Spatial and Temporal Distribution of Mycobacterium tuberculosis Complex Infection in Eurasian Badger (Meles meles) and Cattle in Asturias, Spain
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Study Area
2.3. Description of TB Detection in Badgers and Cattle in Asturias, 2008–2020
2.3.1. Badgers
2.3.1.1. Pathology
2.3.1.2. Bacteriology and Typing
2.3.1.3. Serology (P22 ELISA Assay)
2.3.2. Cattle
2.4. Statistical Analysis
3. Results
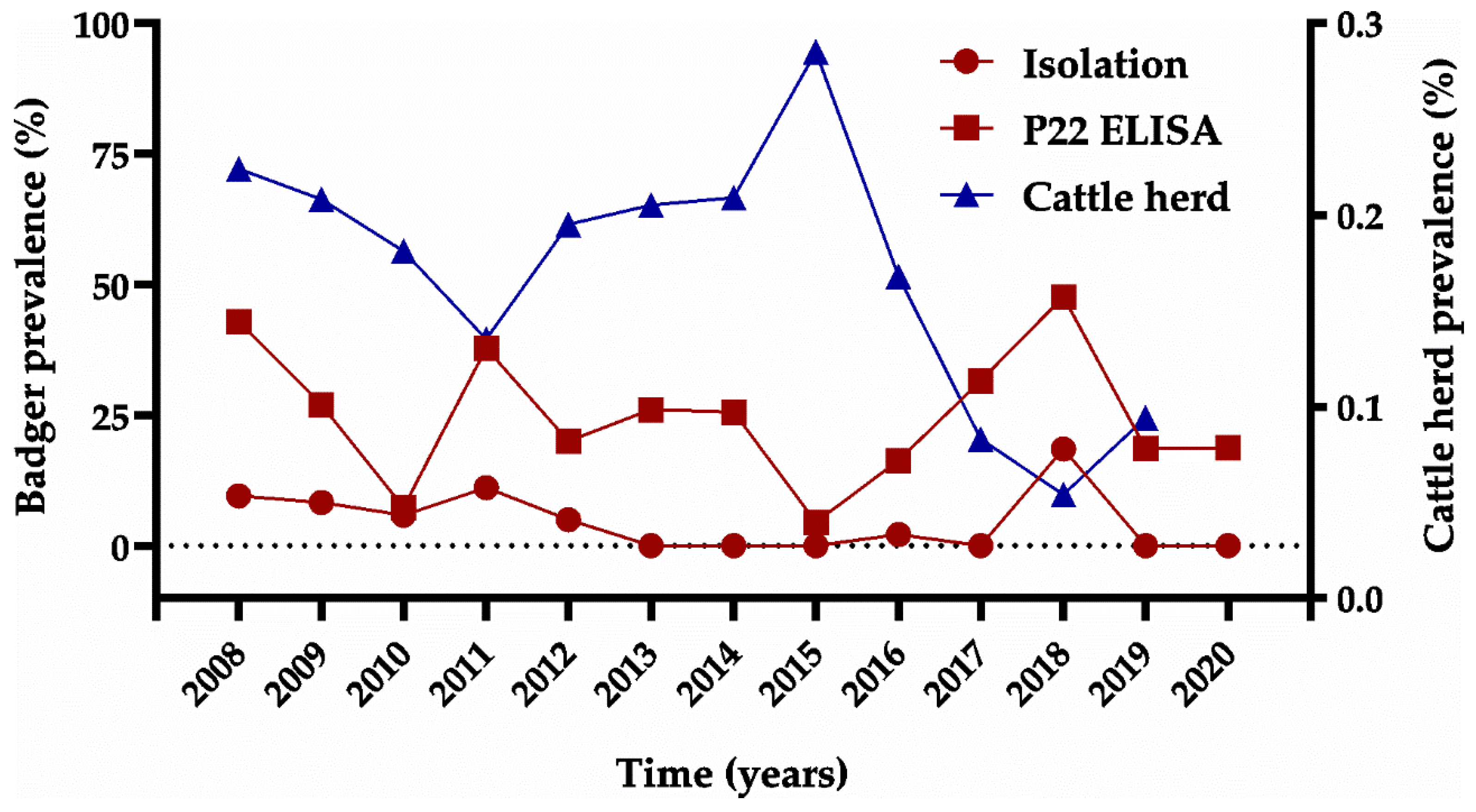
3.1. Badgers
3.1.1. Gross and Microscopic Lesions
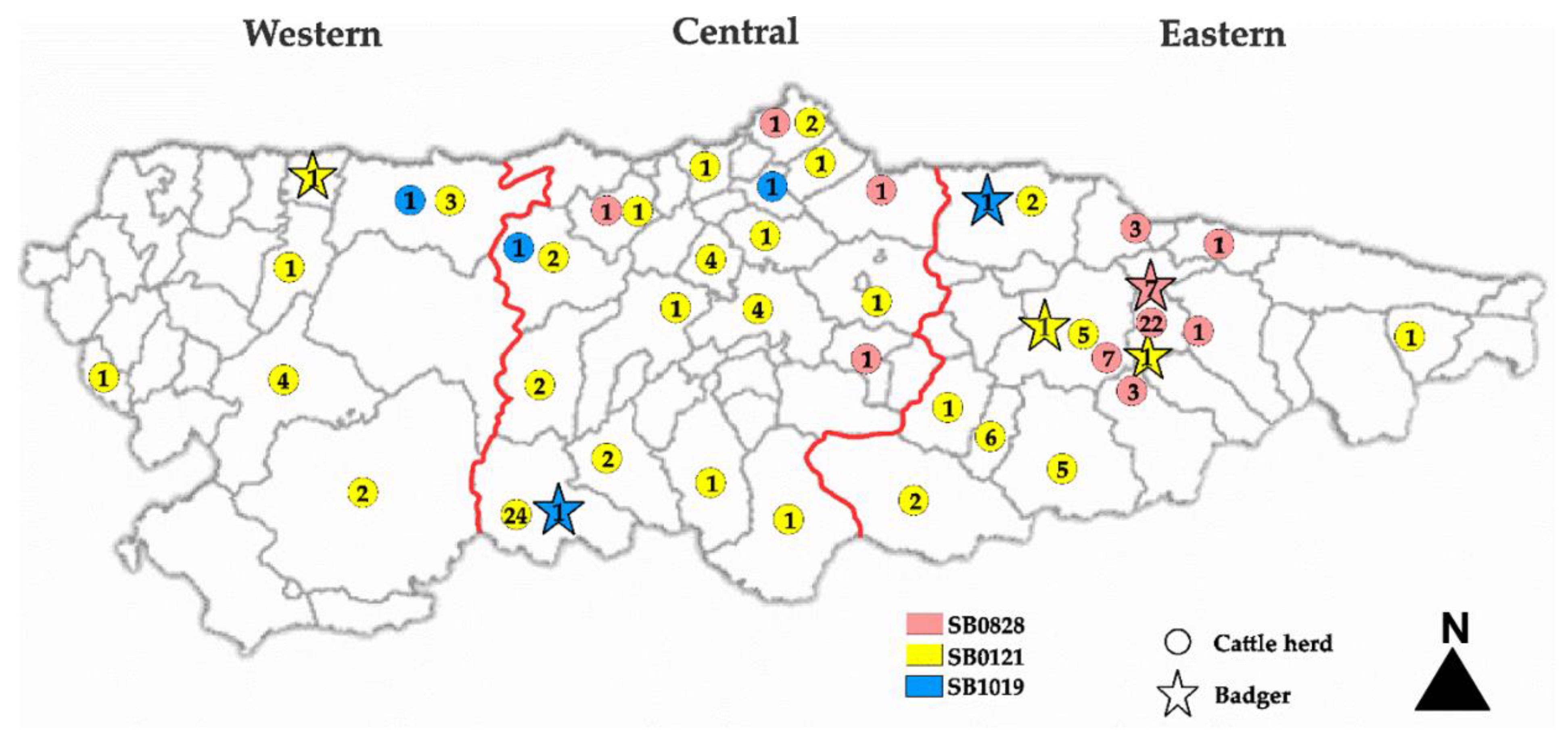
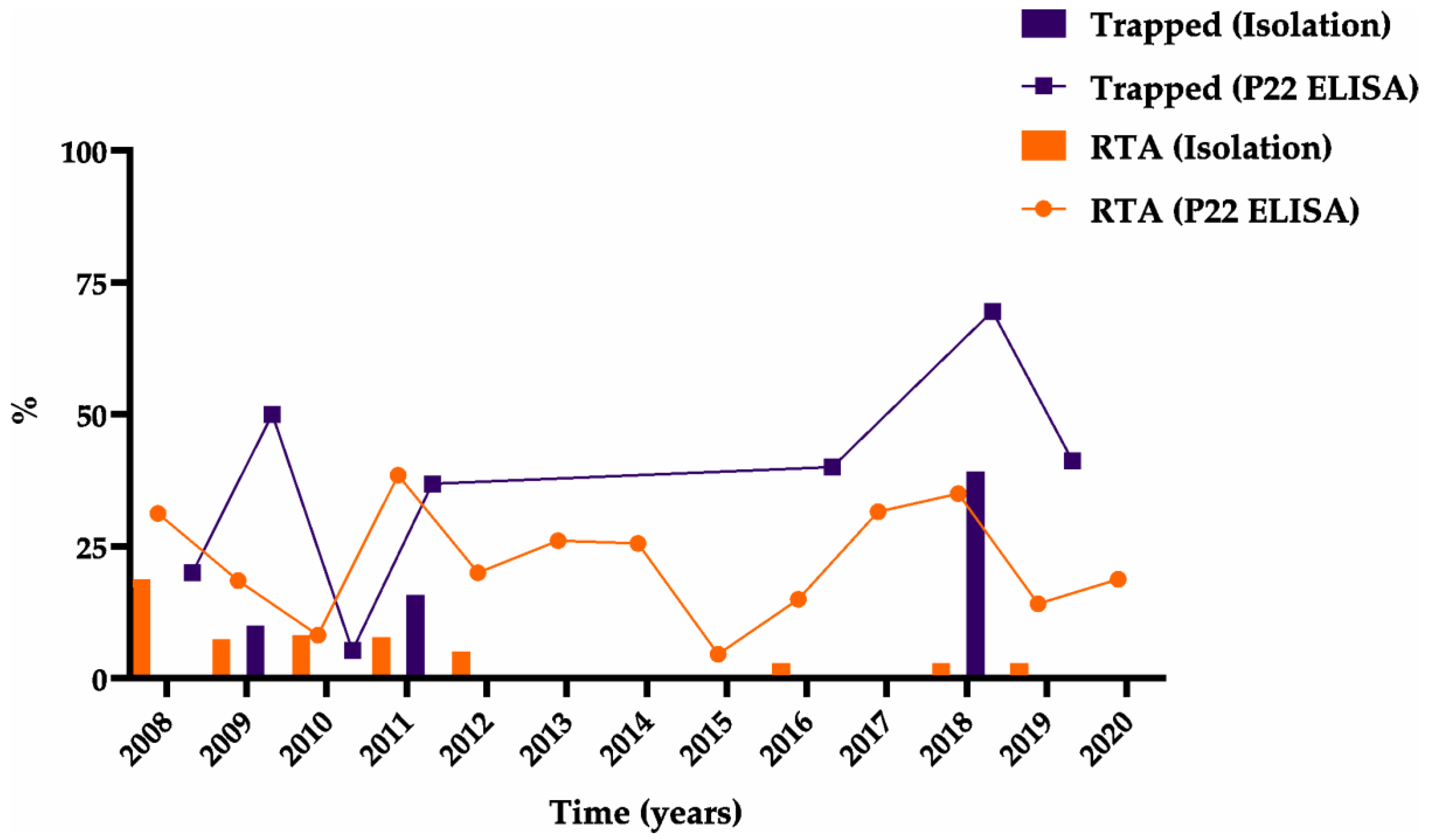
3.1.2. Bacteriological Isolation and Typing
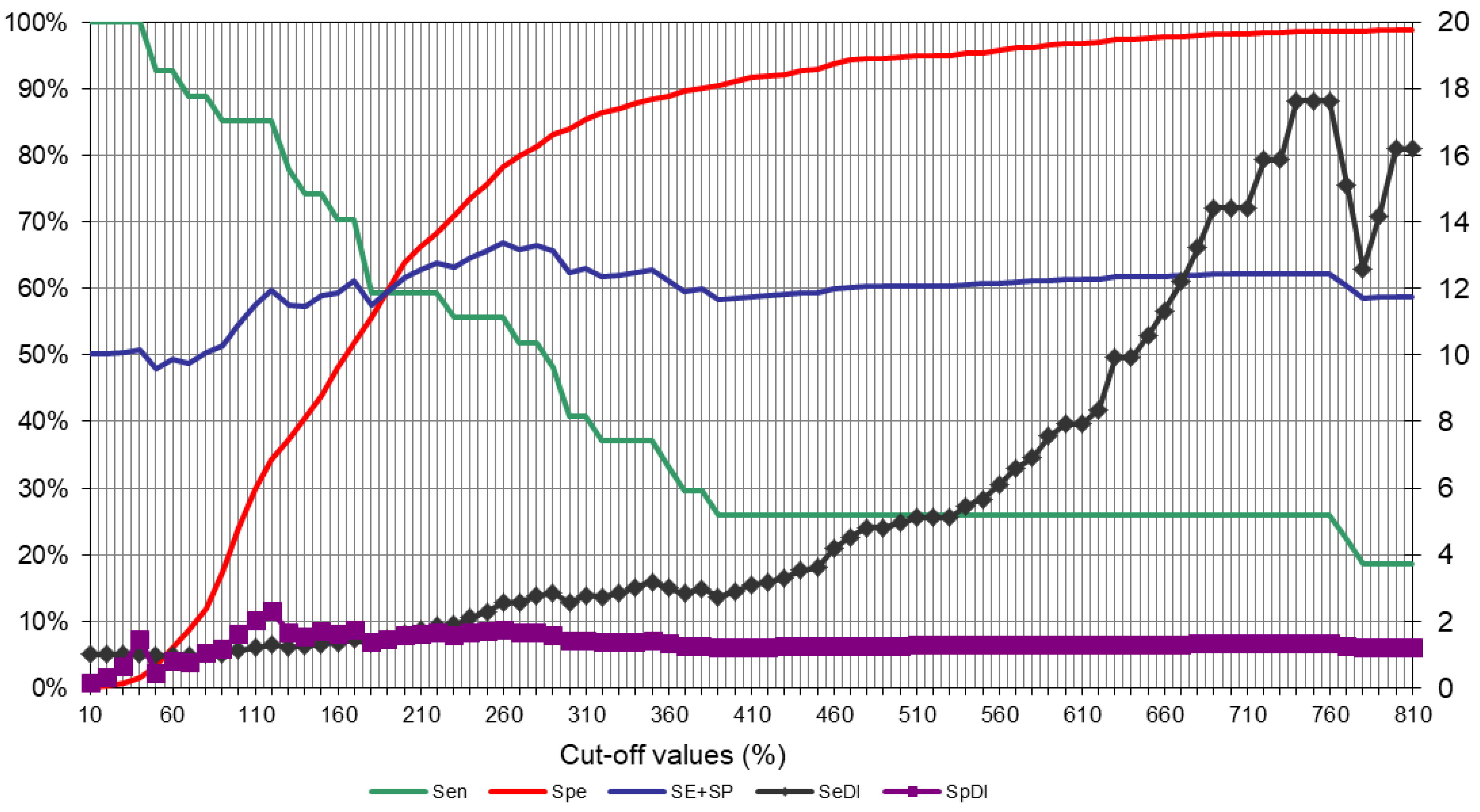
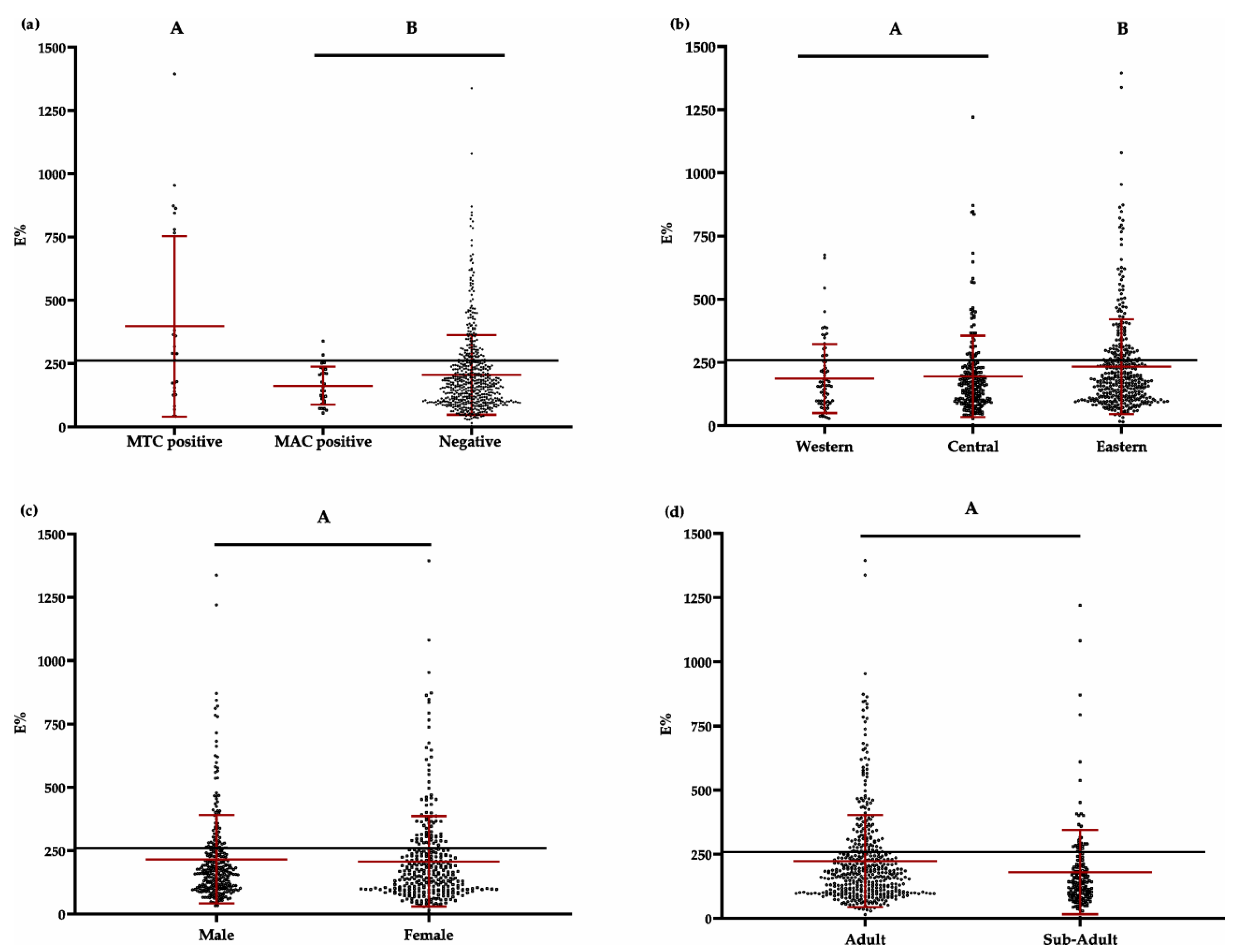
3.1.3. Serological Assay (P22 ELISA)
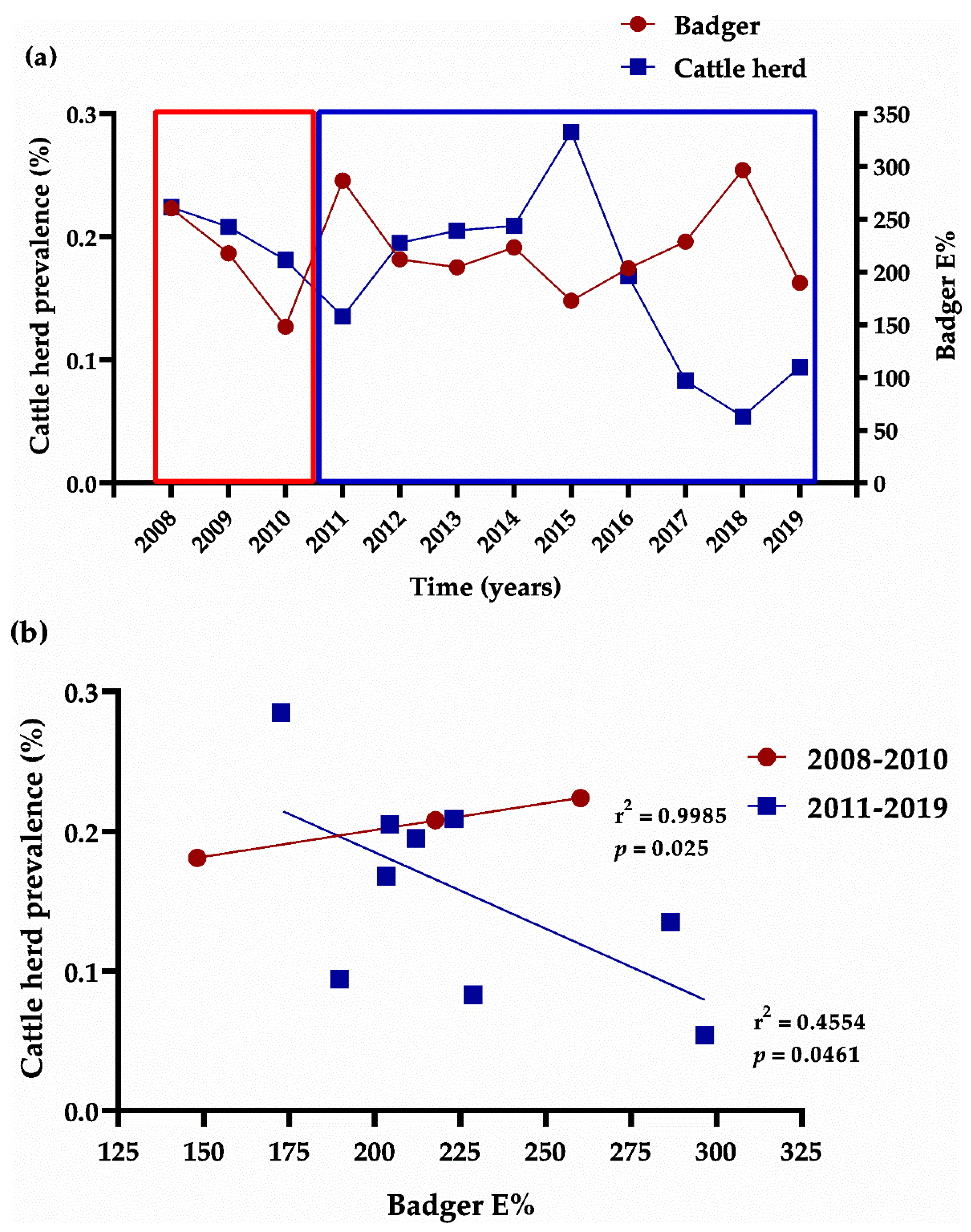
3.2. Cattle
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Olea-Popelka, F.J.; Muwonge, A.; Perera, A.; Dean, A.S.; Mumford, E.; Erlacher-Vindel, E.; Forcella, S.; Silk, B.J.; Ditiu, L.; El Idrissi, A.; et al. Zoonotic tuberculosis in humans begins caused by Mycobacterium bovis- a call for action. Lancet Infect. Dis. 2017, 1, e21–e25. [Google Scholar] [CrossRef]
