Modulation of Bovine Endometrial Cell Receptors and Signaling Pathways as a Nanotherapeutic Exploration against Dairy Cow Postpartum Endometritis
Abstract
Simple Summary
Abstract
1. Introduction
2. Function of Bacterial Pampsin Bovine Endometritis
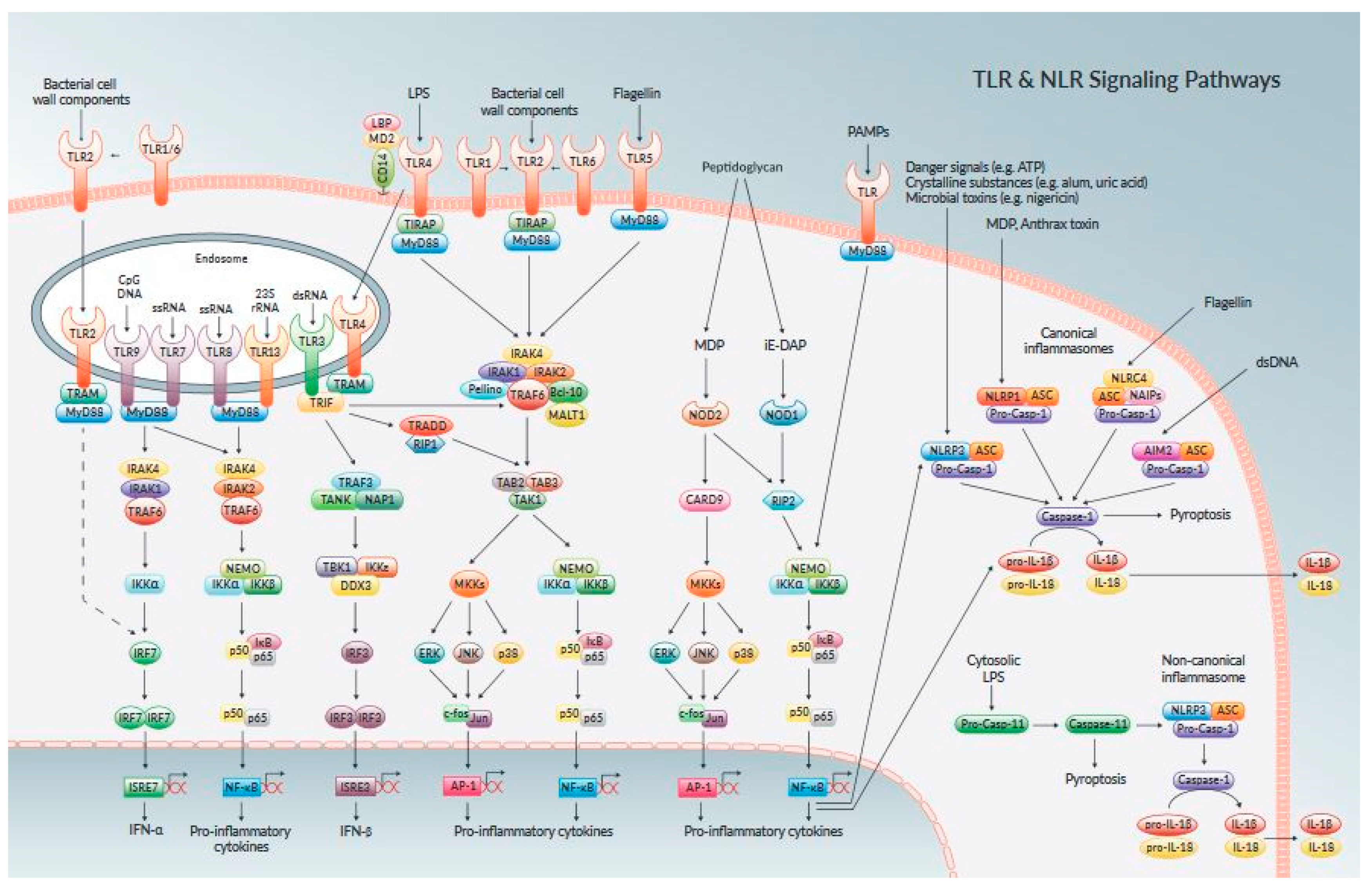
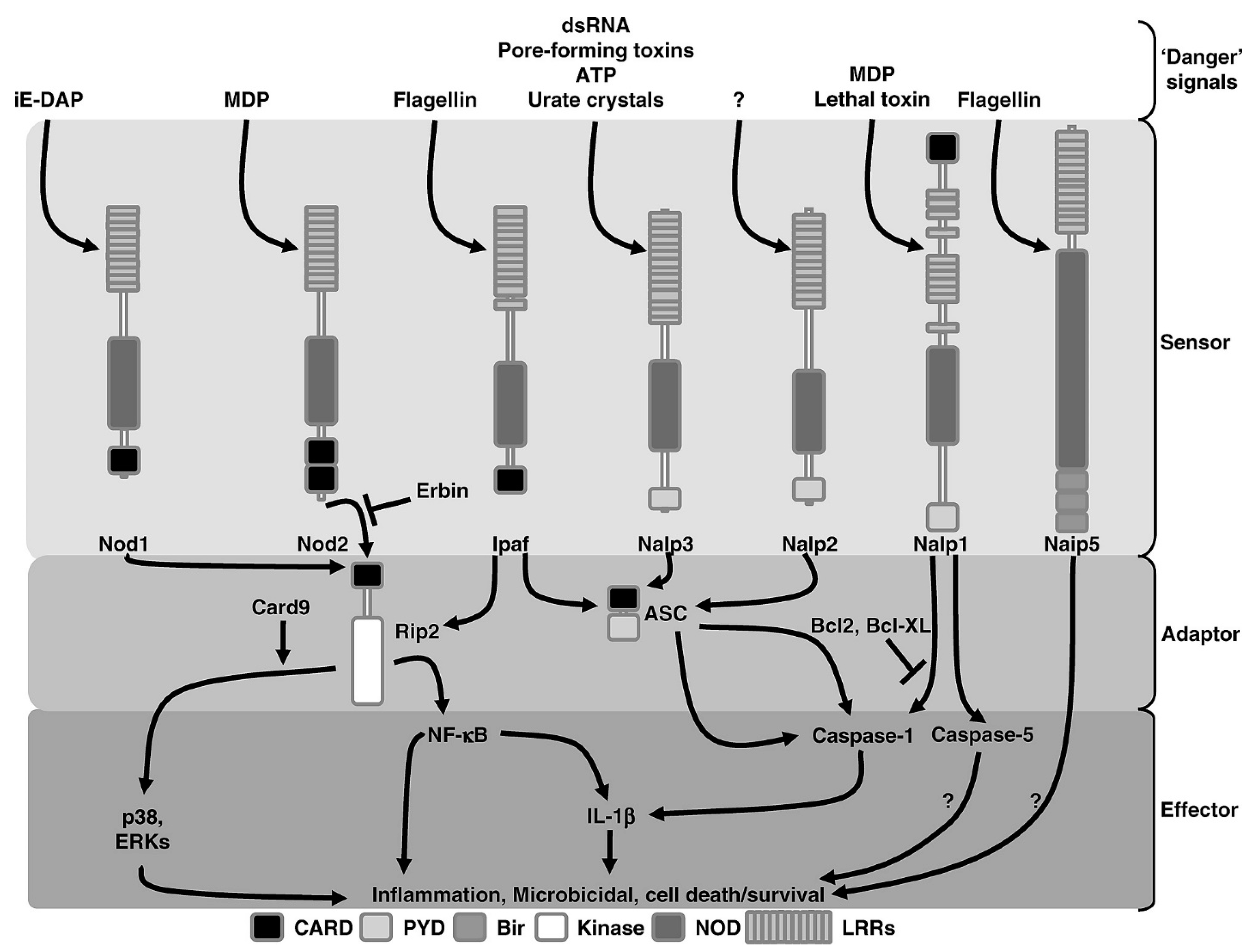
3. Endometrial Cell Receptors and Signalling Biomolecules
4. Evaluation of MAPKSignaling Transduction Pathways in Endometritis Pathogenesis
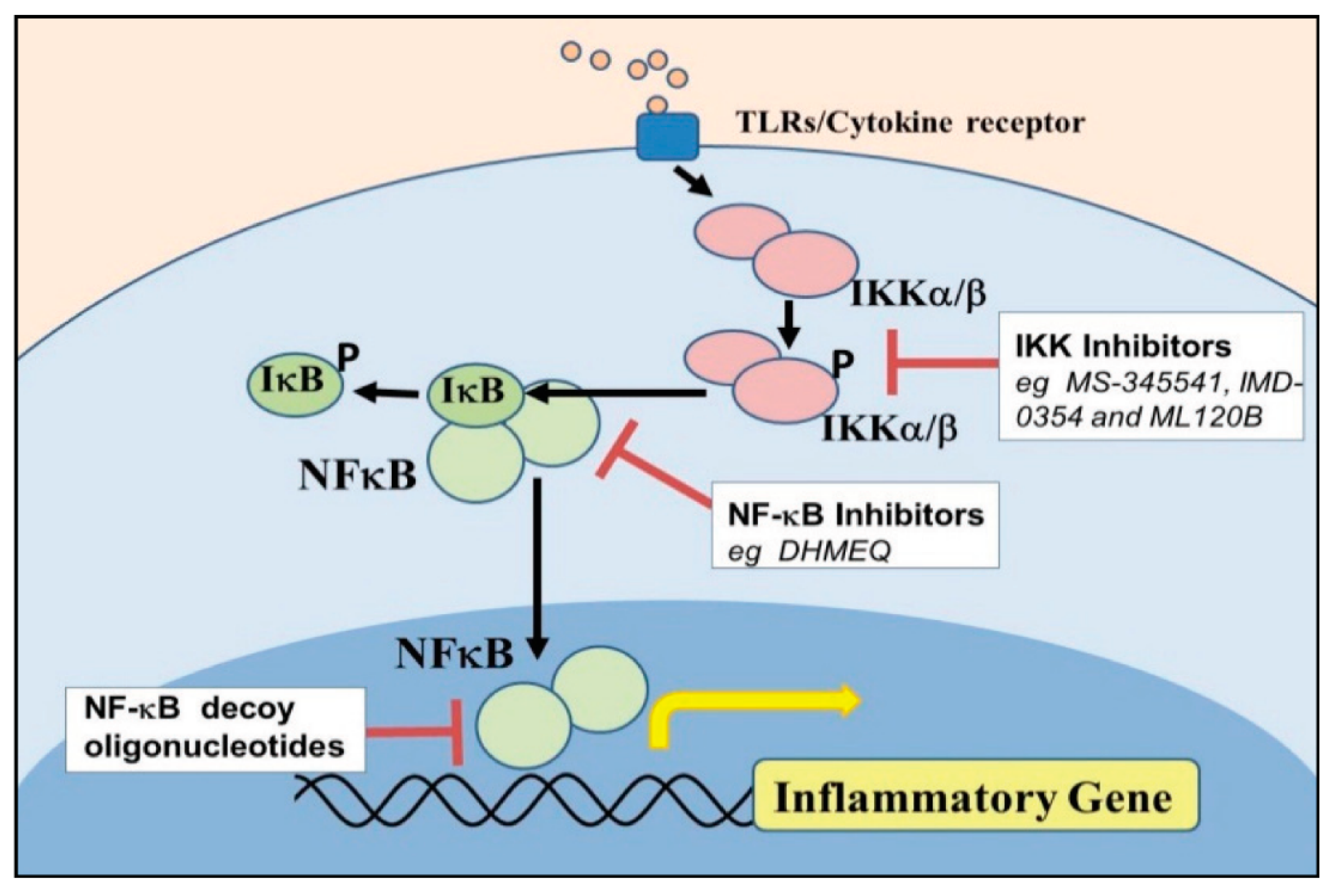
5. Component of Nuclear Factor Kappa Beta [NF–κB] Activation in Endometrium Inflammatory Response
6. Inflammatory Cytokines and Chemokines in Bovine Endometritis
7. Application of Nanotherapeutic in Reproductive Diseases
8. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PAMP | Pathogen Associated Membrane Protein |
| LPS | Lipopolysaccharide |
| IL-β | Interleukin Beta |
| TNFα | Tissue Necrosis Factor-alpha |
| TLR | Toll-like Receptor |
| RLR | Rig-like receptor |
| NLR | NOD Like Receptor |
| MyD88 | Myeloid Differentiation factor 88 |
| Mal | MyD88 associated ligand |
| TIRAP | TIR domain-containing adaptor protein |
| TRAF | tumor necrosis factor receptor (TNFR)-associated factor (TRAF) |
| TRIF | TIR-domain-containing adapter-inducing interferon-β |
| TRAM | TRIF-related adaptor molecule |
| SARM | Sterile alpha and TIR motif-containing |
| NOD | Nucleotide-binding Oligomerization Domains |
| NLRP | NOD Like Receptor Proteins |
| MAPK | mitogen-activated protein kinase |
| ERK | extracellular signal-regulated kinases |
| JNK | c-Jun N-terminal kinases |
References
- de Boer, M.W.; LeBlanc, S.J.; Dubuc, J.; Meier, S.; Heuwieser, W.; Arlt, S.; Gilbert, R.O.; McDougall, S. Invited review: Systematic review of diagnostic tests for reproductive-tract infection and inflammationin dairy cows. J. Dairy Sci. 2014, 97, 3983–3999. [Google Scholar] [CrossRef] [PubMed]
- Gautam, G.; Nakao, T.; Yusuf, M.; Koike, K. Prevalence of endometritis during the postpartum period and its impact on subsequent reproductive performance in two Japanese dairy herds. Anim. Reprod. Sci. 2009, 116, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Gautam, G.; Nakao, T.; Koike, K.; Long, S.T.; Yusuf, M.; Ranasinghe, R.M.; Hayashi, A. Spontaneous recovery or persistence of postpartum endometritis and risk factors for its persistence in Holstein cows. Theriogenology 2010, 73, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Plöntzke, J.; Madoz, L.W.; De la Sota, R.L.; Heuwieser, W.; Drillich, M. Prevalence of clinical endometritis and its impact on reproductive performance in grazing dairy cattle in Argentina. Reprod. Domest. Anim. 2011, 46, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Leblanc, S.J.; Duffield, T.F.; Leslie, K.E.; Bateman, K.G.; Keefe, G.P.; Walton, J.S.; Johnson, W.H. Defining and Diagnosing Postpartum Clinical Endometritis and its Impact on Reproductive Performance in Dairy Cows. J. Dairy Sci. 2002, 85, 2223–2236. [Google Scholar] [CrossRef]
- Okawa, H.; Fujikura, A.; Wijayagunawardane, M.M.P.; Vos, P.L.A.M.; Taniguchi, M.; Takagi, M. Effect of diagnosis and treatment of clinical endometritis based on vaginal discharge score grading system in postpartum Holstein cows. J. Vet. Med. Sci. 2017, 79, 1545–1551. [Google Scholar] [CrossRef]
- Kumar, D.; Satish, S.; Purohit, G.N. A Discussion on Risk Factors, Therapeutic Approach of Endometritis and Metritis in Cattle. Int. J.Curr. Microbiol. Appl. Sci. 2019, 8, 403–421. [Google Scholar] [CrossRef]
- Barański, W.; Podhalicz-Dziegielewska, M.; Zduńczyk, S.; Janowski, T. The diagnosis and prevalence of subclinical endometritis in cows evaluated by different cytologic thresholds. Theriogenology 2012, 78, 1939–1947. [Google Scholar] [CrossRef]
- Dubuc, J.; Duffield, T.F.; Leslie, K.E.; Walton, J.S.; LeBlanc, S.J. Definitions and diagnosis of postpartum endometritis in dairy cows. J. Dairy Sci. 2010, 93, 5225–5233. [Google Scholar] [CrossRef]
- Gilbert, R.O.; Shin, S.T.; Guard, C.L.; Erb, H.N.; Frajblat, M. Prevalence of endometritis and its effects on reproductive performance of dairy cows. Theriogenology 2005, 64, 1879–1888. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, I.M.; Rycroft, A.N.; Dogan, B.; Craven, M.; Bromfield, J.J.; Chandler, A.; Simpson, K.W. Specific strains of Escherichia coli are pathogenic for the endometrium of cattle and cause pelvic inflammatory disease in cattle and mice. PLoS ONE 2010, 5, e9192. [Google Scholar] [CrossRef]
- Donofrio, G.; Herath, S.; Sartori, C.; Cavirani, S.; Flammini, C.F.; Sheldon, I.M. Bovine herpesvirus 4 is tropic for bovine endometrial cells and modulates endocrine function. Reproduction 2007, 134, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, I.M.; Owens, S.E. Postpartum uterine infection and endometritis in dairy cattle. Anim. Reprod. 2017, 14, 622–629. [Google Scholar] [CrossRef]
- Cronin, J.G.; Turner, M.L.; Goetze, L.; Bryant, C.E.; Sheldon, I.M. Toll-Like Receptor 4 and MYD88Dependent Signaling Mechanisms of the Innate Immune System are Essential for the Response to Lipopolysaccharide by Epithelial and Stromal Cells of the Bovine Endometrium. Biol. Reprod. 2012, 86, 1–9. [Google Scholar] [CrossRef]
