Effects of Oral Hyaluronic Acid Administration in Dogs Following Tibial Tuberosity Advancement Surgery for Cranial Cruciate Ligament Injury
Abstract
:Simple Summary
Abstract
1. Introduction
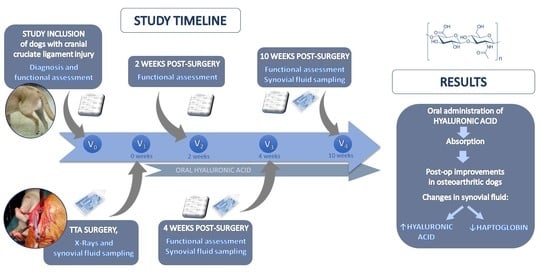
2. Materials and Methods
2.1. Subjects
2.2. Dietary Intervention
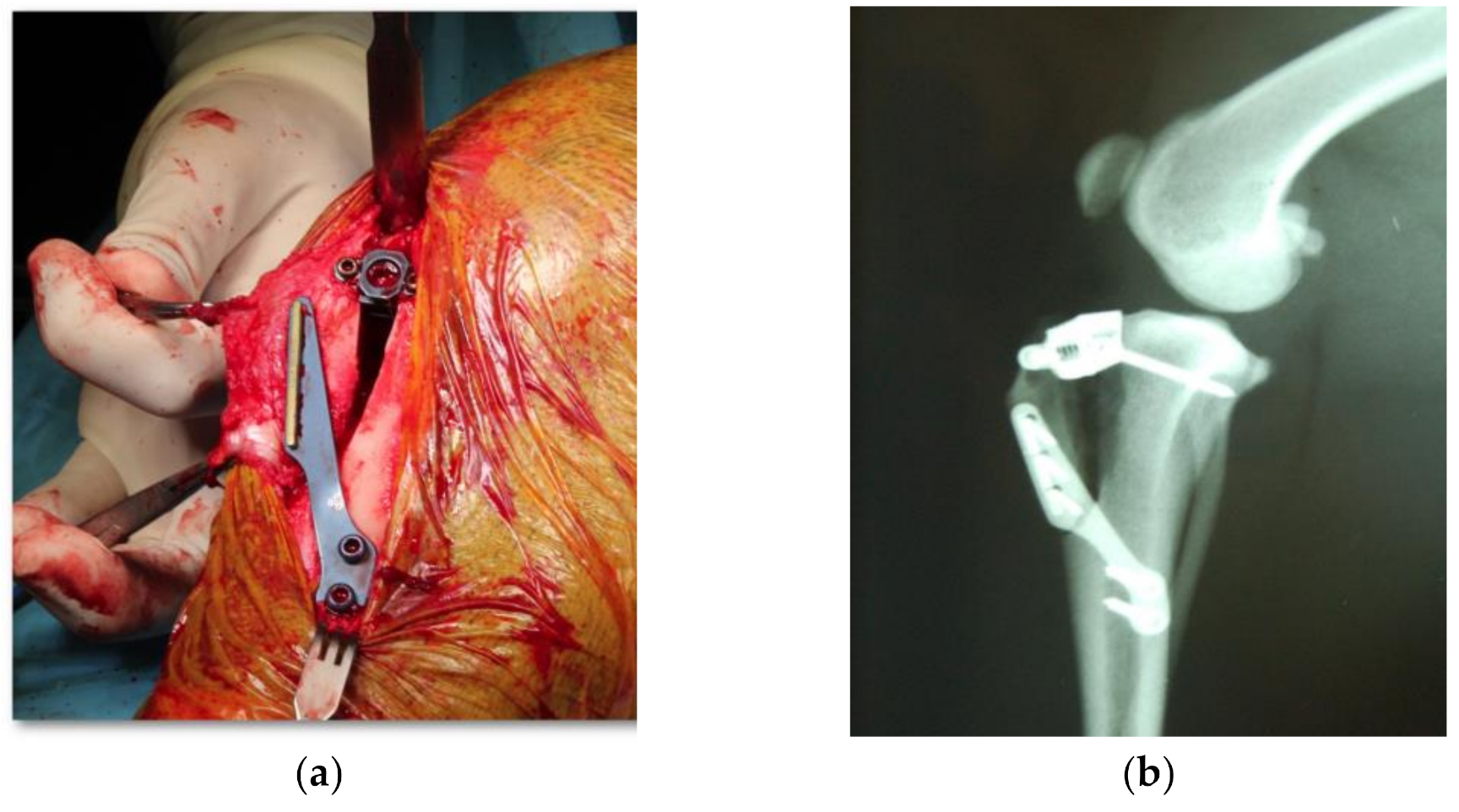
2.3. Experimental Protocol
2.4. Statistical Analysis
3. Results
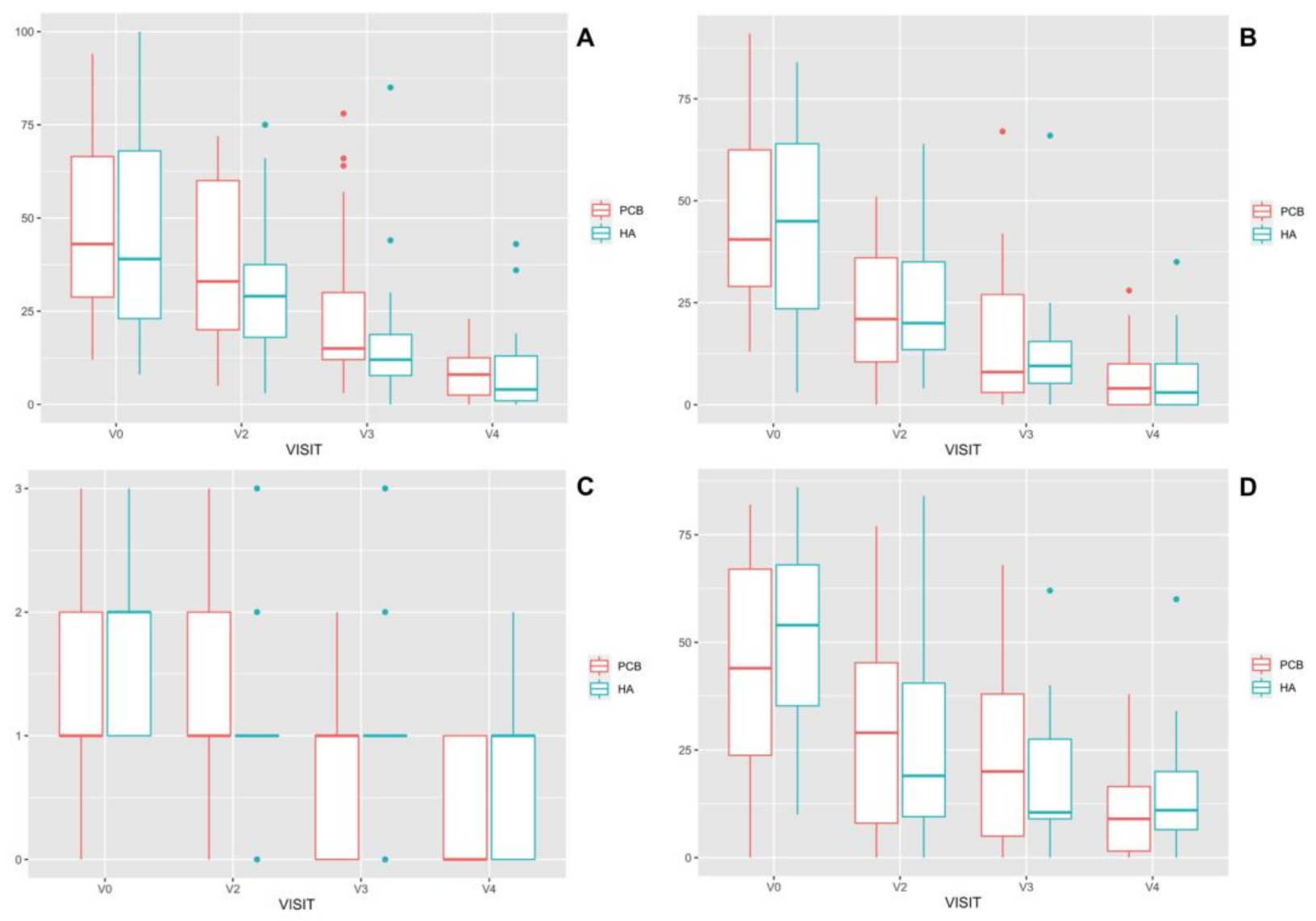
3.1. Clinical Assessments
3.2. Biomarkers in Synovial Fluid
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Plickert, H.; Bondzio, A.; Einspanier, R.; Tichy, A.; Brunnberg, L. Hyaluronic acid concentrations in synovial fluid of dogs with different stages of osteoarthritis. Res. Veter. Sci. 2013, 94, 728–734. [Google Scholar] [CrossRef]
- Bleedorn, J.A.; Muir, P. Synovitis or stifle instability, which comes first? In Advances in the Canine Cranial Cruciate Ligament; Muir, P., Ed.; ACVS Foundation and Willey-Blackwell: Hoboken, NJ, USA, 2010; pp. 81–86. [Google Scholar]
- Wang, C.-T.; Lin, J.; Chang, C.-J.; Lin, Y.-T.; Hou, S.-M. Therapeutic Effects of Hyaluronic Acid on Osteoarthritis of the Knee. A meta-analysis of randomized controlled trials. J. Bone Jt. Surg. Am. Vol. 2004, 86, 538–545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellamy, N.; Campbell, J.; Welch, V.; Gee, T.L.; Bourne, R.; Wells, G.A. Viscosupplementation for the treatment of osteoarthritis of the knee. Cochrane Database Syst. Rev. 2006, 19, CD005321. [Google Scholar] [CrossRef] [Green Version]
- Oe, M.; Tashiro, T.; Yoshida, H.; Nishiyama, H.; Masuda, Y.; Maruyama, K.; Koikeda, T.; Maruya, R.; Fukui, N. Oral hyaluronan relieves knee pain: A review. Nutr. J. 2015, 15, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Archer, C.W.; Francis-West, P. The chondrocyte. Int. J. Biochem. Cell Biol. 2003, 35, 401–404. [Google Scholar] [CrossRef]
- Evanko, S.P.; Tammi, M.I.; Tammi, R.H.; Wight, T.N. Hyaluronan-dependent pericellular matrix. Adv. Drug Deliv. Rev. 2007, 59, 1351–1365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayhan, E.; Kesmezacar, H.; Akgun, I. Intraarticular injections (corticosteroid, hyaluronic acid, platelet rich plasma) for the knee osteoarthritis. World J. Orthop. 