Enteric and Fecal Methane Emissions from Dairy Cows Fed Grass or Corn Silage Diets Supplemented with Rapeseed Oil
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Fecal CH4 Measurements
2.2. Chemical Analysis and Maximum CH4 Emission Potential
2.3. In Vitro Gas Production Measurements
2.4. Microbial Analysis
2.4.1. DNA Isolation
2.4.2. 16S rRNA Gene Amplicon Sequencing
2.4.3. Microbiota Composition Analysis
2.5. Statistical Analysis
3. Results
3.1. Chemical Composition of Feces and Production Data
3.2. Fermentation and Digestibility
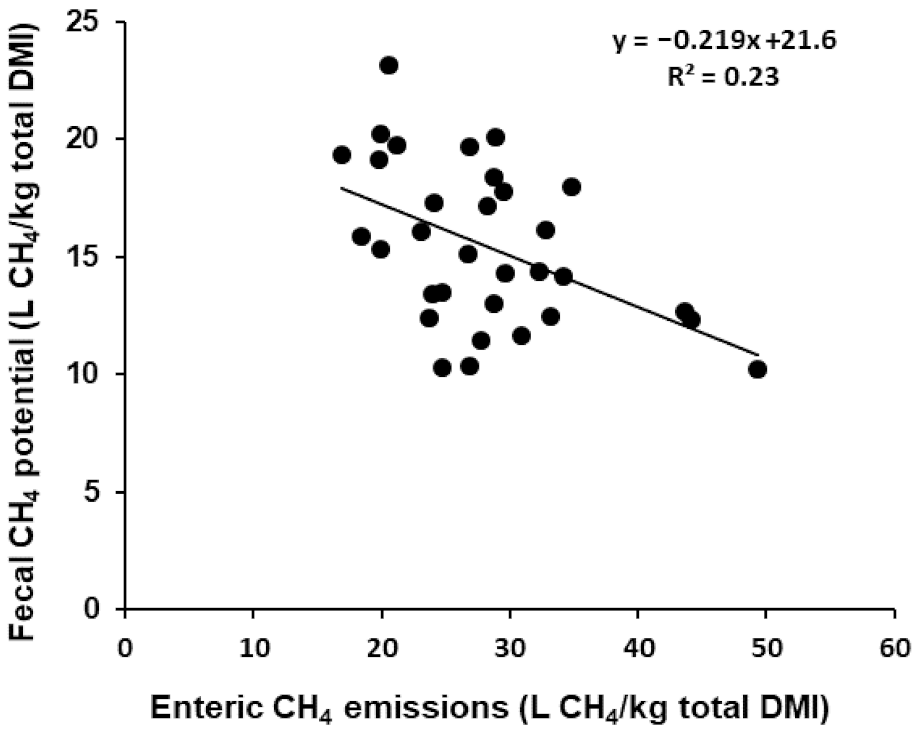
3.3. Methane Emissions
4. Discussion
4.1. Chemical Composition of Feces and Digestibility
4.2. Fermentation in Incubated Feces
4.3. Methane Emissions from Feces
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC. Mitigation of Climate Change. Contribution of Working Group III to the Fifth Assessment Report of Intergovernmental Panel on Climate Change. 2014. Available online: https://www.ipcc.ch/report/ar5/wg3/ (accessed on 17 June 2020).
- Giger-Reverdin, S.; Sauvant, D. Methane Production in Sheep in Relation to Concentrate Feed Composition from Bibliographic Data. In Proceedings of the 8th Seminar of the Sub-Network on Nutrition of the FAO-CIHEAM Inter-Regional Cooperative Research and Development Network on Sheep and Goats, 2000, Cahiers-Options-Mediterraneennes, Grignon, France, 11–13 June 2000; pp. 43–46. [Google Scholar]
- Hindrichsen, I.K.; Wettstein, H.-R.; Machmüller, A.; Jörg, B.; Kreuzer, M. Effect of the carbohydrate composition of feed concentrates on methane emission from dairy cows and their slurry. Environ. Mont Asses 2015, 107, 329–350. [Google Scholar] [CrossRef]
- Hassanat, F.; Benchaar, C. Methane emissions of manure from dairy cows fed red clover or corn silage based diets supplemented with linseed oil. J. Dairy Sci. 2019, 102, 11766–11776. [Google Scholar] [CrossRef]
- IPCC. Chapter 10 Emissions from Livestock and Manure Management. In 2006 IPCC Guidelines for National Greenhouse Gas Inventories, Vol 4. Agriculture, Forestry and Other Land Use, Prepared by the National Greenhouse Gas Inventories Programme; Eggleston, S., Buendia, L., Miwa, K., Ngara, T., Tanabe, K., Eds.; IGES: Hayama, Kanagawa, Japan, 2006; Available online: http://www.ipcc-nggip.iges.or.jp/public/2006gl/vol4.html (accessed on 1 February 2021).