- MAPA. 2020. Available online: https://www.mapa.gob.es/es/ganaderia/temas/sanidad-animal-higiene-ganadera/pnetb_2020final_tcm30-523317.PDF (accessed on 8 January 2021).
- Olea-Popelka, F.J.; Costello, E.; White, P.; McGrath, G.; Collins, J.D.; O’Keeffe, J.; Felton, D.F.; Berke, O.; More, S.; Martin, S.W. Risk factors for disclosureof additional tuberculous cattle in attested-clear herds that had one animal with a confirmed lesion of tuberculosis at slaughter during 2003 in Ireland. Prev. Vet. Med. 2008, 85, 81–91. [Google Scholar] [CrossRef]
- Delahay, R.J.; Smith, G.C.; Barlow, A.M.; Walker, N.; Harris, A.; Clifton-Hadley, R.S.; Cheeseman, C.L. Bovine tuberculosis infection in wild mammals in the South-West region of England: A survey of prevalence and a semi-quantitative assessment of the relative risks to cattle. Vet. J. 2007, 173, 287–301. [Google Scholar] [CrossRef]
- Zanella, G.; Duvauchelle, A.; Hars, J.; Moutou, F.; Boschiroli, M.L.; Durand, B. Patterns of lesions of bovine tuberculosis in wild red deer and wild boar. Vet. Rec. 2008, 163, 43–47. [Google Scholar] [CrossRef]
- Payne, A.; Philipon, S.; Hars, J.; Dufour, B.; Gilot-Fromont, E. Wildlife Interactions on baited places and waterholes in a French area Infected by bovine tuberculosis. Front. Vet. Sci. 2017, 3, 122. [Google Scholar] [CrossRef]