2014, 5, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Caires, R.; Luis, E.; Taberner, F.J.; Fernandez-Ballester, G.; Ferrer-Montiel, A.; Balazs, E.A.; Gomis, A.; Belmonte, C.; de la Peña, E. Hyaluronan modulates TRPV1 channel opening, reducing peripheral nociceptor activity and pain. Nat. Commun. 2015, 6, 8095. [Google Scholar] [CrossRef] [Green Version]
- Gomis, A.; Miralles, A.; Schmidt, R.F.; Belmonte, C. Nociceptive nerve activity in an experimental model of knee joint os-teoarthritis of the guinea pig: Effect of intra-articularhyaluronan application. Pain 2007, 130, 126–136. [Google Scholar] [CrossRef]
- Balogh, L.; Polyak, A.; Mathe, D.; Kiraly, R.; Thuróczy, J.; Terez, M.; Janoki, G.; Ting, Y.; Bucci, L.R.; Schauss, A.G. Absorption, Uptake and Tissue Affinity of High-Molecular-Weight Hyaluronan after Oral Administration in Rats and Dogs. J. Agric. Food Chem. 2008, 56, 10582–10593. [Google Scholar] [CrossRef]
- Sato, T.; Iwaso, H. An effectiveness study of hyaluronic acid (Hyabest J) in the treatment of osteoarthritis of the knee on the patient in the United States. J. New Rem. Clin. 2009, 58, 551–558. [Google Scholar]
- Tashiro, T.; Seino, S.; Sato, T.; Matsuoka, R.; Masuda, Y.; Fukui, N. Oral Administration of Polymer Hyaluronic Acid Alleviates Symptoms of Knee Osteoarthritis: A Double-Blind, Placebo-Controlled Study over a 12-Month Period. Sci. World J. 2012, 2012, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jandi, A.S.; Schulman, A.J. Incidence of Motion Loss of the Stifle Joint in Dogs with Naturally Occurring Cranial Cruciate Ligament Rupture Surgically Treated with Tibial Plateau Leveling Osteotomy: Longitudinal Clinical Study of 412 Cases. Veter. Surg. 2007, 36, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Montavon, P.M.; Damur, D.M.; Tepic, S. Tibial tuberosity advancement (TTA) for the treatment of cranial cruciate disease in dogs: Evidences, technique and initial clinical results. In Proceedings of the 12th European Society of Veterinary Orthopaedics and Traumatology Congress, Munich, Germany, 10–12 September 2004; pp. 254–255. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 23 March 2021).
