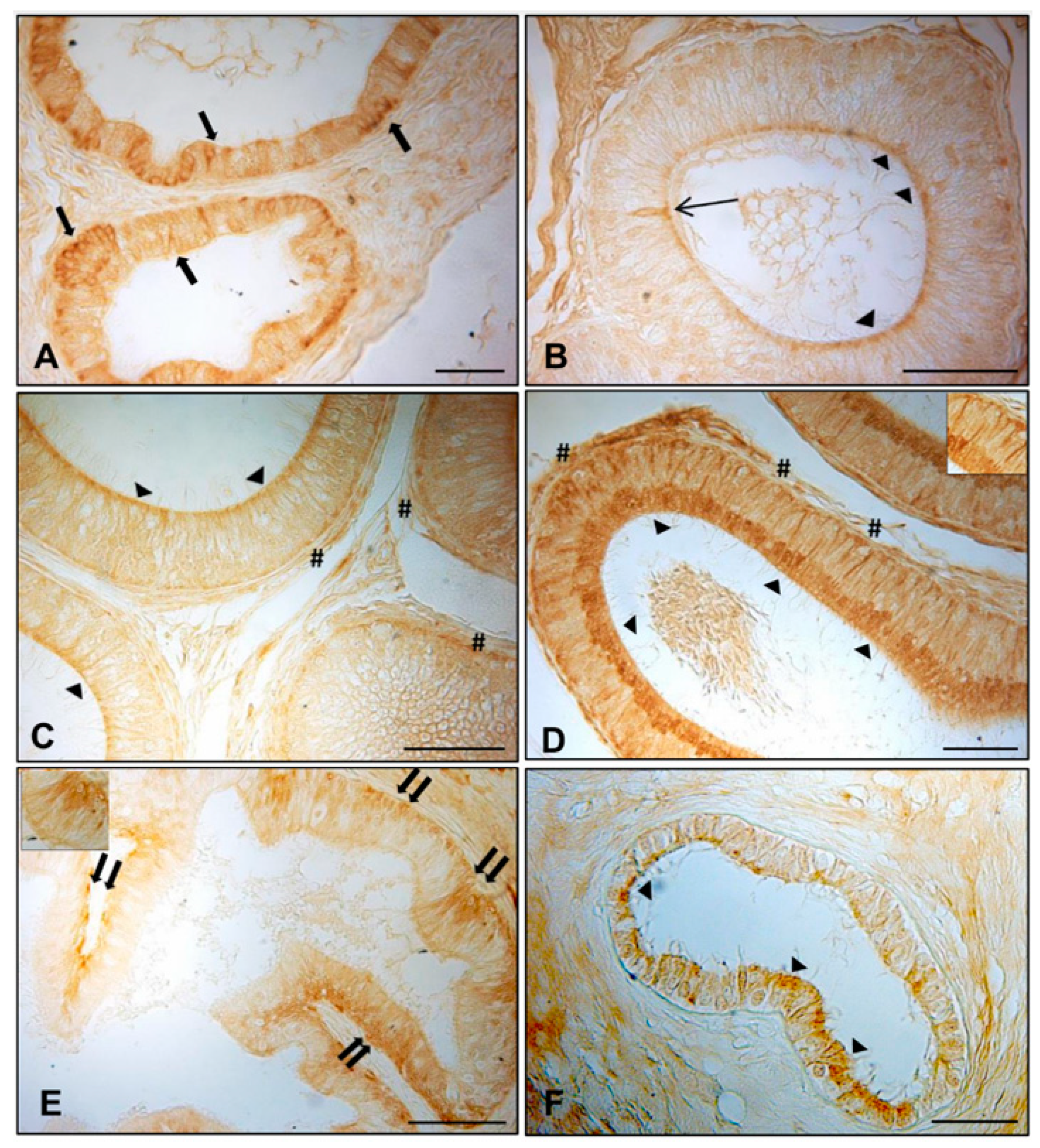
3.1. Immunohistochemistry of AQP7, AQP8, and AQP9 in Normal and Cryptorchid Canine Epididymis
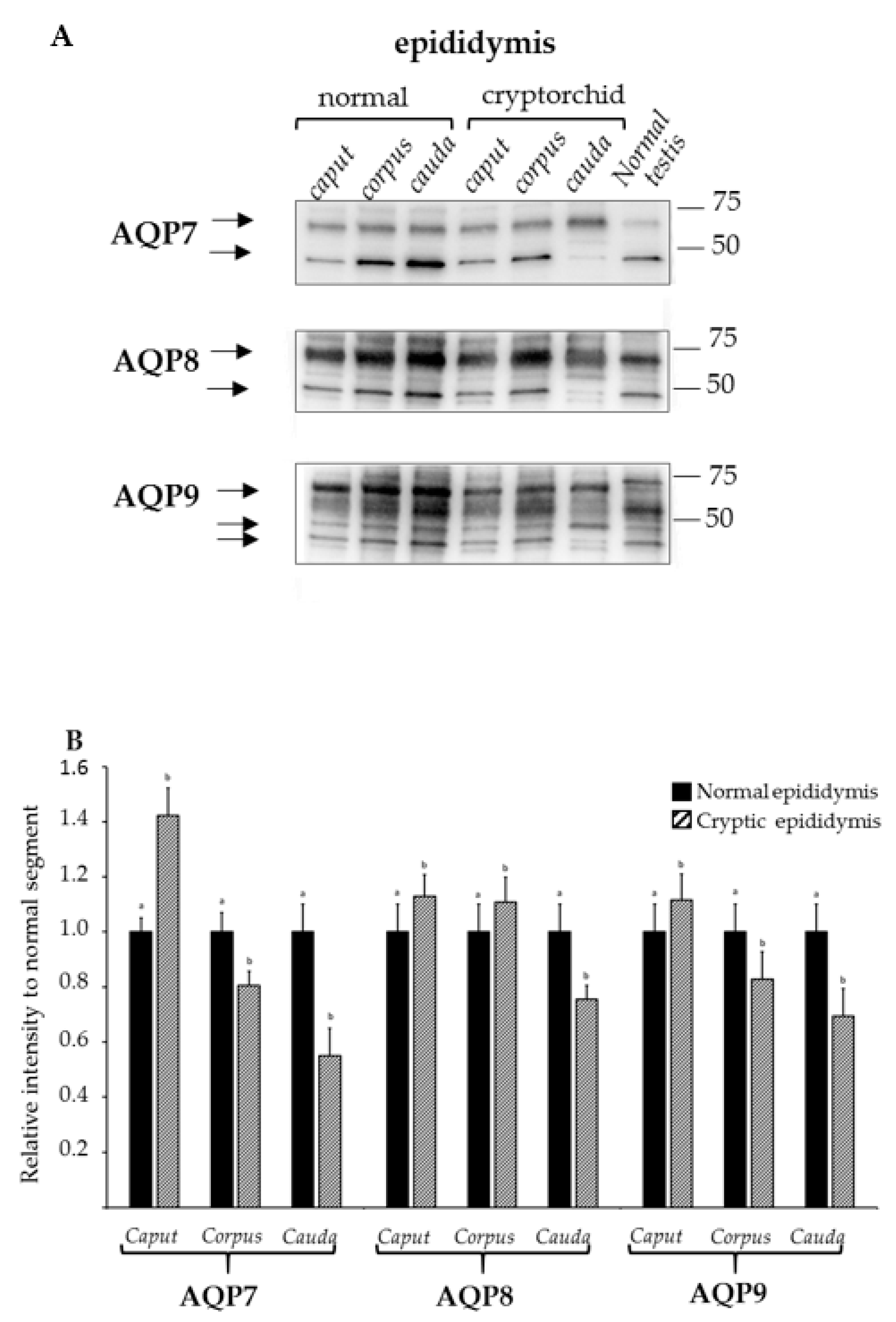
3.2. Analysis of Expression of AQP7, AQP8, and AQP9 in Normal and Cryptorchid Canine Epididymis by Western Blotting
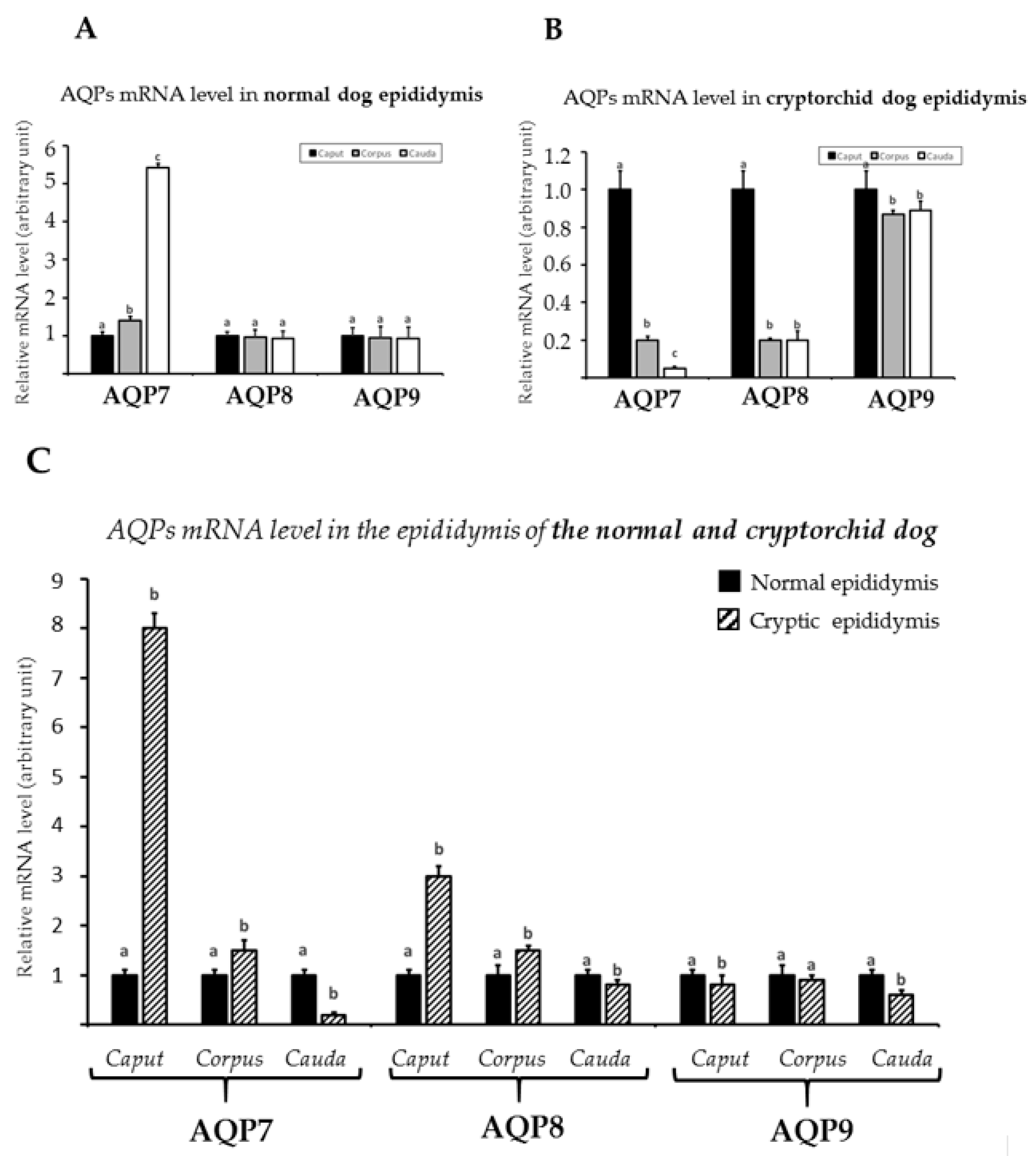
3.3. mRNA Expression of AQP7, AQP8, and AQP9 in Normal and Cryptorchid Canine Epididymis According to Real-Time RT-PCR
3.4. Western Blot Analysis of Hsp70 and Caspase-3 Expression in Normal and Cryptorchid Canine Epididymis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Temple-Smith, P.D.; Zheng, S.S.; Kadioglu, T.; Southwick, G.J. Development and use of surgical procedures to bypass selected regions of the mammalian epididymis: Effects on sperm maturation. J. Reprod. Fertil. Suppl. 1998, 53, 183–195. [Google Scholar] [PubMed]
- Foldesy, R.G.; Bedford, J.M. Biology of the scrotum 1. Temperature and androgen as determinants of the sperm storage capacity of the rat cauda epididymis. Biol. Reprod. 1982, 26, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, R.; Légaré, C.; Lamontagne-Proulx, J.; Breton, S.; Soulet, D. Revisiting structure/functions of the human epididymis. Andrology 2019, 7, 748–757. [Google Scholar] [CrossRef] [PubMed]
- Hemeida, N.A.; Sack, W.O.; McEntee, K. Ductuli efferentes in the epididymis of boar, goat, ram, bull, and stallion. Am. J. Vet. Res. 1978, 39, 1892–1900. [Google Scholar]
- Guttroff, R.F.; Cooke, P.S.; Hess, R.A. Blind-ending tubules and branching patterns of the rat ductuli efferentes. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 1992, 232, 423–431. [Google Scholar] [CrossRef]
- Hess, R.A.; Fernandes, S.A.F.; Gomes, G.R.D.O.; Oliveira, C.A.; Lazari, M.D.F.M.; Porto, C.S. Estrogen and Its Receptors in Efferent Ductules and Epididymis. J. Androl. 2011, 32, 600–613. [Google Scholar] [CrossRef]
- Clulow, J.; Jones, R.C.; Hansen, L.A.; Man, S.Y. Fluid and electrolyte reabsorption in the ductuli efferentes testis. J. Reprod. Fertil. Suppl. 1998, 53, 1–14. [Google Scholar]
- Hess, R. The efferent ductules: Structure and functions. In The Epididymis. From Molecules to Clinical Practice; Robaire, B., Hinton, B.T., Eds.; Kluwer Academic/Plenum: New York, NY, USA, 2002; pp. 49–80. [Google Scholar]
- Breton, S.; Ruan, Y.C.; Park, Y.-J.; Kim, B. Regulation of epithelial function, differentiation, and remodeling in the epididymis. Asian J. Androl. 2016, 18, 3–9. [Google Scholar] [CrossRef]
- Binato de Souza, A.P.; Schorr-Lenz, Â.M.; Lucca, F.; Bustamante-Filho, I.C. The epididymis and its role on sperm quality and male fertility. Anim. Reprod. 2017, 14, 1234–1244. [Google Scholar] [CrossRef]