- Sobrino, R.; Martín-Hernando, M.P.; Vicente, J.; Aurtenetxe, O.; Garrido, J.M.; Gortázar, C. Bovine tuberculosis in a badger (Meles meles) in Spain. Vet. Rec. 2008, 163, 159–160. [Google Scholar] [CrossRef]
- Balseiro, A.; Rodríguez, O.; González-Quirós, P.; Merediz, I.; Sevilla, I.A.; Davé, D.; Dalley, D.J.; Lesellier, S.; Chambers, M.A.; Bezos, J. Infection of Eurasian badgers (Meles meles) with Mycobacterium bovis and Mycobacterium avium complex in Spain. Vet. J. 2011, 190, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Corner, L.A.L.; Clegg, T.A.; More, S.J.; Williams, D.H.; O’Boyle, I.; Costello, E.; Sleeman, D.P.; Griffin, J.M. The effect of varying levels of population control on the prevalence of tuberculosis in badgers in Ireland. Res. Vet. Sci. 2008, 85, 238–249. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, H.E.; Woodroffe, R.; Donnelly, C.A. The duration of the effects of repeated widespread badger culling on cattle tuberculosis following the cessation of culling. PLoS ONE 2010, 5, e9090. [Google Scholar] [CrossRef] [PubMed]
- Gortázar, C.; Delahay, R.J.; McDonald, R.A.; Boadella, M.; Wilson, G.J.; Gavier-Widen, D.; Acevedo, P. The status of tuberculosis in European wild mammals. Mammal Rev. 2012, 42, 193–206. [Google Scholar] [CrossRef]
