Semicarbazide Accumulation, Distribution and Chemical Forms in Scallop (Chlamys farreri) after Seawater Exposure
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Exposure Experiment
2.2.1. Scallops and Temporary Culture
2.2.2. Medicated Bath
2.2.3. Sampling of Scallop
2.3. Sample Preparation
2.3.1. Determination of Semicarbazide
2.3.2. Determination of Tissue-Bound Semicarbazide
2.4. Instruments and Chromatographic Conditions
2.5. Calculation of the Bioconcentration Factor
2.6. Statistical Analysis
3. Results
3.1. Exposure of Scallops to Semicarbazide in Aquaculture Seawater
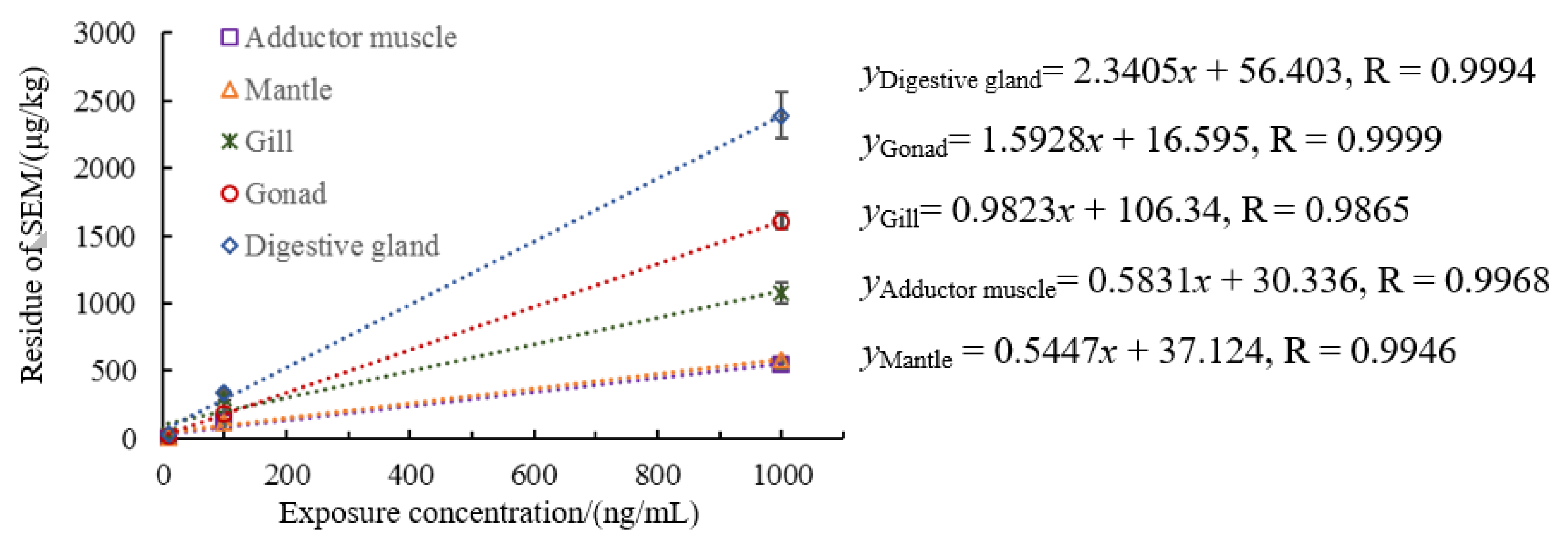
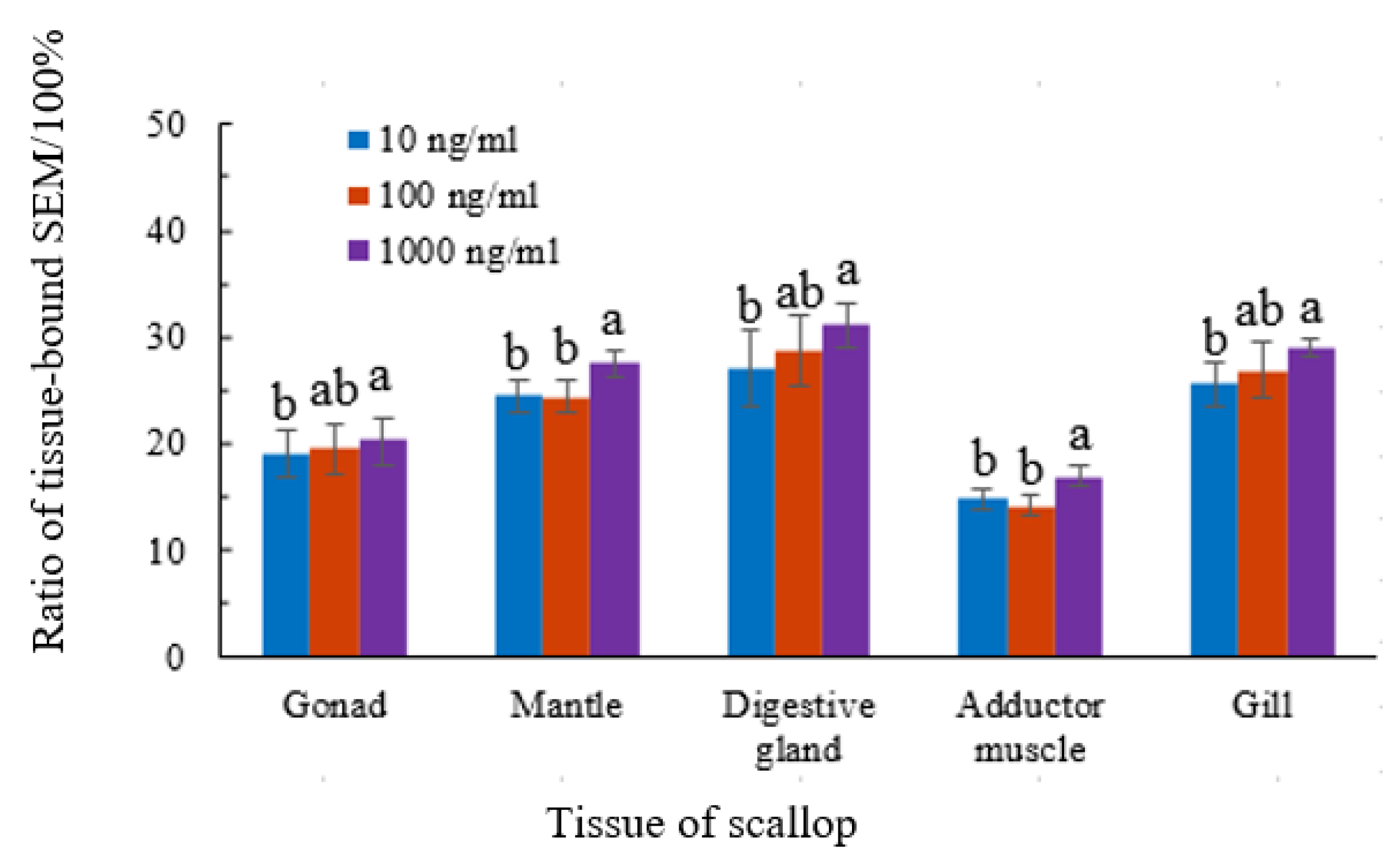
3.2. Morphology of Semicarbazide in Scallop
3.3. Semicarbazide Ci-Ti
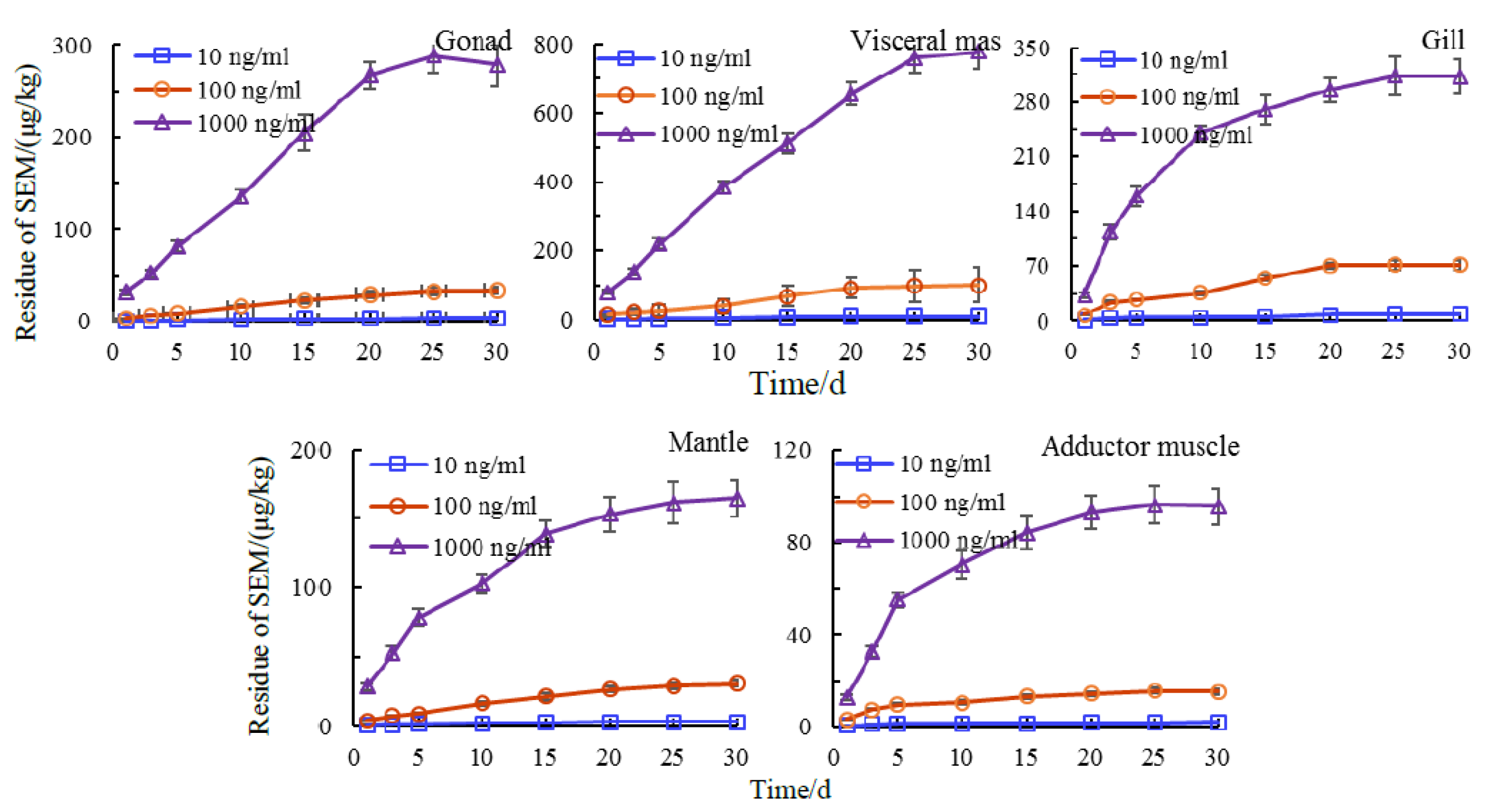
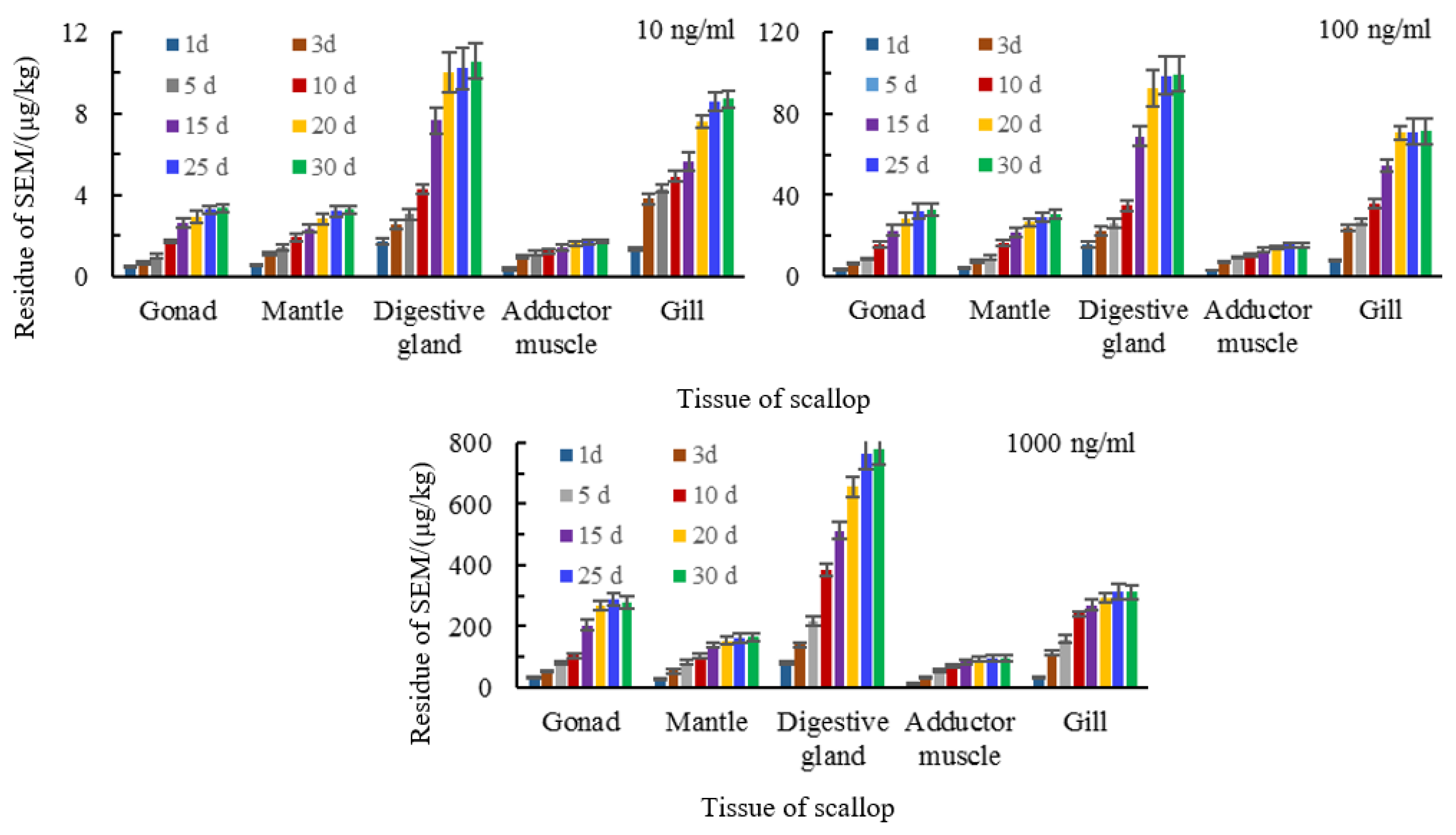
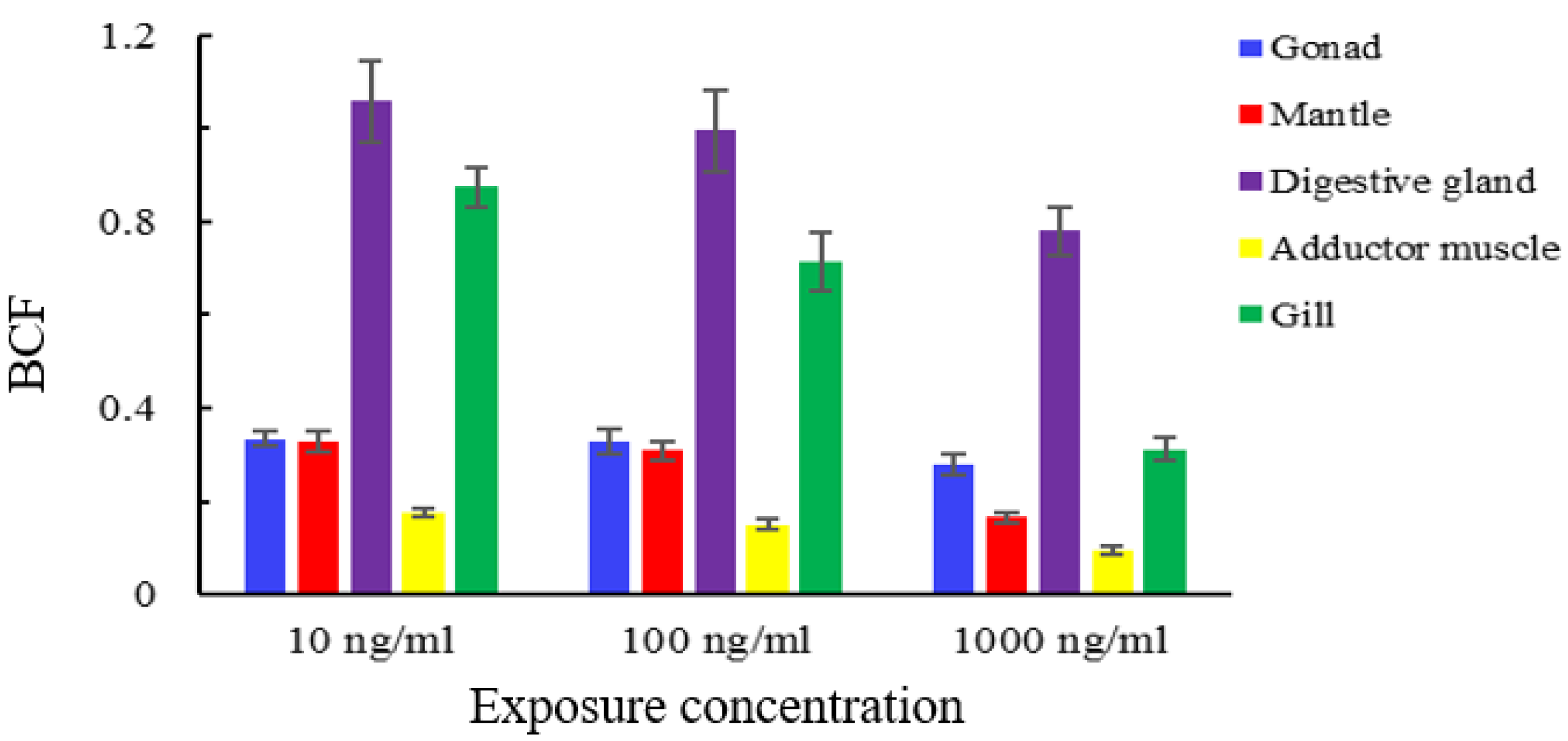
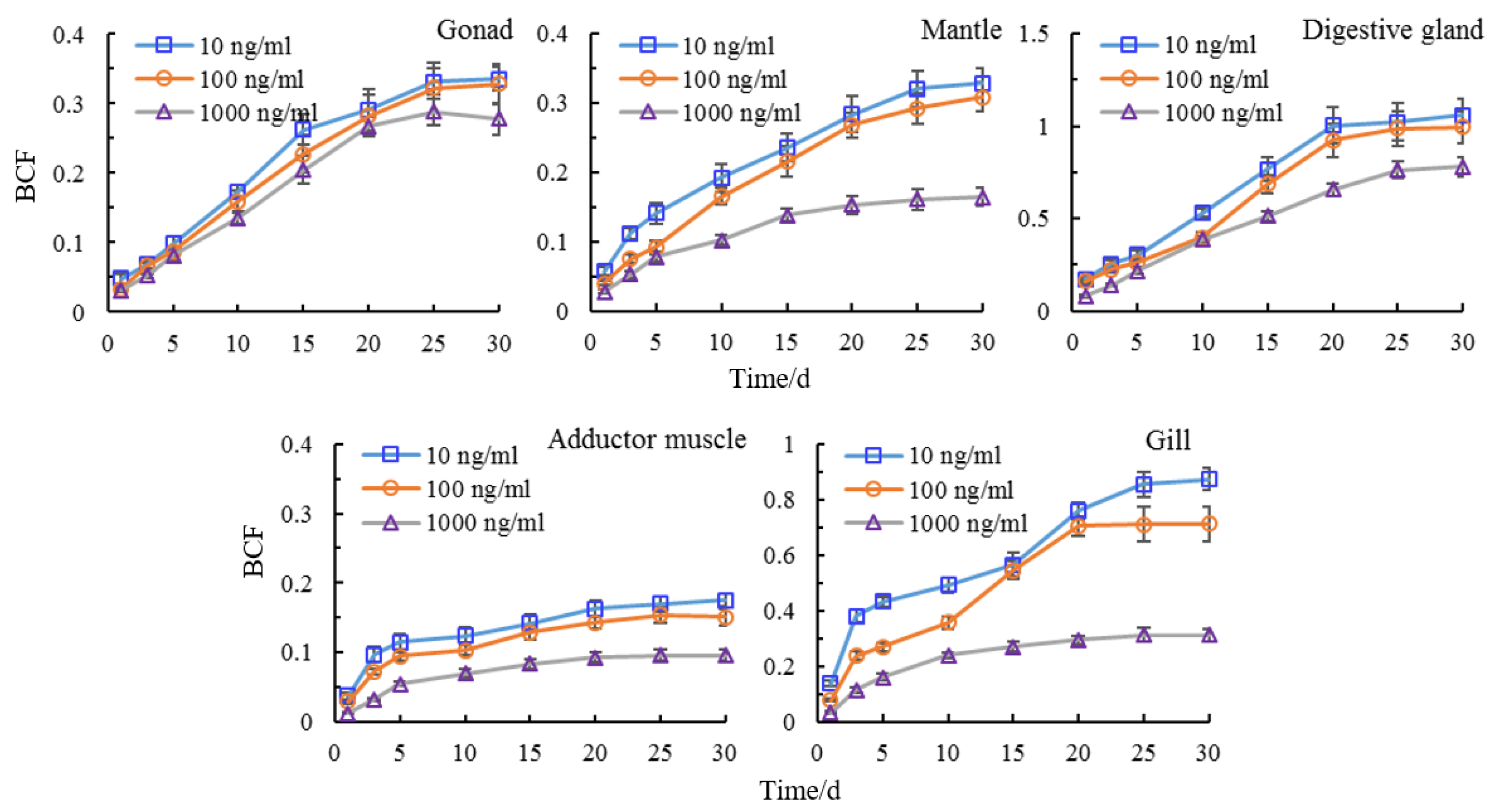
3.4. Tissue Distribution of Semicarbazide
3.5. Bioaccumulation of Semicarbazide in Scallop