- Ramin, M.; Fant, P.; Huhtanen, P. The effects of gradual replacement of barley with oats on enteric methane emissions, rumen fermentation, milk production, and energy utilization in dairy cows. J. Dairy Sci. 2021. [Google Scholar] [CrossRef] [PubMed]
- Sommer, S.G.; Petersen, S.O.; Møller, H.B. Algorithms for calculating methane and nitrous oxide emission from manure management. Nutr. Cycl. Agroecosyst. 2004, 69, 143–154. [Google Scholar] [CrossRef]
- Hristov, A.N.; Oh, J.; Giallongo, F.; Frederick, T.W.; Harper, M.T.; Weeks, H.L.; Branco, A.F.; Moate, P.J.; Deighton, M.H.; Williams, S.R.O.; et al. An inhibitor persistently decreased enteric methane emission from dairy cows with no negative effect on milk production. Proc. Natl. Acad. Sci. USA 2015, 112, 10663–10668. [Google Scholar] [CrossRef]
- Møller, H.B.; Moset, V.; Brask, M.; Weisbjerg, M.R.; Lund, P. Feces composition and manure derived methane yield from dairy cows: Influence of diet with focus on fat supplement and roughage type. Atmos. Environ. 2014, 94, 36–43. [Google Scholar] [CrossRef]
- Chagas, J.C.C.; Ramin, M.; Krizsan, S. Methane emissions from dairy cows fed maize- or grass silage-based diets with or without rapeseed oil supplementation. In Proceedings of the 28th General Meeting of European Grass-Land Federation, Helsinki, Finland, 19–21 October 2020. [Google Scholar]
- Ramin, M.; Huhtanen, P. Development of an in vitro method for determination of methane production kinetics using a fully automated in vitro gas system—A modeling approach. Anim. Feed Sci. Technol. 2012, 174, 190–200. [Google Scholar] [CrossRef]
- Huhtanen, P.; Cabezas-Garcia, E.H.; Utsumi, S.; Zimmerman, S. Comparison of methods to determine methane emissions from dairy cows in farm conditions. J. Dairy Sci. 2015, 98, 3394–3409. [Google Scholar] [CrossRef]
- Puhakka, L.; Jaakkola, S.; Simpura, I.; Kokkonen, T.; Vanhatalo, A. Effects of replacing rapeseed meal with fava bean at 2 concentrate crude protein levels on feed intake, nutrient digestion, and milk production in cows fed grass silage-based diets. J. Dairy Sci. 2016, 99, 7993–8006. [Google Scholar] [CrossRef]
- AOAC International. AOAC Official Methods of Analysis, 17th ed.; The Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Mertens, D.R. Gravimetric determination of amylase-treated neutral detergent fiber in feeds with refluxing in beakers or crucibles: Collaborative study. J. AOAC Int. 2002, 85, 1217–1240. [Google Scholar]
- Huhtanen, P.; Kaustell, K.; Jaakkola, S. The use of internal markers to predict total digestibility and duodenal flow of nutriens in cattle given six different diets. Anim. Feed Sci. Technol. 1994, 48, 211–227. [Google Scholar] [CrossRef]