- Acevedo, P.; Prieto, M.; Quirós, P.; Merediz, I.; de Juan, L.; Infantes-Lorenzo, J.A.; Triguero-Ocaña, R.; Balseiro, A. Tuberculosis epidemiology and badger (Meles meles) spatial ecology in a hotspot-area in Atlantic Spain. Pathogens. 2019, 8, 292. [Google Scholar] [CrossRef]
- Barasona, J.A.; Vicente, J.; Díez-Delgado, I.; Aznar, J.; Gortázar, C.; Torres, M.J. Environmental presence of Mycobacterium tuberculosis complex in aggregation points at the wildlife/livestock interface. Transbound. Emerg. Dis. 2017, 64, 1148–1158. [Google Scholar] [CrossRef] [PubMed]
- Vicente, J.; Barasona, J.A.; Acevedo, P.; Ruíz-Fons, J.F.; Boadella, M.; Diez-Delgado, I.; Beltrán-Beck, B.; González-Barrio, D.; Queirós, J.; Montoro, V.; et al. Temporal trend of tuberculosis in wild ungulates from Mediterranean Spain. Transbound. Emerg. Dis. 2013, 1, 92–103. [Google Scholar] [CrossRef] [PubMed]
- LaHue, N.P.; Baños, J.V.; Acevedo, P.; Gortázar, C.; Martínez-López, B. Spatially explicit modeling of animal tuberculosis at the wildlife-livestock interface in Ciudad Real province, Spain. Prev. Vet. Med. 2016, 128, 101–111. [Google Scholar] [CrossRef]