- Champely, S. pwr: Basic Functions for Power Analysis. R Package Version 1.3-0. Available online: https://CRAN.R-project.org/package=pwr (accessed on 23 March 2021).
- Mair, P.; Wilcox, R. Robust statistical methods in R using the WRS2 package. Behav. Res. Methods 2019, 52, 464–488. [Google Scholar] [CrossRef] [PubMed]
- Pozo, M.A.; Balazs, E.A.; Belmonte, C. Reduction of sensory responses to passive movements of inflamed knee joints by hylan, a hyaluronan derivative. Exp. Brain Res. 1997, 116, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Santangelo, K.S.; Johnson, A.L.; Ruppert, A.S.; Bertone, A.L. Effects of hyaluronan treatment on lipopolysaccharide-challenged fibroblast-like synovial cells. Arthritis Res. Ther. 2007, 9, R1. [Google Scholar] [CrossRef] [Green Version]
- Neuenschwander, H.M.; Moreira, J.J.; Vendruscolo, C.P.; Fülber, J.; Seidel, S.R.T.; Michelacci, Y.M.; Baccarin, R.Y.A. Hyaluronic acid has chondroprotective and joint-preserving effects on LPS-induced synovitis in horses. J. Veter. Sci. 2019, 20, e67. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.T.; Lin, Y.T.; Chiang, B.L.; Lin, Y.H.; Hou, S.M. High molecular weight hyaluronic acid down-regulates the gene expression of osteoarthritis-associated cytokines and enzymes in fibroblast-like synoviocites from patients with early osteoarthritis. Osteoarthr. Cartil. 2006, 37, 241–246. [Google Scholar]
- Day, R.; Brooks, P.; Conaghan, P.G.; Petersen, M. A double blind, randomized, multicentre, parallel group study of the effectiveness and tolerance of intraarticular hyaluronan in osteoarthritis of the knee. J. Rheumatol. 2004, 31, 775–782. [Google Scholar]
- Adams, M.E.; Lussier, A.J.; Peyron, J.G. A Risk-Benefit Assessment of Injections of Hyaluronan and Its Derivatives in the Treatment of Osteoarthritis of the Knee. Drug Saf. 2000, 23, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M.; Maeshima, T.; Kubota, T.; Kurihara, H.; Masuda, Y.; Nomura, Y. Absorption of Orally Administered Hyaluronan. J. Med. Food 2016, 19, 1172–1179. [Google Scholar] [CrossRef] [PubMed]
- Wilke, V.L.; Robinson, D.A.; Evans, R.B.; Rothschild, M.F.; Conzemius, M.G. Estimate of the annual economic impact of treatment of cranial cruciate ligament injury in dogs in the United States. J. Am. Veter. Med. Assoc. 2005, 227, 1604–1607. [Google Scholar] [CrossRef]