- Chandler, E.C.; Ernst, R.K. Bacterial lipids: Powerful modifiers of the innate immune response. F1000Research 2017, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Koh, Y.Q.; Mitchell, M.D.; Almughlliq, F.B.; Vaswani, K.; Peiris, H.N. Regulation of inflammatory mediator expression in bovine endometrial cells: Effects of lipopolysaccharide, interleukin1beta, and tumor necrosis factor-alpha. Physiol. Rep. 2018, 6, e13676. [Google Scholar] [CrossRef]
- Harju, K.; Ojaniemi, M.; Rounioja, S.; Glumoff, V.; Paananen, R.; Vuolteenaho, R.; Hallman, M. Expression of toll-like receptor four and endotoxin responsiveness in mice during the perinatal period. Pediatr. Res. 2005, 57, 644–648. [Google Scholar] [CrossRef]
- Oguejiofor, C.F.; Cheng, Z.; Abudureyimu, A.; Fouladi-Nashta, A.A.; Wathes, D.C. Global transcriptomic profiling of bovine endometrial immune response in vitro. I. Effect of lipopolysaccharide on innate immunity. Biol. Reprod. 2015, 93, 100. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ryu, Y.H.; Baik, J.E.; Yang, J.S.; Kang, S.S.; Im, J.; Yun, C.H.; Kim, D.W.; Lee, K.; Chung, D.K.; Ju, H.R.; et al. Differential Immunostimulatory effects of Gram-positive bacteria due to their lipoteichoic acids. Int. Immunopharmacol. 2009, 9, 127–133. [Google Scholar] [CrossRef]
- Müller-Anstett, M.A.; Müller, P.; Albrecht, T.; Nega, M.; Wagener, J.; Gao, Q.; Kaesler, S.; Schaller, M.; Biedermann, T.; Götz, F. Staphylococcal peptidoglycan co-localizes with Nod2 and TLR2 and activates innate immune response via both receptors in primary murine keratinocytes. PLoS ONE 2010, 5, e13153. [Google Scholar] [CrossRef] [PubMed]
- Moranta, D.; Regueiro, V.; March, C.; Llobet, E.; Margareto, J.; Larrarte, E.; Garmendia, J.; Bengoechea, J.A. Klebsiella pneumoniae capsule polysaccharide impedes the expression of beta-defensins by airway epithelial cells. Infect. Immun. 2010, 78, 1135–1146. [Google Scholar] [CrossRef] [PubMed]
- Bicalho, M.L.S.; Machado, V.S.; Oikonomou, G.; Gilbert, R.O.; Bicalho, R.C. Association between virulence factors of Escherichia coli, Fusobacterium necrophorum, and Arcano-bacterium pyogenes and uterine diseases of dairy cows. Vet. Microbiol. 2012, 157, 125–131. [Google Scholar] [CrossRef]
- de Oliveira-Nascimento, L.; Massari, P.; Wetzler, L.M. The role of TLR2 in infection and immunity. Front. Immunol. 2012, 3, 79. [Google Scholar] [CrossRef]
- Seibert, S.A.; Mex, P.; Köhler, A.; Kauf- mann, S.H.; Mittrücker, H.W. TLR2-, TLR4- and Myd88- independent acquired humoral and cellular immunity against Salmonella enterica Serovar typhimurium. Immunol. Lett. 2010, 127, 126–134. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. The roles of TLRs, RLRs, and NLRs in pathogen recognition. Int. Immunol. 2009, 21, 317–337. [Google Scholar] [CrossRef]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen recognition and innate immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef] [PubMed]
- Heil, F.; Hemmi, H.; Hochrein, H. Species-specific recognition of single-stranded RNA via Toll-like receptor 7 and 8. Science 2004, 303, 1526–1529. [Google Scholar] [CrossRef]
- Lanzarotto, F.; Akbar, A.; Ghosh, S. Does innate immune response defect underlies inflammatory bowel disease in the Asian population? Postgrad. Med. J. 2005, 81, 483–485. [Google Scholar] [CrossRef][Green Version]
- Williams, E.J.; Sibley, K.; Miller, A.N.; Lane, E.A.; Fishwick, J.; Nash, D.M. The effect of Escherichia coli lipopolysaccharide and tumor necrosis factor-alpha on ovarian function. Am. J. Reprod. Immunol. 2008, 60, 462–473. [Google Scholar] [CrossRef] [PubMed]
- Wira, C.R.; Grant-Tschudy, K.S.; Crane-Godreau, M.A. Epithelial cells in the female reproductive tract: A central role as sentinels of immune protection. Am. J.Reprod. Immunol. 2005, 53, 65–76. [Google Scholar] [CrossRef]
- Lane, M.C.; Alteri, C.J.; Smith, S.N.; Mobley, H.L. Expression of flagella coincides with uropathogenic Escherichia coli ascension to the upper urinary tract. Proc. Natl. Acad. Sci. USA 2007, 104, 16669–16674. [Google Scholar] [CrossRef]
- Leendertse, M.; Willems, R.J.; Giebelen, I.A.; van den Pangaart, P.S.; Wiersinga, W.J.; de Vos, A.F.; Florquin, S.; Bonten, M.J.; van der Poll, T. TLR2- dependent MyD88 signaling contributes to early host defense in murine Enterococcus faecium peritonitis. J. Immunol. 2008, 180, 4865–4874. [Google Scholar] [CrossRef]
- Maldonado, R.F.; Sá-Correia, I.; Valvano, M.A. Lipopolysaccharide modification in gram-negative bacteria during chronic infection. FEMS Microbiol. Rev. 2016, 40, 480–493. [Google Scholar] [CrossRef]
- De Tejada, G.M.; Heinbockel, L.; Ferrer-Espada, R.; Heine, H.; Alexander, C.; Bárcena-Varela, S.; Goldmann, T.; Correa, W.; Wiesmüller, K.H.; Gisch, N.; et al. Lipoproteins/ peptides are sepsis-inducing toxins from bacteria that can be neutralized by synthetic anti-endotoxin peptides. Sci Rep. 2015, 5, 14292. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Kida, Y.; Kuwano, K. A triacylated lipoprotein from Mycoplasma genitalium activates NF-κB through Toll-like receptor 1 (TLR1) and TLR2. Infect. Immun. 2008, 76, 3672–3678. [Google Scholar] [CrossRef]
- Whelehan, C.J.; Meade, K.G.; Eckersall, P.D.; Young, F.J.; O’Farrelly, C. Experimental Staphylococcus aureus infection of the mammary gland induces region-specific changes in innate immune gene expression. Vet. Immunol. Immunopathol. 2011, 140, 181–189. [Google Scholar] [CrossRef]