- Belleannée, C.; Labas, V.; Teixeira-Gomes, A.-P.; Gatti, J.-L.; Dacheux, J.-L.; Dacheux, F. Identification of luminal and secreted proteins in bull epididymis. J. Proteom. 2011, 74, 59–78. [Google Scholar] [CrossRef]
- Dacheux, J.-L.; Belleannée, C.; Guyonnet, B.; Labas, V.; Teixeira-Gomes, A.-P.; Ecroyd, H.; Druart, X.; Gatti, J.-L.; Dacheux, F. The contribution of proteomics to understanding epididymal maturation of mammalian spermatozoa. Syst. Biol. Reprod. Med. 2012, 58, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Cooper, T.G.; Barfield, J.P. Utility of infertile male models for contraception and conservation. Mol. Cell. Endocrinol. 2006, 250, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Cornwall, G.A.; Hann, S.R. Specialized gene expression in the epididymis. J. Androl. 1995, 16, 379–383. [Google Scholar] [PubMed]
- Kirchhoff, C. Gene expression in the epididymis. Int. Rev. Cytol. 1999, 188, 133–202. [Google Scholar] [PubMed]
- Bedford, J.M. Effects of elevated temperature on the epididymis and testis: Experimental studies. Adv. Exp. Med. Biol. 1991, 286, 19–32. [Google Scholar] [PubMed]
- Jara, M.; Esponda, P.; Carballada, R. Abdominal temperature induces region-specific p53-independent apoptosis in the cauda epididymidis of the mouse. Biol. Reprod. 2002, 67, 1189–1196. [Google Scholar] [CrossRef]
- Ali, J.I.; Weaver, D.J.; Weinstein, S.H.; Grimes, E.M. Scrotal Temperature and Semen Quality in Men with and without Varicocele. Arch. Androl. 1990, 24, 215–219. [Google Scholar] [CrossRef]
- Lerchl, A.; Keck, C.; Spiteri-Grech, J.; Nieschlag, E.; Bergh, A. Diurnal variations in scrotal temperature of normal men and patients with varicocele before and after treatment. Int. J. Androl. 1993, 16, 195–200. [Google Scholar] [CrossRef]
- Kocak, I.; Dundar, M.; Hekimgil, M.; Okyay, P. Assessment of germ cell apoptosis in cryptorchid rats. Asian J. Androl. 2002, 4, 183–186. [Google Scholar]
- Barbe, M.F.; Tytell, M.; Gower, D.J.; Welch, W.J. Hyperthermia protects against light damage in the rat retina. Science 1988, 241, 1817–1820. [Google Scholar] [CrossRef]
- Cao, W.; Huang, P.; Zhang, L.; Wu, H.-Z.; Zhang, J.; Shi, F.-X. Acute heat stress increases HSP70 expression in the testis, epididymis and vas deferens of adult male mice. Zhonghua Nan Ke Xue 2009, 15, 200–206. [Google Scholar]
- Ngoula, F.; Lontio, F.A.; Tchoffo, H.; Tsague, F.P.M.; Djeunang, R.-M.; Vemo, B.N.; Moffo, F.; Motchewo, N.D. Heat Induces Oxidative Stress: Reproductive Organ Weights and Serum Metabolite Profile, Testes Structure, and Function Impairment in Male Cavy (Cavia porcellus). Front. Vet. Sci. 2020, 7, 37. [Google Scholar] [CrossRef]