- Krizsan, S.J.; Rinne, M.; Nyholm, L.; Huhtanen, P. New recommendations for the ruminal in situ determination of indigestible neutral detergent fibre. Anim. Feed Sci. Technol. 2015, 205, 31–41. [Google Scholar] [CrossRef]
- Lima, D.M.F.; Rodrigues, J.A.D.; Boe, K.; Alvarado-Morales, M.; Ellegaard, L.; Angelidaki, I. Anaerobic modelling for improving synergy and robustness of a manure co-digestion process. Braz. J. Chem. Eng. 2016, 33, 871–883. [Google Scholar] [CrossRef]
- Walters, W.; Hyde, E.R.; Berg-Lyons, D.; Ackermann, G.; Humphrey, G.; Parada, A.; Gilbert, J.A.; Jansson, J.K.; Caporaso, J.G.; Fuhrman, J.A.; et al. Improved Bacterial 16S rRNA Gene (V4 and V4-5) and Fungal Internal Transcribed Spacer Marker Gene Primers for Microbial Community Surveys. mSystems 2015, 1, e00009-15. [Google Scholar] [CrossRef] [PubMed]
- Hartinger, T.; Edwards, J.E.; Gómez Expósito, R.; Smidt, H.; ter Braak, C.J.F.; Gresner, N.; Südekum, K.-H. Differently Pre-treated Alfalfa Silages Affect the in vitro Ruminal Microbiota Composition. Front. Microbiol. 2019, 10, 2761. [Google Scholar] [CrossRef]
- Ramiro-Garcia, J.; Hermes, G.; Giatsis, C.; Sipkema, D.; Zoetendal, E.G.; Schaap, P.J.; Smidt, H. NG-Tax, a highly accurate and validated pipeline for analysis of 16S rRNA amplicons from complex biomes. F1000Research 2016, 5, 1791. [Google Scholar] [CrossRef] [PubMed]
- Poncheewin, W.; Hermes, G.D.; Van Dam, J.C.; Koehorst, J.J.; Smidt, H.; Schaap, P.J. NG-Tax 2.0: A semantic framework for high-throughput amplicon analysis. Front. Gene 2020, 10, 1366. [Google Scholar] [CrossRef]
- Benchaar, C.; Hassanat, F. Methane emissions of stored manure from dairy cows fed conventional or brown midrib corn silage. J. Dairy Sci. 2019, 102, 10632–10638. [Google Scholar] [CrossRef]
- Massé, D.I.; Jarret, G.; Hassanat, F.; Benchaar, C.; Saady, N.M.C. Effect of increasing levels of corn silage in an alfalfa-based dairy cow diet and of manure management practices on manure fugitive methane emissions. Agric. Ecosyst. Environ. 2016, 221, 109–114. [Google Scholar] [CrossRef]
- Benchaar, C.; Hassanat, F.; Martineau, R.; Gervais, R. Linseed oil supplementation to dairy cows fed red clover silage- or corn silage-based diets: Effects on methane production, rumen fermentation, nutrient digestibility, N balance, and milk production. J. Dairy Sci. 2015, 98, 7993–8008. [Google Scholar] [CrossRef]
- Brask, M.; Lund, P.; Hellwing, A.L.F.; Poulsen, M.; Weisbjerg, M.R. Enteric methane production, digestibility and rumen fermentation in dairy cows fed different forages with and without rapeseed fat supplementation. Anim. Feed Sci. Technol. 2013, 184, 67–79. [Google Scholar] [CrossRef]