- Swangchan-Uthai, T.; Lavender, C.R.; Cheng, Z.; Fouladi-Nashta, A.A.; Wathes, D.C. Time course of defense mechanisms in bovine endometrium in response to lipopolysaccharide. Biol. Reprod. 2012, 87, 135. [Google Scholar] [CrossRef]
- Chau, T.A.; McCully, M.L.; Brintnell, W.; An, G.; Kasper, K.J.; Vines, E.D. Toll-like receptor 2 ligands on the staphylococcal cell wall downregulate super antigen-induced T cell activation and prevent toxic shock syndrome. Nat. Med. 2009, 15, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Kawai, T.; Akira, S. Pathogen recognition by the innate immune system. Int. Rev. Immunol. 2011, 30, 16–34. [Google Scholar] [CrossRef] [PubMed]
- Kharayat, N.S.; Sharma, G.C.; Kumar, G.R.; Bisht, D.; Chaudhary, G.; Singh, S.K.; Krishnaswamy, N. Differential expression of endometrial toll-like receptors (TLRs) and antimicrobial peptides (AMPs) in the buffalo (Bubalusbubalis) with endometritis. Vet. Res. Commun. 2019, 43, 261–269. [Google Scholar] [CrossRef]
- Mogensen, T.H. Pathogen Recognition and Inflammatory Signaling in Innate Immune Defenses. Clin. Microbiol. Rev. 2009, 22, 240–273. [Google Scholar] [CrossRef]
- Herath, S.; Fischer, D.P.; Werling, D.; Williams, E.J.; Lilly, S.T.; Dobson, H.; Bryant, C.E.; Sheldon, I.M. Expression and function of Toll-like receptor 4 in the endometrial cells of the uterus. Endocrinology 2006, 147, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Davies, D.; Meade, K.G.; Herath, S.; Eckersall, P.D.; Gonzalez, D.; White, J.O.; Conlan, R.S.; O’Farrelly, C.; Sheldon, I.M. Toll-like receptor and antimicrobial peptide expression in the bovine endometrium. Reprod. Biol. Endocrinol. 2008, 6, 53. [Google Scholar] [CrossRef]
- Ritter, N. Peri-Partal Expression of Endometrial Toll-like Receptors and B-Defensins in Cattle. Ph.D. Thesis, University of Veterinary Medicine Hannover, Hanover, Germany, 2007. [Google Scholar]
- Olmos-ortiz, A.; Flores-espinosa, P.; Mancilla-herrera, I.; Vega-Sánchez, R.; Díaz, L.; Zaga-Clavellina, V. Innate Immune Cells and Toll-like Receptor-Dependent Responses at the Maternal-Fetal Interface. Int. J. Mol. Sci. 2019, 20, 3654. [Google Scholar] [CrossRef]
- Jacca, S.; Franceschi, V.; Colagiorgi, A.; Sheldon, M.; Donofrio, G. Bovine Endometrial Stromal Cells Support Tumor Necrosis Factor Alpha-Induced Bovine Herpesvirus Type 4 Enhanced Replication1. Biol. Reprod. 2013, 88. [Google Scholar] [CrossRef]
- Matsumoto, M.; Kikkawa, S.; Kohase, M.; Miyake, K.; Seya, M. Establishment of a monoclonal antibody against human Toll-like receptor three blocks double-stranded RNA-mediated signaling. Biochem. Biophys. Res. Commun. 2002, 293, 1364–1369. [Google Scholar] [CrossRef]
- Beutler, B. Inferences, questions, and possibilities in Toll-like receptor signaling. Nature 2004, 430, 257–263. [Google Scholar] [CrossRef]
- Verstak, B.; Nagpal, K.; Bottomley, S.P.; Golenbock, D.T.; Hertzog, P.J.; Mansell, A. MyD88 adapter like (Mal)/TIRAP interaction with TRAF6 is critical for TLR2- and TLR4-mediated NF-kappaB pro-inflammatory responses. J. Biol. Chem. 2009, 284, 24192–24203. [Google Scholar] [CrossRef] [PubMed]
- Young, O.M.; Tang, Z.; Niven-Fairchild, T.; Tadesse, S.; Krikun, G.; Norwitz, E.R.; Mor, G.; Abrahams, V.M.; Guller, S. Toll-like receptor-mediated responses by placental Hofbauer cells (HBCs): A potential pro-inflammatory role for fetal M2 macrophages. Am. J. Reprod. Immunol. 2015, 73, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, I.M.; Cronin, J.G.; Bromfield, J.J. Tolerance and Innate Immunity Shape the Development of Postpartum Uterine Disease and the Impact of Endometritis in Dairy Cattle. Ann. Rev. Anim. Biosci. 2019, 7, 361–384. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.L.; Cronin, J.C.; Healey, G.D.; Sheldon, I.M. Epithelial and stromal cells of bovine endometrium has roles in innate immunity and initiate inflammatory responses bacterial lipopeptides in vitro via Toll-like receptors TLR2, TLR1, and TLR6. Endocrinology 2014, 155, 1453–1465. [Google Scholar] [CrossRef] [PubMed]
- Hasan, U.; Chaffois, C.; Gaillard, C.; Saulnier, V.; Merck, E.; Tancredi, S.; Guiet, C.; Brière, F.; Vlach, J.; Lebecque, S.; et al. Human TLR10 is a functional receptor, expressed by B cells and plasmacytoid dendritic cells, which activates gene transcription through MyD88. J. Immunol. 2005, 174, 2942–2950. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.R.; Stuart, L.M.; Wilkinson, K.; van Gils, J.M.; Deng, J.; Halle, A.; Rayner, K.J.; Boyer, L.; Zhong, R.; Frazier, W.A.; et al. CD36 ligands promote sterile inflammation through assembly of a toll-like receptor 4 and 6 heterodimers. Nat. Immunol. 2010, 11, 155–175. [Google Scholar] [CrossRef] [PubMed]
- Botos, I.; Segal, D.M.; Davies, D.R. The structural biology of toll-like receptors. Structure 2011, 19, 447–459. [Google Scholar] [CrossRef]
- Sirard, J.; Cecile, V.; RodrigueDessein, M.C. Nod-Like Receptors: Cytosolic Watchdogs forImmunity against Pathogens. PLoS Pathog 2007, 3, e152. [Google Scholar] [CrossRef]
- Inohara, N.; Chamaillard, N.; McDonald, C. Nuñez G NOD-LRR proteins: Role in host-microbial interactions and inflammatory disease. Annu. Rev. Biochem. 2005, 74, 355–364. [Google Scholar] [CrossRef]