- Pastor-Soler, N.; Isnard-Bagnis, C.; Herak-Kramberger, C.; Sabolic, I.; Van Hoek, A.; Brown, D.; Breton, S. Expression of aquaporin 9 in the adult rat epididymal epithelium is modulated by androgens. Biol. Reprod. 2002, 66, 1716–1722. [Google Scholar] [CrossRef]
- Hermo, L.; Krzeczunowicz, D.; Ruz, R. Cell Specificity of Aquaporins 0, 3, and 10 Expressed in the Testis, Efferent Ducts, and Epididymis of Adult Rats. J. Androl. 2004, 25, 494–505. [Google Scholar] [CrossRef] [PubMed]
- Hermo, L.; Smith, C.E. Thirsty Business: Cell, Region, and Membrane Specificity of Aquaporins in the Testis, Efferent Ducts, and Epididymis and Factors Regulating Their Expression. J. Androl. 2011, 32, 565–575. [Google Scholar] [CrossRef] [PubMed]
- Morishita, Y.; Sakube, Y.; Sasaki, S.; Ishibashi, K. Molecular Mechanisms and Drug Development in Aquaporin Water Channel Diseases: Aquaporin Superfamily (Superaquaporins): Expansion of Aquaporins Restricted to Multicellular Organisms. J. Pharmacol. Sci. 2004, 96, 276–279. [Google Scholar] [CrossRef] [PubMed]
- Yakata, K.; Hiroaki, Y.; Ishibashi, K.; Sohara, E.; Sasaki, S.; Mitsuoka, K.; Fujiyoshi, Y. Aquaporin-11 containing a divergent NPA motif has normal water channel activity. Biochim. Biophys. Acta 2007, 1768, 688–693. [Google Scholar] [CrossRef]
- Arrighi, S.; Bosi, G.; Accogli, G.; DeSantis, S. Seasonal and Ageing-Depending Changes of Aquaporins 1 and 9 Expression in the Genital Tract of Buffalo Bulls (Bubalus bubalis). Reprod. Domest. Anim. 2016, 51, 515–523. [Google Scholar] [CrossRef]
- Klein, C.; Troedsson, M.; Rutllant, J. Region-Specific Expression of Aquaporin Subtypes in Equine Testis, Epididymis, and Ductus Deferens. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2013, 296, 1115–1126. [Google Scholar] [CrossRef]
- Ford, J.; Carnes, K.; Hess, R.A. Ductuli efferentes of the male Golden Syrian hamster reproductive tract. Andrology 2014, 2, 510–520. [Google Scholar] [CrossRef]
- Gao, Y.; Deng, Q.; Zhang, Y.; Zhang, S.; Zhu, Y.; Zhang, J. The expression of the multiple splice variants of AQP8 in porcine testes at different developmental stages. J. Appl. Genet. 2014, 55, 511–514. [Google Scholar] [CrossRef] [PubMed]
- Schimming, B.; Pinheiro, P.; De Matteis, R.; Machado, C.; Domeniconi, R. Immunolocalization of Aquaporins 1 and 9 in the Ram Efferent Ducts and Epididymis. Reprod. Domest. Anim. 2015, 50, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Schimming, B.; Baumam, C.; Pinheiro, P.; De Matteis, R.; Domeniconi, R. Aquaporin 9 is expressed in the epididymis of immature and mature pigs. Reprod. Domest. Anim. 2017, 52, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Skowronski, M.T.; Leska, A.; Robak, A.; Nielsen, S. Immunolocalization of Aquaporin-1, -5, and -7 in the Avian Testis and Vas Deferens. J. Histochem. Cytochem. 2009, 57, 915–922. [Google Scholar] [CrossRef]