- Arndt, C.; Powell, J.M.; Aguerre, M.J.; Wattiaux, M.A. Performance, digestion, nitrogen balance, and emission of manure ammonia, enteric methane, and carbon dioxide in lactating cows fed diets with varying alfalfa silage-to-corn silage ratios. J. Dairy Sci. 2015, 98, 418–430. [Google Scholar] [CrossRef]
- Bayat, A.R.; Tapio, I.; Vilkki, J.; Shingfield, K.J.; Leskinen, H. Plant oil supplements reduce methane emissions and improve milk fatty acid composition in dairy cows fed grass silage-based diets without affecting milk yield. J. Dairy Sci. 2018, 101, 1136–1151. [Google Scholar] [CrossRef] [PubMed]
- Ahring, B.K. Perspectives for anaerobic digestion. Adv. Biochem. Eng. Biotechnol. 2003, 81, 1–30. [Google Scholar] [PubMed]
- Barret, M.; Gagnon, N.; Morissette, B.; Topp, E.; Kalmokoff, M.; Brooks, S.P.J.; Matias, F.; Massé, D.I.; Masse, L.; Talbot, G. Methanoculleus spp. as a biomarker of methanogenic activity in swine manure storage tanks. FEMS Microbiol. Ecol. 2012, 80, 427–440. [Google Scholar] [CrossRef] [PubMed]
- Ramin, M.; Lerose, D.; Tagliapietra, F.; Huhtanen, P. Comparison of rumen fluid inoculum vs. faecal inoculum on predicted methane production using a fully automated in vitro gas production system. Live Sci. 2015, 181, 65–71. [Google Scholar] [CrossRef]
- Lu, X.; Wang, H.; Ma, F.; Zhao, G.; Wang, S. Enhanced anaerobic digestion of cow manure and rice straw by the supplementation of an iron oxide–zeolite system. Energy Fuels 2017, 31, 599–606. [Google Scholar] [CrossRef]
- Ramin, M.; Huhtanen, P. Development of equations for predicting methane emissions from ruminants. J. Dairy Sci. 2013, 96, 2476–2493. [Google Scholar] [CrossRef]
- Danielsson, R.; Ramin, M.; Bertilsson, J.; Lund, P.; Huhtanen, P. Evaluation of an in vitro system for predicting methane production in vivo. J. Dairy Sci. 2017, 100, 8881–8894. [Google Scholar] [CrossRef]
- Mu, Y.; Lin, X.; Wang, Z.; Hou, Q.; Wang, Y.; Hu, Z. High-production dairy cattle exhibit different rumen and fecal bacterial community and rumen metabolite profile than low-production cattle. Microbiologypen 2019, 8, e00673. [Google Scholar] [CrossRef]
- Noel, S.J.; Olijhoek, D.W.; Mclean, F.; Løvendahl, P.; Lund, P.; Højberg, O. Rumen and Fecal Microbial Community Structure of Holstein and Jersey Dairy Cows as Affected by Breed, Diet, and Residual Feed Intake. Animals 2019, 9, 498. [Google Scholar] [CrossRef]
- Beauchemin, K.A.; McGinn, S.M.; Benchaar, C.; Holtshausen, L. Crushed sunflower, flax, or canola seeds in lactating dairy cow diets: Effects on methane production, rumen fermentation, and milk production. J. Dairy Sci. 2009, 92, 2118–2127. [Google Scholar] [CrossRef] [PubMed]
- Grainger, C.; Beauchemin, K.A. Can enteric methane emissions from ruminants be lowered without lowering their production? Anim. Feed Sci. Technol. 2011, 166–167, 308–320. [Google Scholar] [CrossRef]