- Muñoz-Mendoza, M.; Marreros, N.; Boadella, M.; Gortázar, C.; Menéndez, S.; de Juan, L.; Bezos, J.; Romero, B.; Copano, M.F.; Amado, J.; et al. Wild boar tuberculosis in Iberian Atlantic Spain: A different picture from Mediterranean habitats. BMC Vet. Res. 2013, 9, 176. [Google Scholar] [CrossRef] [PubMed]
- Balseiro, A.; González-Quirós, P.; Rodríguez, O.; Copano, M.F.; Merediz, I.; De-Juan, L.; Chambers, M.A.; Delahay, R.J.; Marreros, N.; Royo, L.J.; et al. Spatial relationships between Eurasian badgers (Meles meles) and cattle infected with Mycobacterium bovis in Northern Spain. Vet. J. 2013, 197, 739–745. [Google Scholar] [CrossRef]
- Byrne, A.W.; Kenny, K.; Fogarty, U.; O’Keeffe, J.J.; More, S.J.; McGrath, G.; Teeling, M.; Martin, S.W.; Dohoo, I.R. Spatial and temporal analyses of metrics of tuberculosis infection in badgers (Meles meles) from the Republic of Ireland: Trends in apparent prevalence. Prev. Vet. Med. 2015, 122, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Delahay, R.J.; Walker, N.; Smith, G.C.; Wilkinson, D.; Clifton-Hadley, R.S.; Cheeseman, C.L.; Tomlinson, A.J.; Chambers, M.A. Long-term temporal trends and estimated transmission rates for Mycobacterium bovis infection in an undisturbed high-density badger (Meles meles) population. Epidemiol. Infect. 2013, 141, 1445–1456. [Google Scholar] [CrossRef]