- Chamaillard, M.M.; Hashimoto, Y.; Horie, J.; Masumoto, S.; Qiu, L.; Saab, Y.; Ogura, A.; Kawasaki, K.; Fukase, S.; Kusumoto, M.A.; et al. An essential role forNOD1 in host recognition of bacterial peptidoglycan containing diaminopimelic acid. Nat. Immunol. 2003, 4, 702–707. [Google Scholar] [CrossRef] [PubMed]
- Uehara, A.; Yang, S.; Fujimoto, Y.; Fukase, K.; Kusumoto, S.; Shibata, K.; Sugawara, S.; Takada, H. Muramyldipeptide and diaminopimelic acid-containing desuramylpeptides in combination with chemically synthesized Toll-like receptor agonists synergistically induced production of interleukin-8 in a NOD2- and NOD1- dependent manner, respectively, in human monocytic cells in culture. Cell Microbiol. 2005, 7, 53–61. [Google Scholar]
- Brodsky, I.E.; Monack, D. NLR-mediated control of inflammasome assembly in the host response against bacterial pathogens. Semin. Immunol. 2009, 21, 199–207. [Google Scholar] [CrossRef]
- Kanneganti, T.D.; Lamkanfi, M.; Núñez, G. Intracellular NOD-like Receptors in Host Defenseand Disease. Immunity 2007, 27, 549–559. [Google Scholar] [CrossRef]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef] [PubMed]
- Boughan, P.K.; Argent, R.H.; Body-Malapel, M.; Park, J.H.; Ewings, K.E.; Bowie, A.G.; Ong, S.J.; Cook, S.J.; Sorensen, O.E.; Manzo, B.A. Nucleotide-binding oligomerization domain-1 and epidermal growth factor receptor: Critical regulators of beta-defensins during Helicobacter pylori infection. J. Biol. Chem. 2006, 281, 11637–11648. [Google Scholar] [CrossRef] [PubMed]
- Schroder, K.; Tschopp, J. The inflammasomes. Cell 2010, 140, 821–832. [Google Scholar] [CrossRef]
- Man, S.M.; Kanneganti, T.D. Regulation of inflammasome activation. Immunol Rev. 2015, 265, 6–21. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Katsiki, N.; Butler, A.E.; Sahebkar, A. Effects of antidiabetic drugs on NLRP3inflammasome activity, with a focus on diabetic kidneys. Drug Discov. Today 2018, 24, 256–262. [Google Scholar] [CrossRef]
- Kobayashi, K.S.; Chamaillard, M.; Ogura, Y.; Henegariu, O.; Inohara, N.; Nunez, G.; Flavell, R.A. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science 2005, 307, 731–734. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Li, D.; Wang, J.; Guo, J.; Li, Y.; Cao, Y.; Fu, Y. Melatonin inhibits endoplasmic reticulum stress-associated TXNIP/NLRP3 inflammasome activation in lipopolysaccharide-induced endometritis in mice. Int. Immunopharmacol. 2018, 64, 101–109. [Google Scholar] [CrossRef]
- Vizlin-Hodzic, D.; Zhai, Q.; Illes, S.; Södersten, K.; Truve, K.; Parris, T.Z.; Sobhan, P.K.; Salmela, S.; Kosalai, S.T.; Kanduri, C.; et al. Early-onset of inflammation during ontogeny of bipolar disorder: The NLRP2 inflammasome gene distinctly differentiates between patients and healthy controls in the transition between iPS cell and neural stem cell stages. Transl. Psychiatry 2017, 7, e1010. [Google Scholar] [CrossRef]
- Minkiewicz, J.; de Rivero Vaccari, J.P.; Keane, R.W. Human astrocytes express a novel NLRP2 inflammasome. Glia 2013, 61, 1113–1121. [Google Scholar] [CrossRef]
- Mahadevan, S.; Sathappan, V.; Utama, B.; Lorenzo, I.; Kaskar, K.; Van Den Veyver, I.B. Maternally expressed NLRP2 links the subcortical maternal complex (SCMC) to fertility, embryogenesis, and epigenetic reprogramming. Sci. Rep. 2017, 7, 1–17. [Google Scholar] [CrossRef]
- Place, D.E.; Kanneganti, T.D. Recent advances in inflammasome biology. Curr.Opin. Immunol. 2018, 50, 32–38. [Google Scholar] [CrossRef]
- Sharma, D.; Kanneganti, T.D. The cell biology of inflammasomes: Mechanisms of inflammasome activation and regulation. J. Cell Biol. 2016, 213, 617–629. [Google Scholar] [CrossRef]
- Hoseini, Z.; Sepahvand, F.; Rashidi, B.; Sahebkar, A.; Masoudifar, A.; Mirzaei, H. NLRP3 inflammasome: It’s regulation and involvement in atherosclerosis. J. Cell Physiol. 2018, 233, 2116–2132. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.D.; Huang, B.; Li, M.; Lamb, A.; Kelleher, N.L.; Chen, L.F. Negative regulation of NF-kappaB action by Set9-mediated lysine methylation of the RelA subunit. EMBO J. 2009, 28, 1055–1066. [Google Scholar] [CrossRef]
- Lamkanfi, M.; Dixit, V.M. Inflammasomes and their roles in health and disease. Annu. Rev. Cell Dev. Biol. 2012, 28, 137–161. [Google Scholar] [CrossRef]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 inflammasome: An overview of mechanisms of activation and regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef]
- Nakahira, K.; Haspel, J.A.; Rathinam, V.A.; Lee, S.J.; Dolinay, T.; Lam, H.C.; Englert, J.A.; Rabinovitch, M.; Cernadas, M.; Kim, H.P.; et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat. Immunol. 2011, 12, 222–230. [Google Scholar] [CrossRef]
- Shimada, K.; Crother, T.R.; Karlin, J.; Dagvadorj, J.; Chiba, N.; Chen, S.; Ramanujan, V.K.; Wolf, A.J.; Vergnes, L.; Ojcius, D.M.; et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity 2012, 36, 401–414. [Google Scholar] [CrossRef]
- Fernandes, A.C.C.; Davoodi, S.; Kaur, M.; Veira, D.; Melo, L.E.H.; Cerri, R.L.A. Effect of repeated intravenous lipopolysaccharide infusions on systemic inflammatory response and endometrium gene expression in Holstein heifers. J. Dairy Sci. 2019, 102, 3531–3543. [Google Scholar] [CrossRef] [PubMed]