- Berg, R.; Sykes, J.; Kass, P.; Vernau, W. Effect of Repeated Arthrocentesis on Cytologic Analysis of Synovial Fluid in Dogs. J. Veter. Intern. Med. 2009, 23, 814–817. [Google Scholar] [CrossRef] [PubMed]
- McCready, D.J.; Ness, M.G. Systematic review of the prevalence, risk factors, diagnosis and management of meniscal injury in dogs: Part 1 [corrected]. J. Small Anim. Pract. 2016, 57, 221. [Google Scholar] [CrossRef] [Green Version]
- McCready, D.J.; Ness, M.G. Systematic review of the prevalence, risk factors, diagnosis and management of meniscal injury in dogs: Part 2. J. Small Anim. Pract. 2016, 57, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Livet, V.; Baldinger, A.; Viguier, É.; Taroni, M.; Harel, M.; Carozzo, C.; Cachon, T. Comparison of Outcomes Associated with Tibial Plateau Levelling Osteotomy and a Modified Technique for Tibial Tuberosity Advancement for the Treatment of Cranial Cruciate Ligament Disease in Dogs: A Randomized Clinical Study. Veter. Comp. Orthop. Traumatol. 2019, 32, 314–323. [Google Scholar] [CrossRef]
- Pinna, S.; Lanzi, F.; Cordella, A.; Diana, A. Relationship between the stage of osteoarthritis before and six months after tibial tuberosity advancement procedure in dogs. PLoS ONE 2019, 14, e0219849. [Google Scholar] [CrossRef] [Green Version]
- Stein, S.; Schmoekel, H. Short-term and eight to 12 months results of a tibial tuberosity advancement as treatment of canine cranial cruciate ligament damage. J. Small Anim. Pract. 2008, 49, 398–404. [Google Scholar] [CrossRef]
- Tepic, S.; Damur, D.; Montavon, P.M. Biomechanics of the stifle joint. In Proceedings of the Abstracts of the 1st World Orthopaedic Veterinary Congress, Munich, Germany, 5–8 September 2002; pp. 189–190. [Google Scholar]
- Iwanaga, T.; Shikichi, M.; Kitamura, H.; Yanase, H.; Nozawa-Inoue, K. Morphology and Functional Roles of Synoviocytes in the Joint. Arch. Histol. Cytol. 2000, 63, 17–31. [Google Scholar] [CrossRef] [Green Version]