- Ninyerola, M.; Pons, X.; Roure, J.M. Atlas Climático Digital de la Península Ibérica. Metolodología y Aplicaciones en Bioclimatología y Geobotánica, 1st ed.; Universidad Autónoma de Barcelona: Bellaterra, Spain, 2005. [Google Scholar]
- Acevedo, P.; González-Quirós, P.; Etherington, T.R.; Prieto, J.M.; Gortázar, C.; Balseiro, A. Generalizing and transferring spatial models: A case study to predict Eurasian badger abundance in Atlantic Spain. Ecol. Model. 2014, 275, 1–8. [Google Scholar] [CrossRef]
- Coetsier, C.; Vannuffel, P.; Bloondel, N.; Denef, J.F.; Cocito, C.; Gala, J.L. Duplex PCR for differential identification of Mycobacterium bovis, M. avium, and M. avium subsp. paratuberculosis in formalin-fixed paraffin-embedded tissues from cattle. J. Clin. Microbiol. 2000, 38, 3048–3054. [Google Scholar] [CrossRef]
- Rodríguez-Campos, S.; Aranaz, A.; de Juan, L.; Sáez-Llorente, J.L.; Romero, B.; Bezos, J.; Jiménez, A.; Mateos, A.; Domínguez, L. Limitations of spoligotyping and variable number tandem repeat typing for molecular tracing of Mycobacterium bovis in a high diversity setting. J. Clin. Microbiol. 2011, 49, 3361–3364. [Google Scholar] [CrossRef]
- Mycobacterium bovis Spoligotype Database Website. Available online: www.Mbovis.org (accessed on 2 October 2019).