- Igosheva, N.; Abramov, A.Y.; Poston, L.; Eckert, J.J.; Fleming, T.P.; Duchen, M.R.; McConnell, J. Maternal diet-induced obesity alters mitochondrial activity and redox status in mouse oocytes and zygotes. PLoS ONE 2010, 5, e10074. [Google Scholar] [CrossRef]
- Kim, B.; Kan, R.; Anguish, L.; Nelson, L.M.; Coonrod, S.A. Potential role for MATER in cytoplasmiclattice formation in murine oocytes. PLoS ONE 2010, 5, e12587. [Google Scholar] [CrossRef]
- Levy, M.; Thaiss, C.A.; Zeevi, D.; Dohnalova, L.; Zilberman-Schapira, G.; Mahdi, J.A.; David, E.; Savidor, A.; Korem, T.; Herzig, Y.; et al. Microbiota modulated metabolites shape the intestinal microenvironments by regulating NLRP6 inflammasome signaling. Cell 2015, 163, 1428–1443. [Google Scholar] [CrossRef]
- Chen, G.Y.; Liu, M.; Wang, F.; Bertin, J.; Nunez, G. A functional role for Nlrp6 in intestinalinflammation and tumorigenesis. J. Immunol. 2011, 186, 7187–7194. [Google Scholar] [CrossRef]
- Anand, P.K.; Malireddi, R.K.; Lukens, J.R.; Vogel, P.; Bertin, J.; Lamkanfi, M.; Kanneganti, T.D. NLRP6Negatively regulates innate immunity and host defense against bacterial pathogens. Nature 2012, 488, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Murdoch, S.; Djuric, U.; Mazhar, B.; Seoud, M.; Khan, R.; Kuick, R.; Bagga, R.; Kircheisen, R.; Ao, A.; Ratti, B.; et al. Mutations in NALP7 cause recurrent hydatidiform moles and reproductive wastage in humans. Nat. Genet. 2006, 38, 300–302. [Google Scholar] [CrossRef] [PubMed]
- Akoury, E.; Zhang, L.; Ao, A.; Slim, R. NLRP7 and KHDC3L, the two maternal-effect proteins responsible for recurrent hydatidiform moles, co-localize to the oocyte cytoskeleton. Hum. Reprod. 2015, 30, 159–169. [Google Scholar] [CrossRef]
- Wang, C.M.; Dixon, P.H.; Decordova, S.; Hodges, M.D.; Sebire, N.J.; Ozalp, S.; Fallahian, M.; Sensi, A.; Ashrafi, F.; Repiska, V.; et al. Identification of 13 novel NLRP7mutations in 20 families with recurrent hydatidiform mole; missense mutations cluster in the leucine-rich region. J. Med. Genet. 2009, 46, 569–575. [Google Scholar] [CrossRef]
- Loyi, T.; Kumar, H.; Nandi, S.; Patra, M.K. Expression of pathogen recognition receptors and pro-inflammatory cytokine transcripts in clinical and sub-clinical endometritis cows. Anim. Biotechnol. 2015, 26, 194–200. [Google Scholar] [CrossRef]
- Miller, B.A.; Brewer, A.; Nanni, P.; Lim, J.J.; Callanan, J.J.; Grossmann, J.; Chapwanya, A. Characterization of circulating plasma proteins in dairy cows with cytological endometritis. J. Proteom. 2019, 205, 103421. [Google Scholar] [CrossRef] [PubMed]
- Peti, W.; Page, R. Molecular basis of MAP kinase regulation. Protein Sci. 2013, 22, 1698–1710. [Google Scholar] [CrossRef]
- Fang, L.; Wu, H.M.; Ding, P.S.; Liu, R.Y. TLR2 mediates phagocytosis and autophagy through JNKsignaling pathway in Staphylococcus aureus-stimulated RAW264.7 cells. Cell. Signal. 2014, 26, 806–814. [Google Scholar] [CrossRef]
- Siljamaki, E.; Raiko, L.; Toriseva, M. p38delta mitogen-activated protein kinase regulates the expression of tight junction protein ZO-1 in differentiating human epidermal keratinocytes. Arch. Dermatol. Res. 2014, 306, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Coulombe, P.; Meloche, S. Atypical mitogen-activated protein kinases: Structure, regulation, andfunctions. Biochim. Biophys. ActaMol. Cell Res. 2007, 1773, 1376–1387. [Google Scholar] [CrossRef] [PubMed]
- Gaestel, M.; Kotlyarov, A.; Kracht, M. Targeting innate immunity protein kinase signaling ininflammation. Nat. Rev. Drug Discov. 2009, 8, 480–499. [Google Scholar] [CrossRef] [PubMed]
- Zlobin, A.; Bloodworth, J.C.; Osipo, C. Mitogen-Activated Protein Kinase (MAPK) Signaling BT. In Predictive Biomarkers in Oncology: Applications in Precision Medicine; Badve, S., Kumar, G.L., Eds.; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Thalhamer, T.; McGrath, M.A.; Harnett, M.M. MAPKs and their relevance to arthritis and inflammation. Rheumatology 2008, 47, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Escós, A.; Risco, A.; Alsina-Beauchamp, D.; Cuenda, A. p38 γ and p38 δ Mitogen Activated ProteinKinases (MAPKs), New Stars in the MAPK Galaxy. Front. Cell DevBiol. 2016, 4, 31. [Google Scholar] [CrossRef]
- Ittner, A.; Block, H.; Reichel, C.A.; Varjosalo, M.; Gehart, H.; Sumara, G.; Gstaiger, M.; Krombach, F.; Zarbock, A.; Ricci, R. Regulation of PTEN activity by p38delta-PKD1 signaling in neutrophils confer inflammatory responses in the lung. J. Exp. Med. 2012, 209, 2229–2246. [Google Scholar] [CrossRef]
- Kourea, H.P.; Nikolaou, M.; Tzelepi, V. Expression of phosphorylated Akt, mTOR, and MAPK intype I endometrial carcinoma: Clinical significance. Anticancer. Res. 2015, 35, 2321–2331. [Google Scholar]
- Knight, T.; Irving, J.A.E. Ras/Raf/MEK/ERK pathway activation in childhood acute lymphoblasticleukemia and its therapeutic targeting. Front. Oncol. 2014, 4, 160. [Google Scholar] [CrossRef]
- Remy, G.; Risco, A.M.; Inesta-Vaquera, F.A.; González-Terán, B.; Sabio, G.; Davis, R.J.; Cuenda, A. Differential activation of p38MAPK isoformsby MKK6 and MKK3. Cell. Signal. 2010, 22, 660–667. [Google Scholar] [CrossRef]