- Fraser, J.R.E.; Laurent, T.C.; Laurent, U.B.G. Hyaluronan: Its nature, distribution, functions and turnover. J. Intern. Med. 1997, 242, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Prehm, P. Release of hyaluronate from eukaryotic cells. Biochem. J. 1990, 267, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Henderson, E.B.; Grootveld, M.; Farrell, A.; Smith, E.C.; Thompson, P.W.; Bake, D.R. A pathological role for damaged hyaluronan in synovitis. Ann. Rheum. Dis. 1991, 50, 196–200. [Google Scholar] [CrossRef] [Green Version]
- Kuroki, K.; Cook, J.L.; Kreeger, J.M. Mechanisms of action and potential uses of hyaluronan in dogs with osteoarthritis. J. Am. Veter. Med. Assoc. 2002, 221, 944–950. [Google Scholar] [CrossRef] [PubMed]
- Garner, B.C.; Kuroki, K.; Stoker, A.M.; Cook, C.R.; Cook, J.L. Expression of proteins in serum, synovial fluid, synovial membrane, and articular cartilage samples obtained from dogs with stifle joint osteoarthritis secondary to cranial cruciate ligament disease and dogs without stifle joint arthritis. Am. J. Veter. Res. 2013, 74, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Olszewska-Slonina, D.M.; Jung, S.; Olszewski, K.J.; Cwynar, A.; Drewa, G. Evaluation of selected parameters of lipid perox-idation and paraoxonase activity in blood of patients with joint osteoarthritis. Protein Pept. Lett. 2018, 25, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Soran, N.; Altindag, O.; Çakir, H.; Çelik, H.; Demirkol, A.; Aksoy, N. Assessment of paraoxonase activities in patients with knee osteoarthritis. Redox Rep. 2008, 13, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Ertürk, C.; Altay, M.A.; Bilge, A.; Çelik, H. Is there a relationship between serum ox-LDL, oxidative stress, and PON1 in knee osteoarthritis? Clin. Rheumatol. 2017, 36, 2775–2780. [Google Scholar] [CrossRef]
- Kajikawa, T.; Furuta, A.; Onishi, T.; Tajima, T.; Sugii, S. Changes in concentrations of serum amyloid A protein, α1-acid glycoprotein, haptoglobin, and C-reactive protein in feline sera due to induced inflammation and surgery. Veter. Immunol. Immunopathol. 1999, 68, 91–98. [Google Scholar] [CrossRef]
- Crisman, M.V.; Scarratt, W.K.; Zimmerman, K.L. Blood Proteins and Inflammation in the Horse. Veter. Clin. N. Am. Equine Pr. 2008, 24, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Kitasato, A.; Tajima, Y.; Kuroki, T.; Tsutumi, R.; Adachi, T.; Mishima, T.; Kanematsu, T. Inflammatory cytokines promote inducible nitric oxide synthase-mediated DNA damage in hamster gallbladder epithelial cells. World J. Gastroenterol. 2007, 13, 6379–6384. [Google Scholar] [CrossRef]