- Álvarez, J.; García, I.G.; Aranaz, A.; Bezos, J.; Romero, B.; de Juan, L.; Mateos, A.; Gómez-Mampaso, E.; Domínguez, L.J. Genetic diversity of Mycobacterium avium isolates recovered from clinical samples and from the environment: Molecular characterization for diagnostic purposes. Clin. Microbiol. 2008, 46, 1246–1251. [Google Scholar] [CrossRef][Green Version]
- Infantes-Lorenzo, J.A.; Dave, D.; Moreno, I.; Anderson, P.; Lesellier, S.; Gormley, E.; Domínguez, L.; Balseiro, A.; Gortázar, C.; Domínguez, M.; et al. New serological platform for detecting antibodies against Mycobacterium tuberculosis complex in European badgers. Vet. Med. Sci. 2019, 5, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, P.; Hopkins, B.; Jones, J.; Galloway, T.; Pike, R.; Rolfe, S.; Hewinson, G. Temporal and spatial Mycobacterium bovis prevalence patterns as evidenced in the All Wales Badgers Found Dead (AWBFD) survey of infection 2014–2016. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Thomas, J.; Balseiro, A.; Gortázar, C.; Risalde, M.A. Diagnostic of tuerculosis in wildlife: A systematic review. Vet. Res. 2021, 52, 31. [Google Scholar] [CrossRef]
- Courcier, E.A.; Pascual-Linaza, A.V.; Arnold, M.E.; McCormick, C.M.; Corbett, D.M.; O’Hagan, M.J.H.; Collins, S.F.; Trimble, N.A.; McGeown, C.F.; McHugh, G.E.; et al. Evaluating the application of the dual path platform VetTB tests for badgers (Meles meles) in the test and vaccinate or remove (TVR) wildlife research intervention project in Northern Ireland. Res. Vet. Sci. 2020, 130, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Drewe, J.A.; Tomlinson, A.J.; Walker, N.J.; Delahay, R.J. Diagnostic accuracy and optimal use of three tests for tuberculosis in live badgers. PLoS ONE. 2010, 5, e11196. [Google Scholar] [CrossRef]
- Buzdugan, S.N.; Chambers, M.A.; Delahay, R.J.; Drewe, J.A. Diagnosis of tuberculosis in groups of badgers: An exploration of the impact of trapping efficiency, infection prevalence and the use of multiple tests. Epidemiol. Infect. 2016, 144, 1717–1727. [Google Scholar] [CrossRef] [PubMed]
- Chambers, M.A.; Waterhouse, S.; Lyashchenko, K.; Delahay, R.; Sayers, R.; Hewinson, R.G. Performance of TB immunodiagnostic tests in Eurasian badgers (Meles meles) of different ages and the influence of duration of infection on serological sensitivity. BMC Vet. Res. 2009, 5, 42. [Google Scholar] [CrossRef]
- Aznar, I.; Frankena, K.; More, S.J.; O’Keeffe, J.; McGrath, G.; de Jong, M.C.M. Quantification of Mycobacterium bovis transmission in a badger vaccine field trial. Prev. Vet. Med. 2017, 149, 29–37. [Google Scholar] [CrossRef]
- Infantes-Lorenzo, J.A.; Moreno, I.; de los Ángeles Risalde, M.; Roy, A.; Villar, M.; Romero, B.; Ibarrola, N.; de la Fuente, J.; Puentes, E.; de Juan, L.; et al. Proteomic characterisation of bovine and avian purified protein derivatives and identification of specific antigens for serodiagnosis of bovine tuberculosis. Clin. Proteomics. 2017, 14, 36. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.; Buss, P.; Hofmeyr, J.; Olea-Popelka, F.; Parsons, S.; van Helden, P. Antemortem diagnosis of Mycobacterium bovis infection in free-ranging African lions (Panthera leo) and implications for transmission. J. Wildl. Dis. 2015, 51, 493–497. [Google Scholar] [CrossRef]
- Murphy, D.; Gormley, E.; Collins, D.M.; McGrath, G.; Sovsic, E.; Costello, E.; Corner, L.A.L. Tuberculosis in cattle herds are sentinels for Mycobacterium bovis infection in European badgers (Meles meles): The Irish Greenfield Study. Vet. Microbiol. 2011, 151, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Corner, L.A.L.; Murphy, D.; Gormley, E. Mycobacterium bovis infection in the Eurasian badger (Meles meles): The disease, pathogenesis, epidemiology and control. J. Comp. Pathol. 2011, 144, 1–24. [Google Scholar] [CrossRef]