- Yeste, M.; Morató, R.; Rodríguez-Gil, J.; Bonet, S.; Prieto-Martínez, N. Aquaporins in the male reproductive tract and sperm: Functional implications and cryobiology. Reprod. Domest. Anim. 2017, 52, 12–27. [Google Scholar] [CrossRef] [PubMed]
- Sales, A.; Lobo, C.; Carvalho, A.; Moura, A.; Rodrigues, A. Review Structure, function, and localization of aquaporins: Their possible implications on gamete cryopreservation. Genet. Mol. Res. 2013, 12, 6718–6732. [Google Scholar] [CrossRef]
- Pelagalli, A.; Squillacioti, C.; Ali’, S.; Liguori, G.; Mirabella, N. Cellular distribution of aquaporins in testes of normal and cryptorchid dogs: A preliminary study on dynamic roles. Anim. Reprod. Sci. 2019, 204, 22–30. [Google Scholar] [CrossRef]
- Domeniconi, R.F.; Orsi, A.M.; Justulin, L.A.; Beu, C.C.L.; Felisbino, S.L. Aquaporin 9 (AQP9) Localization in the Adult Dog Testis Excurrent Ducts by Immunohistochemistry. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2007, 290, 1519–1525. [Google Scholar] [CrossRef]
- Sang, Y.; Ortega, M.T.; Blecha, F.; Prakash, O.; Melgarejo, T. Molecular cloning and characterization of three β-defensins from canine testes. Infect. Immun. 2005, 73, 2611–2620. [Google Scholar] [CrossRef]
- Lee, W.-Y.; Lee, R.; Park, H.-J.; Do, J.T.; Park, C.; Kim, J.-H.; Jhun, H.; Lee, J.-H.; Hur, T.; Song, H. Characterization of male germ cell markers in canine testis. Anim. Reprod. Sci. 2017, 182, 1–8. [Google Scholar] [CrossRef]
- Assisi, L.; Pelagalli, A.; Squillacioti, C.; Liguori, G.; Annunziata, C.; Mirabella, N. Orexin A-Mediated Modulation of Reproductive Activities in Testis of Normal and Cryptorchid Dogs: Possible Model for Studying Relationships between Energy Metabolism and Reproductive Control. Front. Endocrinol. 2019, 10, 816. [Google Scholar] [CrossRef] [PubMed]
- Bencharif, D.; Dordas-Perpinya, M. Canine semen cryoconservation: Emerging data over the last 20 years. Reprod. Domest. Anim. 2020, 55, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Domeniconi, R.F.; Orsi, A.M.; Justulin, L.A., Jr.; Beu, C.C.L.; Felisbino, S.L. Immunolocalization of aquaporins 1, 2 and 7 in rete testis, efferent ducts, epididymis and vas deferens of adult dog. Cell Tissue Res. 2008, 332, 329–335. [Google Scholar] [CrossRef]
- Arrighi, S.; Aralla, M.; Fracassetti, P.; Mobasheri, A.; Cremonesi, F. Aquaporin water channels in the canine gubernaculum testis. Acta Histochem. 2013, 115, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, N.; Silberstein, C.; Beaulieu, V.; Piétrement, C.; Van Hoek, A.N.; Brown, D.; Breton, S. Postnatal Expression of Aquaporins in Epithelial Cells of the Rat Epididymis. Biol. Reprod. 2006, 74, 427–438. [Google Scholar] [CrossRef]