- Environment Canada. Greenhouse Gas Sources and Sinks in Canada, National Inventory Report 1990–2013 Part 2; Environment Canada: Ottawa, ON, Canada, 2015. [Google Scholar]
- Massé, D.I.; Masse, L.; Claveau, S.; Benchaar, C.; Thomas, O. Methane emissions from manure storages. Trans. ASABE 2008, 51, 1775–1781. [Google Scholar] [CrossRef]

| Item | Diet 1 | |||
|---|---|---|---|---|
| GS | GS-RSO | GSCS | GSCS-RSO | |
| Ingredient | ||||
| Grass silage | 558 | 534 | 277 | 264 |
| Corn silage | 0 | 0 | 290 | 278 |
| Crimped barley | 336 | 316 | 328 | 311 |
| Rapeseed meal | 91.0 | 91.0 | 90.0 | 90.0 |
| Rapeseed oil | 0 | 44.0 | 0 | 42.0 |
| Minerals 2 | 15.0 | 15.0 | 15.0 | 15.0 |
| Chemical composition In dry matter, g/kg | ||||
| Organic matter | 922 | 924 | 935 | 935 |
| Neutral detergent fiber | 371 | 355 | 348 | 332 |
| Indigestible NDF | 61.9 | 59.4 | 68.8 | 66.1 |
| pdNDF 3 | 309 | 296 | 279 | 266 |
| Crude fat 4 | 33.8 | 76.1 | 33.0 | 73.1 |
| Item | Diet 1 | SEM | p-Value 3 | ||||
|---|---|---|---|---|---|---|---|
| GS | GS-RSO | GSCS | GSCS-RSO | Forage | Oil | ||
| Intake, kg/d | |||||||
| Dry matter | 21.2 | 19.2 | 20.0 | 18.5 | 0.50 | 0.07 | <0.01 |
| Organic matter | 19.5 | 17.7 | 18.6 | 17.2 | 0.46 | 0.17 | <0.01 |
| Neutral detergent fiber | 7.42 | 6.63 | 6.64 | 5.94 | 0.194 | <0.01 | <0.01 |
| Indigestible NDF | 1.17 | 1.08 | 1.26 | 1.14 | 0.036 | 0.06 | <0.01 |
| pdNDF 2 | 6.37 | 5.68 | 5.45 | 4.86 | 0.167 | <0.01 | <0.01 |
| Milk yield, kg/d | 31.5 | 32.7 | 29.6 | 29.2 | 0.88 | <0.01 | 0.88 |
| Diet 1 | p-Value 10 | ||||||
|---|---|---|---|---|---|---|---|
| Item | GS | GS-RSO | GSCS | GSCS-RSO | SEM | Forage | Oil |
| Fecal output, kg/d | |||||||
| Dry matter | 5.13 | 5.35 | 5.32 | 5.85 | 0.232 | 0.15 | 0.13 |
| Organic matter | 4.45 | 4.69 | 4.70 | 5.22 | 0.203 | 0.06 | 0.07 |
| NDS 2 | 2.01 | 2.18 | 1.97 | 2.32 | 0.119 | 0.64 | 0.04 |
| Neutral detergent fiber | 2.44 | 2.51 | 2.72 | 2.89 | 0.127 | 0.01 | 0.33 |
| Indigestible NDF | 1.17 | 1.08 | 1.26 | 1.14 | 0.036 | 0.06 | <0.01 |
| pdNDF 3 | 1.27 | 1.42 | 1.46 | 1.74 | 0.098 | 0.01 | 0.03 |
| pdOM 4, kg/d | 3.27 | 3.60 | 3.43 | 4.07 | 0.177 | 0.09 | 0.01 |
| CH4 emission | |||||||
| Feces, L/kg DM | 3.99 | 3.31 | 3.70 | 3.60 | 0.17 | 0.98 | 0.05 |
| Feces, L/kg OM | 4.66 | 3.81 | 4.22 | 4.03 | 0.19 | 0.60 | 0.02 |
| Feces, L/d | 18.3 | 16.1 | 18.6 | 20.2 | 1.99 | 0.30 | 0.90 |
| Enteric, L/d | 608 | 459 | 607 | 487 | 21.7 | 0.53 | <0.01 |
| Enteric + feces, L/d | 637 | 507 | 618 | 508 | 21.4 | 0.69 | <0.01 |
| Maximum CH4 from VFA, L/kg DM feces 5 | 59.4 | 55.5 | 58.0 | 54.4 | 2.63 | 0.64 | 0.16 |
| Maximum CH4 from VFA, L/kg OM feces | 69.0 | 63.5 | 66.0 | 60.6 | 2.97 | 0.32 | 0.09 |