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- EunChae, R.; Ji-Sung, P.; Sib Sankar, G.; Se Chang, P. A simplified modification to rapidly determine the residues of nitrofurans and their metabolites in aquatic animals by HPLC triple quadrupole mass spectrometry. Environ. Sci. Pollut. Res. 2020. [Google Scholar] [CrossRef]
- Bogialli, S.; Di Corcia, A. Recent applications of liquid chromatography–mass spectrometry to residue analysis of antimicrobials in food of animal origin. Anal. Bioanal. Chem. 2009, 395, 947–966. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Yang, S.; Liu, J.; Mi, J.; Dou, L.; Pan, Y.; Mari, G.M.; Wang, Z. Progress in immunoassays for nitrofurans detection food and agricultural immunology. Food Agric. Immunol. 2020, 31, 907–926. [Google Scholar] [CrossRef]
- Wang, K.; Kou, Y.; Wang, M.; Ma, X.; Wang, J. Determination of nitrofuran metabolites in fish by ultraperformance liquid chromatography-photodiode array detection with thermostatic ultrasound-assisted derivatization. ACS Omega 2020, 5, 18887–18893. [Google Scholar] [CrossRef] [PubMed]
- Ralica, K. Antibiotic residues and human health hazard–review. Bulg. J. Agric. Sci. 2020, 26, 664–668. [Google Scholar]
- Commission Regulation (EC) No 1442/95. Amending annexes I, II, III and IV of Council Regulation (EEC) No 2377/90 laying down a community procedure for the establishment of maximum residue limits of veterinary medicinal products in foodstuffs of animal origin. Off. J. Eur. Communities 1995, 143, 26–30.