- Yeung, C.-H. Aquaporins in spermatozoa and testicular germ cells: Identification and potential role. Asian J. Androl. 2010, 12, 490–499. [Google Scholar] [CrossRef]
- Kirchhoff, C. The dog as a model to study human epididymal function at a molecular level. Mol. Hum. Reprod. 2002, 8, 695–701. [Google Scholar] [CrossRef]
- Ellerbrock, K.; Pera, I.; Hartung, S.; Ivell, R. Gene expression in the dog epididymis: A model for human epididymal function. Int. J. Androl. 1994, 17, 314–323. [Google Scholar] [CrossRef]
- Sampaolo, S.; Liguori, G.; Vittoria, A.; Napolitano, F.; Lombardi, L.; Figols, J.; Melone, M.A.B.; Esposito, T.; Di Iorio, G. First study on the peptidergic innervation of the brain superior sagittal sinus in humans. Neuropeptides 2017, 65, 45–55. [Google Scholar] [CrossRef]
- De Luca, A.; Vassalotti, G.; Pelagalli, A.; Pero, M.E.; Squillacioti, C.; Mirabella, N.; Lombardi, P.; Avallone, L. Expression and Localization of Aquaporin-1 along the Intestine of Colostrum Suckling Buffalo Calves. Anat. Histol. Embryol. 2014, 44, 391–400. [Google Scholar] [CrossRef]
- Squillacioti, C.; De Luca, A.; Liguori, G.; Alì, S.; Germano, G.; Vassalotti, G.; Navas, L.; Mirabella, N. Urocortinergic system in the testes of normal and cryptorchid dogs. Ann. Anat. Anat. Anz. 2016, 207, 91–96. [Google Scholar] [CrossRef]
- Légaré, C.; Thabet, M.; Sullivan, R. Expression of heat shock protein 70 in normal and cryptorchid human excurrent duct. Mol. Hum. Reprod. 2004, 10, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Shukla, K.K.; Mahdi, A.A.; Rajender, S. Apoptosis, spermatogenesis and male infertility. Front. Biosci. 2012, 4, 746–754. [Google Scholar] [CrossRef]
- Tekayev, M.; Bostancieri, N.; Saadat, K.A.; Turker, M.; Yuncu, M.; Ulusal, H.; Cicek, H.; Arman, K. Effects of Moringa oleifera Lam Extract (MOLE) in the heat shock protein 70 expression and germ cell apoptosis on experimentally induced cryptorchid testes of rats. Gene 2019, 688, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Cooper, T.G.; Brooks, D.E. Entry of glycerol into the rat epididymis and its utilization by epididymal spermatozoa. J. Reprod. Fertil. 1981, 61, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Robaire, B.; Hermo, L. Efferent Ducts, Epididymis, and Vas Deferens: Structure, Functions, and Their Regulations; Raven Press: New York, NY, USA, 1988. [Google Scholar]
- Picciarelli-Lima, P.; Oliveira, A.G.; Reis, A.M.; Kalapothakis, E.; Mahecha, G.A.B.; Hess, R.A.; Oliveira, C.A. Effects of 3-beta-diol, an androgen metabolite with intrinsic estrogen-like effects, in modulating the aquaporin-9 expression in the rat efferent ductules. Reprod. Biol. Endocrinol. 2006, 4, 51. [Google Scholar] [CrossRef]