| Maximum CH4 from VFA, L/kg DMI | 14.4 | 15.4 | 15.2 | 16.8 | 0.708 | 0.14 | 0.08 |
| VFA after 9 weeks of in vitro incubation | |||||||
| Total VFA, mM | 199 | 196 | 189 | 188 | 6.9 | 0.22 | 0.79 |
| Acetate, mmol/mol | 608 | 597 | 573 | 575 | 9.4 | <0.01 | 0.62 |
| Propionate, mmol/mol | 191 | 200 | 194 | 198 | 7.4 | 0.94 | 0.40 |
| Butyrate, mmol/mol | 172 | 175 | 204 | 200 | 13.1 | 0.04 | 0.96 |
| Isobutyric acid, mmol/mol | 27.8 | 27.0 | 28.2 | 27.0 | 1.29 | 0.88 | 0.45 |
| pH after 9 weeks of incubation | 5.41 | 5.18 | 5.17 | 5.16 | 0.049 | 0.02 | 0.04 |
| In vivo digestibility using iNDF from feces before 9 weeks in vitro incubation | |||||||
| DMD 8, g/ kg | 755 | 719 | 746 | 680 | 7.7 | <0.01 | <0.01 |
| OMD 6, g/ kg | 775 | 741 | 765 | 699 | 6.7 | <0.01 | <0.01 |
| NDFD 7, g/kg | 627 | 575 | 580 | 488 | 10.9 | <0.01 | <0.01 |
| pdNDFD, g/kg | 760 | 686 | 719 | 582 | 15.5 | <0.01 | <0.01 |
| In vivo digestibility using iNDF from feces after 9 weeks in vitro incubation | |||||||
| DMD, g/ kg | 784 | 746 | 770 | 759 | 9.3 | 0.97 | 0.01 |
| OMD, g/ kg | 805 | 769 | 790 | 777 | 8.4 | 0.67 | <0.01 |
| NDFD, g/kg | 669 | 611 | 614 | 610 | 18.2 | 0.13 | 0.11 |
| pdNDFD, g/kg | 822 | 740 | 767 | 761 | 25.8 | 0.51 | 0.10 |
| Apparent OMD 9, g/kg | 213 | 209 | 205 | 201 | 8.8 | 0.36 | 0.59 |
| Item | Diet 1 | SEM | p-Value 2 | ||||
|---|---|---|---|---|---|---|---|
| GS | GS-RSO | GSCS | GSCS-RSO | Forage | Oil | ||
| Methane emissions, L/kg DM | 11.0 | 12.5 | 13.4 | 12.4 | 1.11 | 0.31 | 0.77 |
| Total gas production, L/kg DM | 95.0 | 103 | 97.0 | 104 | 6.08 | 0.77 | 0.18 |
| Methane/total gas production | 0.118 | 0.163 | 0.144 | 0.122 | 0.0257 | 0.78 | 0.66 |
| Abundance (%) | Diet 1 | SEM | p-Value 2 | ||||
|---|---|---|---|---|---|---|---|
| GS | GS-RSO | GSCS | GSCS-RSO | Forage | Oil | ||
| Archaea/Bacteria | 4.42 | 5.26 | 4.23 | 4.23 | 0.781 | 0.44 | 0.59 |
| Archaea | |||||||
| Methanobrevibacter | 97.5 | 97.7 | 98.9 | 98.5 | 0.72 | 0.13 | 0.92 |
| Methanosphaera | 2.52 | 2.16 | 1.10 | 1.46 | 0.722 | 0.15 | 1.0 |
| Methanocorpusculum | 0.0 | 0.13 | 0.00 | 0.00 | 0.068 | 0.33 | 0.33 |
| Top 15 bacterial families | |||||||
| Atopobiaceae | 1.44 | 2.45 | 2.56 | 2.71 | 0.620 | 0.28 | 0.36 |
| Bacteroidaceae | 1.59 | 1.58 | 1.46 | 2.27 | 0.365 | 0.45 | 0.28 |
| Bacteroidales_RF16_group | 0.93 | 0.57 | 1.03 | 0.87 | 0.219 | 0.37 | 0.24 |
| Bifidobacteriaceae | 3.04 | 1.97 | 8.9 | 0.72 | 1.544 | 0.15 | <0.01 |
| Christensenellaceae | 4.89 | 5.61 | 4.93 | 5.05 | 0.415 | 0.53 | 0.32 |
| Eggerthellaceae | 1.30 | 1.65 | 0.95 | 0.72 | 0.239 | 0.01 | 0.80 |
| Erysipelotrichaceae | 2.97 | 2.88 | 2.27 | 2.62 | 0.369 | 0.21 | 0.73 |
| Family_XIII | 4.29 | 4.87 | 3.15 | 4.01 | 0.527 | 0.07 | 0.18 |