- FDA. Topical nitrofurans; extralabel animal drug use; order of prohibition. Fed. Regist. Dep. Heal. Hum. Serv. 2002, 67, 5470–5471. [Google Scholar]
- Ministry of Agriculture of China. Announcement No.235 of the Ministry of Agriculture of China, Maximum Residue Limits of Veterinary Drugs in Animal Food; China Standards Press: Beijing, China, 2002.
- Yu, W.L.; Liu, W.H.; Tian, W.R.; Li, X.T.; Wang, X.H. Semicarbazide universality study and its speculated formation pathway. J. Food Saf. 2019, 39, 1–8. [Google Scholar] [CrossRef]
- Hron, R.; Jursic, B.S. Preparation of substituted semicarbazides from corresponding amines and hydrazines via phenyl carbamates. Tetrahedron Lett. 2014, 55, 1540–1543. [Google Scholar] [CrossRef]
- Subashchandrabose, S.; Babu, N.R.; Saleem, H.; Padusha, M.S.A. Vibrational studies on (E)-1-((pyridine-2-yl) methylene) semicarbazide using experimental and theoretical method. J. Mol. Struct. 2015, 1094, 254–263. [Google Scholar] [CrossRef]
- Adam, B.; Benjamin, P.Y.L.; David, L.; Stephen, W.S. Semicarbazide formation in azodicarbonamide-treated flour: A model study. J. Agric. Food Chem. 2004, 52, 5730–5734. [Google Scholar]
- Chen, L.; Cui, H.; Dong, Y.L.; Guo, D.Q.; He, Y.J.; Li, X.J.; Yuan, Z.B.; Zou, H. Simultaneous detection of azodicarbonamide and the metabolic product semicarbazide in flour by capillary electrophoresis. Food Anal. Method 2016, 9, 1106–1111. [Google Scholar] [CrossRef]
- Wei, T.F.; Li, G.K.; Zhang, Z.M. Rapid determination of trace semicarbazide in flour products by high-performance liquid chromatography based on a nucleophilic substitution reaction. J. Sep. Sci. 2017, 40, 1993–2001. [Google Scholar] [CrossRef] [PubMed]
- Hoenicke, K.; Gatermann, R.; Hartig, L.; Mandix, M.; Otte, S. Formation of semicarbazide (SEM) in food by hypochlorite treatment: Is SEM a specific marker for nitrofurazone abuse? Food Addit. Contam. 2004, 21, 526–537. [Google Scholar] [CrossRef] [PubMed]
- Bendall, J.G. Semicarbazide is non-specific as a marker metabolite to reveal nitrofurazone abuse as it can form under Hofmann conditions. Food Addit. Contam. 2009, 26, 47–56. [Google Scholar] [CrossRef]
- Vlastos, D.; Moshou, H.; Epeoglou, K. Evaluation of genotoxic effects of semicarbazide on cultured human lymphocytes and rat bone marrow. Food Chem. Toxicol. 2009, 48, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Richard, H.S.; Ludovica, V.; Walburga, S.; Lucie, R. Why semicarbazide (SEM) is not an appropriate marker for the usage of nitrofurazone on agricultural animals. Food Addit. Contam. A 2015, 32, 1842–1850. [Google Scholar]
- Kwon, J.W. Semicarbazide: Natural occurrence and uncertain evidence of its formation from food processing. Food Control. 2017, 72, 268–275. [Google Scholar] [CrossRef]
- Magyar, K.; Mészáros, Z.; Mátyus, P. Semicarbazide-sensitive amine oxidase. Its physiological significance. Pure Appl. Chem. 2001, 73, 1393–1400. [Google Scholar] [CrossRef]
- Dawson, D.A.; Rinaldi, A.C.; Pöch, G. Biochemical and toxicological evaluation of agent-cofactor reactivity as a mechanism of action of osteolathyrism. Toxicology 2002, 177, 267–284. [Google Scholar] [CrossRef]
- Macedo, C.E.; Martinez, R.C.; Albrechet-Souza, L.; Molina, V.A.; Brandão, M.L. 5-HT2- and D1-mechanisms of the basolateral nucleus of the amygdala enhance conditioned fear and impair unconditioned fear. Appl. Behav. Brain Res. 2007, 177, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Wang, W.; Tian, H.; Zhang, X.N.; Guo, L.L.; Ru, S.G. An emerging water contaminant, semicarbazide, exerts an anti-estrogenic effect in zebrafish (Danio rerio). Bull. Env. Contam. Toxicol. 2014, 93, 280–288. [Google Scholar] [CrossRef]
- Yu, M.; Feng, Y.L.; Zhang, X.N.; Wang, J.; Tian, H.; Wang, W.; Ru, S.G. Semicarbazide disturbs the reproductive system of male zebrafish (Danio rerio) through the GABAergic system. Reprod. Toxicol. 2017, 73, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Yue, Z.H.; Yu, M.; Zhao, X.N.; Wang, J.; Ru, S.G. The anti-androgenic effect of chronic exposure to semicarbazide on male Japanese flounder (Paralichthys olivaceus) and its potential mechanisms. Comp. BioChem. Phys. C 2018, 210, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Yue, Z.H.; Yu, M.; Zhao, H.F.; Wang, J.; Zhang, X.N.; Tian, H.; Wang, W.; Ru, S.G. The anti-estrogenicity of chronic exposure to semicarbazide in female Japanese flounders (Paralichthys olivaceus), and its potential mechanisms. Mar. Pollut. Bull. 2018, 129, 806–812. [Google Scholar] [CrossRef]