- Ruz, R.; Gregory, M.; Smith, C.E.; Cyr, D.G.; Lubahn, D.B.; Hess, R.A.; Hermo, L. Expression of aquaporins in the efferent ductules, sperm counts, and sperm motility in estrogen receptor-α deficient mice fed lab chow versus casein. Mol. Reprod. Dev. 2006, 73, 226–237. [Google Scholar] [CrossRef] [PubMed]
- Fujii, T.; Hirayama, H.; Fukuda, S.; Kageyama, S.; Naito, A.; Yoshino, H.; Moriyasu, S.; Yamazaki, T.; Sakamoto, K.; Hayakawa, H.; et al. Expression and localization of aquaporins 3 and 7 in bull spermatozoa and their relevance to sperm motility after cryopreservation. J. Reprod. Dev. 2018, 64, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Arrighi, S.; Romanello, M.G.; Domeneghini, C. Morphological examination of epididymal epithelium in the mule (E. hinnus) in comparison with parental species (E. asinus and E. caballus). Histol. Histopathol. 1991, 6, 325–337. [Google Scholar]
- Schön, J.; Blottner, S. Seasonal variations in the epididymis of the roe deer (Capreolus capreolus). Anim. Reprod. Sci. 2009, 111, 344–352. [Google Scholar] [CrossRef]
- Arrighi, S. Primary cilia in the basal cells of equine epididymis: A serendipitous finding. Tissue Cell 2013, 45, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Hermo, L.; Schellenberg, M.; Liu, L.Y.; Dayanandan, B.; Zhang, T.; Mandato, C.A.; Smith, C.E. Membrane Domain Specificity in the Spatial Distribution of Aquaporins 5, 7, 9, and 11 in Efferent Ducts and Epididymis of Rats. J. Histochem. Cytochem. 2008, 56, 1121–1135. [Google Scholar] [CrossRef] [PubMed]
- Hashem, M.A. Biochemical and expression studies on Aquaporin 9 (AQP9) in wild and AQP9 knockout mice. Vet. Arhiv 2010, 80, 93–112. [Google Scholar]
- Verkman, A.S.; Mitra, A.K. Structure and function of aquaporin water channels. Am. J. Physiol. Renal Physiol. 2000, 278, F13–F28. [Google Scholar] [CrossRef] [PubMed]
- Arrighi, S. Are the basal cells of the mammalian epididymis still an enigma? Reprod. Fertil. Dev. 2014, 26, 1061–1071. [Google Scholar] [CrossRef]
- Elkjær, M.-L.; Nejsum, L.N.; Gresz, V.; Kwon, T.-H.; Jensen, U.B.; Frøkiær, J.; Nielsen, S. Immunolocalization of aquaporin-8 in rat kidney, gastrointestinal tract, testis, and airways. Am. J. Physiol. Renal Physiol. 2001, 281, F1047–F1057. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, D.W. Structure and function of the epithelium lining the ductuli efferentes, ductus epididymis and ductus deferens in the rat. In Handbook of Physiology, Section 7, Endocrinology. Vol. V. Male Reproductive System; Hamilton, D.W., Creep, R.O., Eds.; American Physiological Society: Washington, DC, USA, 1975; pp. 259–301. [Google Scholar]