| Lachnospiraceae | 20.4 | 24.8 | 16.2 | 22.9 | 1.76 | 0.10 | <0.01 |
| Lactobacillaceae | 1.48 | 1.55 | 0.68 | 0.70 | 0.210 | <0.01 | 0.84 |
| Muribaculaceae | 1.65 | 2.84 | 2.30 | 3.98 | 0.474 | 0.07 | <0.01 |
| Peptostreptococcaceae | 6.26 | 6.47 | 5.97 | 5.78 | 0.744 | 0.51 | 0.98 |
| Prevotellaceae | 9.93 | 6.45 | 10.2 | 10.7 | 1.10 | 0.05 | 0.19 |
| Rikenellaceae | 8.00 | 4.54 | 7.66 | 5.40 | 0.709 | 0.71 | <0.01 |
| Ruminococcaceae | 28.3 | 26.8 | 28.1 | 27.0 | 1.31 | 0.96 | 0.32 |
| Top 15 Bacterial genera | |||||||
| Bifidobacterium | 3.04 | 1.96 | 8.89 | 0.72 | 1.542 | 0.15 | <0.01 |
| Christensenellaceae_R-7_group | 4.89 | 5.61 | 4.93 | 5.05 | 0.415 | 0.53 | 0.32 |
| Eubacterium_coprostanoligenes_group | 5.36 | 7.82 | 5.77 | 7.54 | 0.447 | 0.88 | <0.01 |
| Lachnospiraceae_NK3A20_group | 12.1 | 16.2 | 9.73 | 10.2 | 1.27 | <0.01 | 0.09 |
| Mogibacterium | 2.27 | 2.48 | 1.74 | 2.23 | 0.237 | 0.11 | 0.15 |
| Olsenella | 1.24 | 2.15 | 2.41 | 2.68 | 0.614 | 0.18 | 0.34 |
| Paeniclostridium | 2.00 | 2.08 | 2.16 | 1.96 | 0.292 | 0.94 | 0.82 |
| Prevotellaceae_UCG-003 | 5.80 | 3.77 | 5.48 | 6.89 | 0.864 | 0.12 | 0.72 |
| Rikenellaceae_RC9_gut_group | 6.31 | 3.72 | 6.42 | 4.45 | 0.686 | 0.54 | <0.01 |
| Romboutsia | 3.46 | 3.62 | 3.08 | 3.19 | 0.447 | 0.38 | 0.77 |
| Ruminococcaceae_UCG-005 | 14.8 | 12.5 | 13.2 | 10.1 | 1.03 | 0.06 | 0.01 |
| Ruminococcaceae_UCG-013 | 2.23 | 1.57 | 3.42 | 3.34 | 0.476 | <0.01 | 0.44 |
| Ruminococcus_2 | 1.95 | 1.12 | 2.24 | 2.38 | 0.764 | 0.32 | 0.65 |
| Turicibacter | 2.12 | 1.90 | 1.71 | 1.76 | 0.306 | 0.38 | 0.79 |
| Unknown_Lachnospiraceae | 2.17 | 2.30 | 1.52 | 3.19 | 0.373 | 0.74 | 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramin, M.; Chagas, J.C.; Smidt, H.; Exposito, R.G.; Krizsan, S.J. Enteric and Fecal Methane Emissions from Dairy Cows Fed Grass or Corn Silage Diets Supplemented with Rapeseed Oil. Animals 2021, 11, 1322. https://doi.org/10.3390/ani11051322
Ramin M, Chagas JC, Smidt H, Exposito RG, Krizsan SJ. Enteric and Fecal Methane Emissions from Dairy Cows Fed Grass or Corn Silage Diets Supplemented with Rapeseed Oil. Animals. 2021; 11(5):1322. https://doi.org/10.3390/ani11051322
Chicago/Turabian StyleRamin, Mohammad, Juana C. Chagas, Hauke Smidt, Ruth Gomez Exposito, and Sophie J. Krizsan. 2021. "Enteric and Fecal Methane Emissions from Dairy Cows Fed Grass or Corn Silage Diets Supplemented with Rapeseed Oil" Animals 11, no. 5: 1322. https://doi.org/10.3390/ani11051322
APA StyleRamin, M., Chagas, J. C., Smidt, H., Exposito, R. G., & Krizsan, S. J. (2021). Enteric and Fecal Methane Emissions from Dairy Cows Fed Grass or Corn Silage Diets Supplemented with Rapeseed Oil. Animals, 11(5), 1322. https://doi.org/10.3390/ani11051322