- Tian, X.H.; Xu, Y.J.; Zheng, H.W.; Cui, Y.M.; Jiang, F.; Gong, X.H. Semicarbazide exposure induces histological damage and enzymatic reactions in apostichopus japonicas. Mod. Food Sci. Technol. 2020, 36, 35–42. [Google Scholar]
- Xu, Y.J.; Sun, X.K.; Tian, X.H.; Gong, X.H.; Zhang, X.Z.; Zhang, L.M. Survey of semicarbazide contamination in castal waters adjacent to the chaohe river estuary. Oceanol. Limnol. Sin. 2010, 41, 1–5. [Google Scholar]
- Tian, X.H.; Xu, Y.J.; Song, X.K.; Gong, X.H.; Liu, Y.H.; Zhou, Q.L.; Wang, Z.Q.; Xia, C.H. Temporal and spatial distribution of semicarbazide in western Laizhou Bay. Mar. Pollut. Bull. 2016, 112, 393–398. [Google Scholar] [CrossRef]
- Tian, X.H.; Xu, Y.J.; Gong, X.H.; Han, D.F.; Wang, Z.Q.; Zhou, Q.L.; Sun, C.X.; Ren, C.B.; Xue, J.L.; Xia, C.H. Environmental status and early warning value of the pollutant Semicarbazide in Jincheng and Sishili Bays, Shandong Peninsula, China. Sci. Total Env. 2017, 576, 868–878. [Google Scholar] [CrossRef]
- Nouws, J.F.; Laurensen, J. Postmortal degradation of furazolidone and furaltadone in edible tissues of calves. Vet. Q 1990, 12, 56–59. [Google Scholar] [CrossRef]
- Kim, D.; Kim, B.; Hyung, S.W.; Lee, C.H.; Kim, J. An optimized method for the accurate determination of nitrofurans in chicken meat using isotope dilution–liquid chromatography/mass spectrometry. J. Food Compos. Anal. 2015, 40, 24–31. [Google Scholar] [CrossRef]
- Rizzo, G.; Baroni, L. Soy, soy foods and their role in vegetarian diets. Nutrients 2018, 10, 43. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.X.; Zhu, Z.; Yang, P.; Lu, S.Y.; Liu, H.H.; Liu, W.J.; Liu, G.H. Simultaneous determination of eight nitrofuran residues in shellfish and fish using ultra-high performance liquid chromatography–tandem mass spectrometry. J. Food Compos. Anal. 2020, 92, 103540. [Google Scholar] [CrossRef]
- Cooper, K.M.; Kennedy, D.G. Stability studies of the metabolites of nitrofuran antibiotics during storage and cooking. Food Addit. Contam. 2007, 24, 935–942. [Google Scholar] [CrossRef]
- Chu, P.S.; Lopez, M.I. Liquid chromatography-tandem mass spectrometry for the determination of protein-bound residues in shrimp dosed with nitrofurans. J. Agric. Food Chem. 2005, 53, 8934–8939. [Google Scholar] [CrossRef]
- Van Poucke, C.; Detavernier, C.; Wille, M.; Kwakman, J.; Sorgeloos, P.; Van Peteghem, C. Investigation into the possible natural occurence of semicarbazide in Macrobrachium rosenbergii prawns. Agr. Food Chem. 2011, 59, 2107–2112. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2020; Sustainability in Action; FAO: Rome, Italy, 2020; pp. 10–15. [Google Scholar] [CrossRef]
- Bureau of Fisheries of Ministry of Agriculture and Rural Affairs of the PRC; National Fisheries Technology Extension Center; China Society of Fisheries. Part Two: Production. In 2020 China Fishery Statistical Yearbook, 1st ed; China Agricultural Press: Beijing, China, 2020; pp. 22–23. [Google Scholar]
- Department of probability and statistics, Institute of mathematics, Chinese Academy of Sciences. The critical value (ra) table of correlation coefficient ρ = 0. In Common Mathematical Statistics, 1st ed.; Science Press: Beijing, China, 1979; p. 18. [Google Scholar]
- Zenker, A.; Cicero, M.R.; Prestinaci, F.; Bottoni, P.; Carere, M. Bioaccumulation and biomagnification potential of pharmaceuticals with a focus to the aquatic environment. J. Environ. Manag. 2014, 133, 378–387. [Google Scholar] [CrossRef]
- Cammilleri, G.; Galluzzo, F.G.; Fazio, F.; Pulvirenti, A.; Vella, A.; Dico, G.M.L.; Macaluso, A.; Ciaccio, G.; Ferrantelli, V. Mercury detection in benthic and pelagic fish collected from western Sicily (Southern Italy). Animals 2019, 9, 594. [Google Scholar] [CrossRef]
- Giangrosso, G.; Cammilleri, G.; Macaluso, A.; Vella, A.; D’Orazio, N.; Graci, S.; Dico, G.M.L.; Galvano, F.; Giangrosso, M.; Ferrantelli, V. Hair mercury levels detection in fishermen from Sicily (Italy) by ICP-MS method after microwave-assisted. Bioinorg Chem. Appl. 2016, 5408014. [Google Scholar] [CrossRef]
- Commission Decision (2003/181/EC). Amending decision 2002/657/EC as regards the setting of minimum required performance limits (MRPLs) for certain residues in food of animal origin. Off. J. Eur. Union 2003, 71, 17–18.