- Ramos, A.S.; Dym, M. Fine structure of the monkey epididymis. Am. J. Anat. 1977, 149, 501–531. [Google Scholar] [CrossRef]
- Jones, R.; Hamilton, D.W.; Fawcett, D.W. Morphology of the epithelium of the extratesticular rete testis, ductuli efferentes and ductus epididymidis of the adult male rabbit. Am. J. Anat. 1979, 156, 373–400. [Google Scholar] [CrossRef]
- Shum, W.W.C.; Ruan, Y.C.; Da Silva, N.; Breton, S. Establishment of Cell-Cell Cross Talk in the Epididymis: Control of Luminal Acidification. J. Androl. 2011, 32, 576–586. [Google Scholar] [CrossRef]
- Shum, W.W.C.; Da Silva, N.; McKee, M.; Smith, P.J.; Brown, D.; Breton, S. Transepithelial Projections from Basal Cells Are Luminal Sensors in Pseudostratified Epithelia. Cell 2008, 135, 1108–1117. [Google Scholar] [CrossRef]
- Leung, G.P.; Cheung, K.H.; Leung, C.T.; Tsang, M.W.; Wong, P.Y. Regulation of epididymal principal cell functions by basal cells: Role of transient receptor potential (Trp) proteins and cyclooxygenase-1 (COX-1). Mol. Cell. Endocrinol. 2004, 216, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Mandon, M.; Hermo, L.; Cyr, D.G. Isolated Rat Epididymal Basal Cells Share Common Properties with Adult Stem Cells. Biol. Reprod. 2015, 93, 115. [Google Scholar] [CrossRef] [PubMed]
- Pinel, L.; Mandon, M.; Cyr, D.G. Tissue regeneration and the epididymal stem cell. Andrology 2019, 7, 618–630. [Google Scholar] [CrossRef] [PubMed]
- Lü, Y.; Bhushan, S.; Tchatalbachev, S.; Marconi, M.; Bergmann, M.; Weidner, W.; Chakraborty, T.; Meinhardt, A. Necrosis Is the Dominant Cell Death Pathway in Uropathogenic Escherichia coli Elicited Epididymo-Orchitis and Is Responsible for Damage of Rat Testis. PLoS ONE 2013, 8, e52919. [Google Scholar] [CrossRef]
- Prieto-Martínez, N.; Vilagran, I.; Morató, R.; Rivera del Álamo, M.M.; Rodríguez-Gil, J.E.; Bonet, S.; Yeste, M. Relationship of aquaporins 3 (AQP3), 7 (AQP7), and 11 (AQP11) with boar sperm resilience to withstand freeze-thawing procedures. Andrology 2017, 5, 1153–1164. [Google Scholar] [CrossRef]
- Chen, J.-A.; Chang, L.-R.; Feng, G.-M.; Lee, S.-T.; Hsieh, C.-Y.; Jeng, S.-F.; Huang, W.-S. Stress alters the expression of aquaporins in cultured rat intestinal epithelial cells. Exp. Ther. Med. 2015, 10, 1967–1972. [Google Scholar] [CrossRef][Green Version]
- Bernardino, R.L.; Carrageta, D.F.; Silva, A.M.; Calamita, G.; Alves, M.G.; Soveral, G.; Oliveira, P.F. Estrogen Modulates Glycerol Permeability in Sertoli Cells through Downregulation of Aquaporin-9. Cells 2018, 7, 153. [Google Scholar] [CrossRef]
- Miki, A.; Kanamori, A.; Negi, A.; Naka, M.; Nakamura, M. Loss of Aquaporin 9 Expression Adversely Affects the Survival of Retinal Ganglion Cells. Am. J. Pathol. 2013, 182, 1727–1739. [Google Scholar] [CrossRef]