- Slattery, M.L.; Mullany, L.E.; Sakoda, L.C.; Wolff, R.K.; Samowitz, W.S.; Herrick, J.S. The MAPK signaling pathway in colorectal cancer: Dysregulated genes and their association with microRNAs. Cancer Inform. 2018, 17. [Google Scholar] [CrossRef] [PubMed]
- Court, N.W.; dos Remedios, C.G.; Cordell, J.; Bogoyevitch, M.A. Cardiac Expression and Subcellular Localization of the p38 Mitogen-activated Protein Kinase Member, Stress-activated Protein Kinase-3(SAPK3). J. Mol. Cell. Cardiol. 2002, 34, 413–426. [Google Scholar] [CrossRef]
- Del Reino, P.; Alsina-Beauchamp, D.; Escós, A.; Cerezo-Guisado, M.I.; Risco, A.; Aparicio, N. Prooncogenic role of alternative p38 mitogen-activated protein kinases p38gamma and p38delta, linking inflammation and cancer in colitis-associated colon cancer. Cancer Res. 2014, 74, 6150–6160. [Google Scholar] [CrossRef]
- Kumar, S.; Boehm, J.; Lee, J. p38 MAP kinases: Key signaling molecules as therapeutic targets for inflammatory diseases. Nat. Rev. Drug Discov. 2003, 2, 717–726. [Google Scholar] [CrossRef]
- Yoon, W.J.; Lee, N.H.; Hyun, C.G. Limonene suppresses lipopolysaccharide-induced production of nitric oxide, prostaglandin E2, and pro-inflammatory cytokines in RAW 264.7 macrophages. J. Oleo Sci. 2010, 59, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Ventura, J.J.; Tenbaum, S.; Perdiguero, E. p38α MAPkinase is essential in lung stem and progenitor cell proliferation and differentiation. Nat. Genet. 2007, 39, 750–758. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Ramos, R.; Van Langendonckt, A.; Defriere, S.; Lousse, J.C.; Colette, S.; Devoto, L. Involvement of the nuclear factor-kB pathway in the pathogenesis of endometriosis. Fertil. Steril. 2010, 94, 1985–1994. [Google Scholar] [CrossRef]
- Karin, M.; Greten, F.R. NF-κB: Linking inflammation and immunity to cancer development and progression. Nat. Rev. Immunol. 2005, 5, 749–759. [Google Scholar] [CrossRef]
- Shen, Y.; Liu, B.; Mao, W.; Gao, R.; Feng, S.; Qian, Y.; Cao, J. PGE2 downregulates LPS-induced inflammatory responses via the TLR4-NF-κB signaling pathway in bovine endometrial epithelial cells. Prostaglandins Leukot. Essent. Fat. Acids 2018, 129, 25–31. [Google Scholar] [CrossRef]
- Huang, B.; Yang, X.D.; Lamb, A.; Chen, L.F. Posttranslational modifications of NF- kappaB: Another Layer of regulation for NF-kB signaling pathway. Cell. Signal. 2010, 22, 1282–1290. [Google Scholar] [CrossRef]
- Bhatt, D.; Ghosh, S. Regulation of the NF-κB-mediated transcription of inflammatory genes. Front. Immunol. 2014, 5, 71. [Google Scholar] [CrossRef]
- Hara, H.; Bakal, C.; Wada, T.; Bouchard, D.; Rottapel, R.; Saito, T.; Penninger, J.M. The Molecular Adapter Carma1 Controls Entry of IκB Kinase into the Central Immune Synapse. J. Exp. Med. 2004, 200, 1167–1177. [Google Scholar] [CrossRef]
- Yang, X.D.; Tajkhorshid, E.; Chen, L.F. Functional interplay between acetylation and methylation of the RelA subunit of NF-kappaB. Mol. Cell. Biol. 2010, 30, 2170–2180. [Google Scholar] [CrossRef]
- Ghosh, S.; Karin, M. Missing pieces in the NF-kB puzzle. Cell 2002, 109, S81–S96. [Google Scholar] [CrossRef]
- Hoesel, B.; Schmid, J.A. The complexity of NF-κB signaling in inflammation and cancer. Mol. Cancer 2013, 12, 1–15. [Google Scholar] [CrossRef]
- Marinho, H.S.; Real, C.; Cyrne, L.; Soares, H.; Antunes, F. Hydrogen peroxide sensing, signaling and regulation of transcription factors. Redox Biol. 2014, 2, 535–562. [Google Scholar] [CrossRef] [PubMed]
- Moreno, R.; Sobotzik, J.M.; Schultz, C.; Schmitz, M.L. Specification of the NF-κB transcriptional response by p65 phosphorylation and TNF-induced nuclear translocation of IKKε. Nucleic Acids Res. 2010, 38, 6029–6044. [Google Scholar] [CrossRef] [PubMed]
- Heissmeyer, V.; Krappmann, D.; Hatada, E.N.; Scheidereit, C. Shared pathways of IκB kinase- inducedSCF(βTrCP)-mediated ubiquitination and degradation for the NF-κB precursor p105 and IκBα. Mol. Cell. Biol. 2001, 21, 1024–1035. [Google Scholar]
- Häcker, H.; Karin, M. Regulation and Function of IKK and IKK-Related Kinases. Science’s STKE 2006, 13. [Google Scholar] [CrossRef]
- Gabler, C.; Fischer, C.; Drillich, M.; Einspanier, R.; Heuwieser, W. Time-dependent mRNA expression of selected pro-inflammatory factors in the endometrium of primiparous cows postpartum. Reprod. Biol. Endocrinol. 2010, 8, 152. [Google Scholar] [CrossRef]
- Almeria, S.; Serrano, B.; Yaniz, J.L.; Darwich, L.; Lopez-Gatius, F. Cytokine gene expression profiles in peripheral blood mononuclear cells from Neospora caninum naturally infected dams throughout gestation. Vet. Parasitol. 2012, 183, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.J.; Barreto, R.S.N.; Perecin, F.; Mansouri-Attia, N.; Pereira, F.T.V.; Meirelles, F.V. Modulation of Maternal Immune System During Pregnancy in the Cow. Reprod. Domest. Anim. 2012, 47, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Nasu, K.; Narahara, H. Pattern recognition via the toll-like receptor system in the human female genital tract. Mediat. Inflamm. 2010, 2010. [Google Scholar] [CrossRef] [PubMed]