- Cheeseman, C.L.; Jones, G.W.; Gallagher, J.; Mallinson, P.J. The population structure, density and prevalence of tuberculosis (Mycobacterium bovis) in badgers (Meles meles) from four areas in south west England. J. Appl. Ecol. 1981, 18, 795–804. [Google Scholar] [CrossRef]
- Bourne, F.J.; Donnelly, C.A.; Cox, D.R.; Gettinby, G.; Mclnerney, J.P.; Morrison, W.I.; Woodroffe, R. TB policy and the ISG’s findings. Vet. Rec. 2007, 161, 633–635. [Google Scholar] [CrossRef]
- Sandoval Barron, E.; Swift, B.; Chantrey, J.; Christley, R.; Gardner, R.; Jewell, C.; McGrath, I.; Mitchell, A.; O’Cathail, C.; Prosser, A.; et al. A study of tuberculosis in road traffic-killed badgers on the edge of the British bovine TB epidemic area. Sci. Rep. 2018, 8, 17206. [Google Scholar] [CrossRef] [PubMed]
- Delahay, R.J.; Walker, N.J.; Forrester, G.J.; Harmsen, B.; Riordan, P.; Macdonald, D.W.; Newman, C.; Cheeseman, C.L. Demographic correlates of bite wounding in Eurasian badgers, Meles meles L., in stable and perturbed populations. Anim. Behav. 2006, 71, 1047–1055. [Google Scholar] [CrossRef]
- Reveillaud, E.; Desvaux, S.; Boschiroli, M.L.; Hars, J.; Faure, E.; Fediaevsky, A.; Cavalerie, L.; Chevalier, F.; Jabert, P.; Poliak, S.; et al. Infection of wildlife by Mycobacterium bovis in France assessment through a national surveillance system, Sylvatub. Front. Vet. Sci. 2018, 5, 262. [Google Scholar] [CrossRef]
- Michelet, L.; de Cruz, K.; Tambosco, J.; Hénault, S.; Boschiroli, M.L. Mycobacterium microti interferes with bovine tuberculosis surveillance. Microorganisms 2020, 8, 1850. [Google Scholar] [CrossRef]
- Michelet, L.; de Cruz, K.; Phalente, Y.; Karoui, C.; Henault, S.; Beral, M.; Boschiroli, M.L. Mycobacterium microti infection in dairy goats, France. Emerg. Infect. Dis. 2016, 22, 569–570. [Google Scholar] [CrossRef] [PubMed]
- Michelet, L.; de Cruz, K.; Karoui, C.; Henault, S.; Boschiroli, M.L. Mycobacterium microti infection in a cow in France. Vet. Rec. 2017, 180, 429. [Google Scholar] [CrossRef]
- Lorente-Leal, V.; Liandris, E.; Pacciarini, M.; Botelho, A.; Kenny, K.; Loyo, B.; Fernández, R.; Bezos, J.; Domínguez, L.; de Juan, L.; et al. Direct PCR on tissue samples to detect Mycobacterium tuberculosis complex: An alternative to the bacteriological culture. J. Clin. Microbiol. 2021, 59, e01404-20. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blanco Vázquez, C.; Barral, T.D.; Romero, B.; Queipo, M.; Merediz, I.; Quirós, P.; Armenteros, J.Á.; Juste, R.; Domínguez, L.; Domínguez, M.; et al. Spatial and Temporal Distribution of Mycobacterium tuberculosis Complex Infection in Eurasian Badger (Meles meles) and Cattle in Asturias, Spain. Animals 2021, 11, 1294. https://doi.org/10.3390/ani11051294
Blanco Vázquez C, Barral TD, Romero B, Queipo M, Merediz I, Quirós P, Armenteros JÁ, Juste R, Domínguez L, Domínguez M, et al. Spatial and Temporal Distribution of Mycobacterium tuberculosis Complex Infection in Eurasian Badger (Meles meles) and Cattle in Asturias, Spain. Animals. 2021; 11(5):1294. https://doi.org/10.3390/ani11051294
Chicago/Turabian StyleBlanco Vázquez, Cristina, Thiago Doria Barral, Beatriz Romero, Manuel Queipo, Isabel Merediz, Pablo Quirós, José Ángel Armenteros, Ramón Juste, Lucas Domínguez, Mercedes Domínguez, and et al. 2021. "Spatial and Temporal Distribution of Mycobacterium tuberculosis Complex Infection in Eurasian Badger (Meles meles) and Cattle in Asturias, Spain" Animals 11, no. 5: 1294. https://doi.org/10.3390/ani11051294
APA StyleBlanco Vázquez, C., Barral, T. D., Romero, B., Queipo, M., Merediz, I., Quirós, P., Armenteros, J. Á., Juste, R., Domínguez, L., Domínguez, M., Casais, R., & Balseiro, A. (2021). Spatial and Temporal Distribution of Mycobacterium tuberculosis Complex Infection in Eurasian Badger (Meles meles) and Cattle in Asturias, Spain. Animals, 11(5), 1294. https://doi.org/10.3390/ani11051294