- Fan, X.; Robaire, B. Orchidectomy Induces a Wave of Apoptotic Cell Death in the Epididymis. Endocrinology 1998, 139, 2128–2136. [Google Scholar] [CrossRef]
- Bongki, K.; Breton, S. Androgens are essential for epithelial cell recovery after efferent duct ligation in the initial segment of the mouse epididymis. Biol. Reprod. 2020, 102, 76–83. [Google Scholar]


| Gene Target | Sequence 5′–3′ | Genbank Accession Number |
|---|---|---|
| AQP7 | XM_531973.5 | |
| Forward | TGTCCCCACCCCCAATG | |
| Reverse | TCGTATATTTGGCAGCTTTCTC | |
| AQP8 | XM_014114532.1 | |
| Forward | AAACATCAGTGGAGGACATTTCAA | |
| Reverse | GCTCCTGGACTGTCACAAAGG | |
| AQP9 | XM_544701.4 | |
| Forward | CCTTCCCTGCGAATCACAA | |
| Reverse | AGGTGCATCGCTTGATGTAGAG |
| NORMAL | AQP7 | AQP8 | AQP9 | |
|---|---|---|---|---|
| Epididymis | Efferent ductules | +++ | +++ | +++ |
| Caput | ++ | ++ | ++ | |
| Corpus | ++ | ++ | ++ | |
| Cauda | +++ | +++ | ++ | |
| CRYPTORCHID | ||||
| Epididymis | Efferent ductules | - | - | - |
| Caput | ++ | +++ | ++ | |
| Corpus | - | - | - | |
| Cauda | +++ | +++ | ++ |
| AQP7 | AQP8 | AQP9 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Epididymal Segment | Cell Types | |||||||||||
| P | B | N | P | B | N | P | B | N | ||||
| NORMAL | Caput | + | - | + | + | - | + | + * | - | - | ||
| Corpus | - | + | - | + | - | - | + * | - | - | |||
| Cauda | + | - | - | + | - | - | + * | +/- | - | |||
| Caput | - | + | - | - | + | - | + | - | - | |||
| CRYPTORCHID | Corpus | - | - | - | - | - | - | - | - | - | ||
| Cauda | + | + | - | + | + | - | + | - | - | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Squillacioti, C.; Mirabella, N.; Liguori, G.; Germano, G.; Pelagalli, A. Aquaporins Are Differentially Regulated in Canine Cryptorchid Efferent Ductules and Epididymis. Animals 2021, 11, 1539. https://doi.org/10.3390/ani11061539
Squillacioti C, Mirabella N, Liguori G, Germano G, Pelagalli A. Aquaporins Are Differentially Regulated in Canine Cryptorchid Efferent Ductules and Epididymis. Animals. 2021; 11(6):1539. https://doi.org/10.3390/ani11061539
Chicago/Turabian StyleSquillacioti, Caterina, Nicola Mirabella, Giovanna Liguori, Giuseppe Germano, and Alessandra Pelagalli. 2021. "Aquaporins Are Differentially Regulated in Canine Cryptorchid Efferent Ductules and Epididymis" Animals 11, no. 6: 1539. https://doi.org/10.3390/ani11061539
APA StyleSquillacioti, C., Mirabella, N., Liguori, G., Germano, G., & Pelagalli, A. (2021). Aquaporins Are Differentially Regulated in Canine Cryptorchid Efferent Ductules and Epididymis. Animals, 11(6), 1539. https://doi.org/10.3390/ani11061539