- Yu, Z.Q.; Xu, Y.J.; Tian, X.H.; Liu, Y.H.; Song, X.K.; Ren, C.B.; Huang, H.; Liu, Y.; Zhang, L.M. Semicarbazide bioaccumulation in seashells of Sishili Bay. Mar. Environ. Sci. 2013, 32, 39–42. [Google Scholar]
- Shu, X.J.; Cheng, B.; Xu, J.J.; Xiong, Y.W.; Song, W.; Song, Y.; Han, G.; Jiang, S.F. Study on the characteristics of semicarbazide (SEM) in Macrobrachium nipponense during the culture period. Freshw. Fish. 2020, 50, 11–16. [Google Scholar]
- Pan, L.Q.; Ren, J.Y.; Liu, J. Responsed of antioxidant systems and LPO level to benzo(a) pyrenye and benzo(k) fluoranthene in the haemolymph of the scallop Chlamys farreri. Environ. Pollut. 2006, 141, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Mccracken, R.J.; Van Rhijn, J.A.; Kennedy, D.G. The occurrence of nitrofuran metabolites in the tissues of chickens exposed to very low dietary concentrations of the nitrofurans. Food Addit. Contam. 2005, 22, 567–572. [Google Scholar] [CrossRef]
- Jakiul Islam, M.; Liza, A.A.; Mohsinul Reza, A.H.M.; Shaheed Reza, M.; Khan, M.N.A.; Kamal, M. Source identification and entry pathways of banned antibiotics nitrofuran and chloramphenicol in shrimp value chain of Bangladesh. Eurasia J. BioSci. 2014, 8, 71–83. [Google Scholar] [CrossRef]
- Rizal, G.M.; Gyeltshen, J.; Gyeltshen, K. Evaluation of animal feeds for the presence of three important antibiotic classes in Bhutan. J. Glob. Antimicrob. Resist. 2018, 15, 228–231. [Google Scholar] [CrossRef] [PubMed]
- Radovnikovic, A.; Conroy, E.R.; Gibney, M.; O’Mahony, J.; Danaher, M. Residue analyses and exposure assessment of the Irish population to nitrofuran metabolites from different food commodities in 2009–2010. Food Addit. Contam. A 2013, 30, 1858–1869. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, L.; Sun, W.; Sun, X.; Peng, J.; Li, Z.; Zhu, P.; Zheng, X. Semicarbazide Accumulation, Distribution and Chemical Forms in Scallop (Chlamys farreri) after Seawater Exposure. Animals 2021, 11, 1500. https://doi.org/10.3390/ani11061500
Xing L, Sun W, Sun X, Peng J, Li Z, Zhu P, Zheng X. Semicarbazide Accumulation, Distribution and Chemical Forms in Scallop (Chlamys farreri) after Seawater Exposure. Animals. 2021; 11(6):1500. https://doi.org/10.3390/ani11061500
Chicago/Turabian StyleXing, Lihong, Weihong Sun, Xiaojie Sun, Jixing Peng, Zhaoxin Li, Panpan Zhu, and Xuying Zheng. 2021. "Semicarbazide Accumulation, Distribution and Chemical Forms in Scallop (Chlamys farreri) after Seawater Exposure" Animals 11, no. 6: 1500. https://doi.org/10.3390/ani11061500
APA StyleXing, L., Sun, W., Sun, X., Peng, J., Li, Z., Zhu, P., & Zheng, X. (2021). Semicarbazide Accumulation, Distribution and Chemical Forms in Scallop (Chlamys farreri) after Seawater Exposure. Animals, 11(6), 1500. https://doi.org/10.3390/ani11061500