- Baravalle, M.E.; Stassi, A.F.; Velazquez, M.M.L.; Belotti, E.M.; Rodríguez, F.M.; Ortega, H.H.; Salvetti, N.R. Altered expression of pro-inflammatory cytokines in ovarian follicles of cows with the cystic ovarian disease. J. Comp. Pathol. 2015, 153, 116–130. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S. Orostachys japonicus extract inhibits the lipopolysaccharide-induced pro-inflammatory factors by suppression of transcription factors. Food Sci. Nutr. 2020, 8, 1812–1817. [Google Scholar] [CrossRef]
- Fengyang, L.; Yunhe, F.; Bo, L.; Zhicheng, L.; Depeng, L.; Dejie, L.; Zhengtao, Y. Steviosidesuppressed inflammatory cytokine secretion by downregulation of NF-kB and MAPK signaling pathways in LPS-Stimulated RAW264.7 cells. Inflammation 2012, 35, 1669–1675. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Wang, Y.; Wang, H.; Dong, J.; Li, Z.; Li, J. Different effects of cortisol on pro-inflammatory gene expressions in LPS-, heat-killed E. coli-, or live E. coli-stimulated bovine endometrial epithelial cells. BMC Vet. Res. 2020, 16, 1–8. [Google Scholar] [CrossRef]
- Kelly, P.; Meade, K.G.; O’Farrelly, C. Non-canonical inflammasome-mediated IL-1β production by primary endometrial epithelial and stromal fibroblast cells is NLRP3 and caspase-4 dependent. Front. Immunol. 2019, 10, 1–17. [Google Scholar] [CrossRef]
- Healy, L.L.; Cronin, J.G.; Sheldon, I.M. Endometrial cells sense and react to tissue damage during infection of the bovine endometrium via interleukin 1. Sci. Rep. 2014, 4, 7060. [Google Scholar] [CrossRef]
- Healy, L.L.; Cronin, J.G.; Sheldon, I.M. Polarized epithelial cells secrete interleukin 6 apically in the bovine endometrium. Biol. Reprod. 2015, 92, 151-1. [Google Scholar] [CrossRef]
- Brodzki, P.; Kostro, K.; Brodzki, A.; Wawron, W.; Marczuk, J.; Kurek, L. Inflammatory cytokines and acutephase proteins concentrations in the peripheral blood and uterus of cows that developed endometritis during early postpartum. Theriogenology 2015, 84, 11–18. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, Z.; Yang, Y.; Ping, M.; Shaukat, A.; Yang, J.; Guo, Y.F.; Zhang, T.; Zhu, X.Y.; Qiu, J.X.; et al. Catalpol ameliorates LPS-induced endometritis by inhibiting inflammation and TLR4/NF-κB signaling. J. Zhejiang Univ. Sci. B 2019, 20, 816–827. [Google Scholar] [CrossRef]
- Clark, I.A.; Vissel, B. The meteorology of cytokine storms, and the clinical usefulness of this knowledge. Semin. Immunopathol. 2017, 39, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.C.; Yun, H.J.; Cho, W.K.; Gu, M.J.; Jin, Y.M. Inhibitory effects of palmultang on inflammatory mediator production related to the suppression of NF-κB and MAPK pathways and induction of HO-1 expression in macrophages. Int. J. Mol. Sci. 2014, 15, 8443–8457. [Google Scholar] [CrossRef] [PubMed]
- Hashem, N.M.; Gonzalez-Bulnes, A. State-of-the-art and prospective of nanotechnologies for smart reproductive management of farm animals. Animals 2020, 10, 840. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, F.; Hosseini-Nasr, M.; Rad-Malekshahi, M.; Samadi, N.; Atyabi, F.; Dinarvand, R. Preparation and antibacterial activity evaluation of rifampicin-loaded poly lactide-co-glycolide nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 161–167. [Google Scholar] [CrossRef]
- Hill, E.K.; Li, J. Current and future prospects for nanotechnology in animal production. J. Anim. Sci. Biotechnol. 2017, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Underwood, C.; van Eps, A.W. Nanomedicine and veterinary science: The reality and the practicality. Vet. J. 2012, 193, 12–23. [Google Scholar] [CrossRef]
- Kim, B.Y.S.; Rutka, J.T.; Chan, W.C.W. Nanomedicine. N. Engl. J. Med. 2010, 363, 2434–2443. [Google Scholar] [CrossRef]
- Pritchard, N.; Kaitu’u-Lino, T.; Harris, L.; Tong, S.; Hannan, N. Nanoparticles in pregnancy: The next frontier in reproductive therapeutics. Hum. Reprod. Update 2020, 27, 280–304. [Google Scholar] [CrossRef]
- Jung, W.K.; Koo, H.C.; Kim, K.W.; Shin, S.; Kim, S.H.; Park, Y.H. Antibacterial activity and mechanism of action of the silver ion in Staphylococcus aureus and Escherichia coli. Appl. Environ. Microbiol. 2008, 74, 2171–2178. [Google Scholar] [CrossRef]
- Keelan, J.A.; Leong, J.W.; Ho, D.; Iyer, K.S. Therapeutic and safety considerations of nanoparticle-mediated drug delivery in pregnancy. Nanomedicine 2015, 10, 2229–2247. [Google Scholar] [CrossRef]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramirez, J.T. The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 16, 2346–2353. [Google Scholar] [CrossRef]
- Rai, M.; Yadav, A.; Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2001, 27, 76–83. [Google Scholar] [CrossRef]
- Kim, J.C.; Shin, H.C.; Cha, S.W.; Koh, W.S.; Chung, M.K.; Han, S.S. Evaluation of developmental toxicity in rats exposed to the environmental estrogen bisphenol A during pregnancy. Life Sci. 2001, 69, 2611–2625. [Google Scholar] [CrossRef]
- Carvalho, S.G.; Araujo, V.H.S.; dos Santos, A.M.; Duarte, J.L.; Silvestre, A.L.P.; FonsecSantos, B.; Villanova, J.C.O.; Gremião, M.P.D.; Chorilli, M. Advances and challenges in nanocarriers and nanomedicines for veterinary application. Int. J. Pharm. 2020, 580, 119214. [Google Scholar] [CrossRef] [PubMed]
- Cerbu, C.; Kah, M.; White, J.C.; Astete, C.E.; Sabliov, C.M. Fate of Biodegradable Engineered Nanoparticles Used in Veterinary Medicine as Delivery Systems from a One Health Perspective. Molecules 2021, 26, 523. [Google Scholar] [CrossRef]
- Greatti, V.R.; Oda, F.; Sorrechia, R.; Kapp, B.R.; Seraphim, C.M.; Weckwerth, A.C.V.B.; Chorilli, M.; Da Silva, P.B.; Eloy, J.O.; Kogan, M.J.; et al. Poly-ε-caprolactone Nanoparticles Loaded with 4-Nerolidylcatechol (4-NC) for Growth Inhibition of Microsporum canis. Antibiotics 2020, 9, 894. [Google Scholar] [CrossRef]
- Tu’uhevaha, J.; Kaitu’u-Lino, T.J.; Pattison, S.; Ye, L.; Tuohey, L.; Sluka, P.; MacDiarmid, J.; Brahmbhatt, H.; Johns, T.; Horne, A.W.; et al. Targeted nanoparticle delivery of doxorubicin into placental tissues to treat ectopic pregnancies. Endocrinology 2013, 154, 911–919. [Google Scholar] [CrossRef]
- Youssef, F.S.; El-Banna, H.A.; Elzorba, H.Y.; Galal, A.M. Application of some nanoparticles in the field of veterinary medicine. Int. J. Vet. Sci. Med. 2019, 7, 78–93. [Google Scholar] [CrossRef] [PubMed]
- Pamungkas, F.A.; Sianturi, R.S.G.; Wina, E.; Kusumaningrum, D.A. Chitosan nanoparticle of hCG (Human Chorionic Gonadotrophin) hormone in increasing induction of dairy cattle ovulation. J. Ilmu Ternak Dan Vet. 2016, 21, 34. [Google Scholar] [CrossRef]
- Ebeid, K.; Meng, X.; Thiel, K.W.; Do, A.V.; Geary, S.M.; Morris, A.S.; Pham, E.L.; Wongrakpanich, A.; Chhonker, Y.S.; Murry, D.J.; et al. Synthetically lethal nanoparticles for treatment of endometrial cancer. Nat. Nanotechnol. 2018, 13, 72–81. [Google Scholar] [CrossRef]
- Paul, J.W.; Hua, S.; Ilicic, M.; Tolosa, J.M.; Butler, T.; Robertson, S.; Smith, R. Drug delivery to the human and mouse uterus using immunoliposomes targeted to the oxytocin receptor. Am. J. Obstet. Gynecol. 2017, 216, 283.e1–283.e14. [Google Scholar] [CrossRef]
- Hassanein, E.M.; Hashem, N.M.; El-Azrak, K.E.D.M.; Gonzalez-Bulnes, A.; Hassan, G.A.; Salem, M.H. Efficiency of GnRH–loaded chitosan nanoparticles for inducing LH secretion and fertile ovulations in protocols for artificial insemination in rabbits does. Animals 2021, 11, 440. [Google Scholar] [CrossRef]
- Singh, A.K.; Chakravarty, B.; Chaudhury, K. Nanoparticle-assisted combinatorial therapy for effective treatment of endometriosis. J. Biomed. Nanotechnol. 2015, 11, 789–804. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Dixon, M.; Zucchelli, M.; Hambiliki, F.; Levkov, L.; Hovatta, O.; Kere, J. Expression analysis of the NLRP gene family suggests a role in human preimplantation development. PLoS ONE 2008, 3, e2755. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, G.; Zhu, X.; Jiang, K.; Wu, H.; Deng, G.; Qiu, C. Sodium selenite induces apoptosis ROS-mediated NF-κB signaling and activation of the Bax-caspase-9-caspase-3 axis in 4T1 cells. J. Cell. Physiol. 2019, 234, 2511–2522. [Google Scholar] [CrossRef]
- Diwan, M.; Tafaghodi, M.; Samuel, J. Enhancement of immune responses by co-delivery of a CpG oligodeoxynucleotide and tetanus toxoid in biodegradable nanospheres. J. Control. Release 2002, 85, 247–262. [Google Scholar] [CrossRef]
- Schlosser, E.; Mueller, M.; Fischer, S.; Basta, S.; Busch, D.H.; Gander, B.; Groettrup, M. TLR ligands and antigen need to be co-encapsulated into the same biodegradable microsphere for the generation of potent cytotoxic T lymphocyte responses. Vaccine 2008, 26, 1626–1637. [Google Scholar] [CrossRef] [PubMed]
- Florindo, H.F.; Pandit, S.; Goncalves, L.M.; Videira, M.; Alpar, O.; Almeida, A.J. Antibody and cytokine-associated immune responses to S. equi antigens entrapped in PLA nanospheres. Biomaterials 2009, 30, 5161–5169. [Google Scholar] [CrossRef]
- Rawat, M.; Singh, D.; Saraf, S.; Saraf, S. Nanocarriers: Promising Vehicle for Bioactive Drugs. Biol. Pharm. Bull. 2006, 29, 1790–1798. [Google Scholar] [CrossRef] [PubMed]
- Riehemann, K.; Schneider, S.W.; Luger, T.A.; Godin, B.; Ferrari, M.; Fuchs, H. Nanomedicine–challenge and perspectives. Angew. Chem. Int. Engl. 2009, 48, 872–897. [Google Scholar] [CrossRef] [PubMed]
- Mahapatro, A.; Singh, D.K. Biodegradable nanoparticles are an excellent vehicle for site-directed in-vivo delivery of drugs and vaccines. J. Nanobiotechnol. 2011, 9, 55. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oladejo, A.O.; Li, Y.; Wu, X.; Imam, B.H.; Yang, J.; Ma, X.; Yan, Z.; Wang, S. Modulation of Bovine Endometrial Cell Receptors and Signaling Pathways as a Nanotherapeutic Exploration against Dairy Cow Postpartum Endometritis. Animals 2021, 11, 1516. https://doi.org/10.3390/ani11061516
Oladejo AO, Li Y, Wu X, Imam BH, Yang J, Ma X, Yan Z, Wang S. Modulation of Bovine Endometrial Cell Receptors and Signaling Pathways as a Nanotherapeutic Exploration against Dairy Cow Postpartum Endometritis. Animals. 2021; 11(6):1516. https://doi.org/10.3390/ani11061516
Chicago/Turabian StyleOladejo, Ayodele Olaolu, Yajuan Li, Xiaohu Wu, Bereket Habte Imam, Jie Yang, Xiaoyu Ma, Zuoting Yan, and Shengyi Wang. 2021. "Modulation of Bovine Endometrial Cell Receptors and Signaling Pathways as a Nanotherapeutic Exploration against Dairy Cow Postpartum Endometritis" Animals 11, no. 6: 1516. https://doi.org/10.3390/ani11061516
APA StyleOladejo, A. O., Li, Y., Wu, X., Imam, B. H., Yang, J., Ma, X., Yan, Z., & Wang, S. (2021). Modulation of Bovine Endometrial Cell Receptors and Signaling Pathways as a Nanotherapeutic Exploration against Dairy Cow Postpartum Endometritis. Animals, 11(6), 1516. https://doi.org/10.3390/ani11061516