- Koch, A.; Zacharowski, K.; Boehm, O.; Stevens, M.; Markus, F.; Lipfert, P.; von Giesen, H.-J.; Wolf, A.; Freynhagen, R. Nitric oxide and pro-inflammatory cytokines correlate with pain intensity in chronic pain patients. Inflamm. Res. 2007, 56, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Farrell, A.J.; Blake, D.R.; Palmer, R.M. Increased concentrations of nitrite in synovial fluid and serum samples suggest in-creased nitric oxide synthesis in rheumatic diseases. Ann. Rheum. Dis. 1992, 51, 1219–1221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grabowski, P.S.; Macpherson, H.; Ralston, S.H. Nitric oxide production in cells derived from the human joint. Rheumatology 1996, 35, 207–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bredt, D.S.; Snyder, S.H. Nitric oxide—A physiological messenger molecule. Annu. Rev. Biochem. 1994, 63, 175. [Google Scholar] [CrossRef] [PubMed]
- Abramson, S.B. Nitric oxide in inflammation and pain associated with osteoarthritis. Arthritis Res. Ther. 2008, 10, S2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spreng, D. Role of nitric oxide production and matrix protease activity in cruciate ligament degeneration. In Advances in the Canine Cranial Cruciate Ligament; Muir, P., Ed.; ACVS Foundation and Willey-Blackwell: Hoboken, NJ, USA, 2010; pp. 71–76. [Google Scholar]
| Visit | V0 | V1 | V2 | V3 | V4 |
|---|---|---|---|---|---|
| Diagnostic consultation (specialty consultation, radiology, and blood tests) | X | ||||
| Signing of informed consent (study information and document signing) | X | ||||
| Functional assessment (assessment by owner and veterinarian) | X | X | X | X | |
| TTA surgery (surgical treatment, postoperative radiology, and arthrocentesis) | X | ||||
| Synovial fluid aspiration | X | X |
| Biomarker (Units) | Mean ± SD |
|---|---|
| HA (µg/mL) | 1614.62 ± 393.60 |
| HAP (g/L) | 0.22 ± 0.36 |
| NO (µmol/L) | 9.38 ± 7.57 |
| PON-1 (IU/mL) | 0.86 ± 0.61 |
| Biomarker | Group PCB | Group HA | |||
|---|---|---|---|---|---|
| n | Median (Min–Max) | n | Median (Min–Max) | ||
| HA (μg/mL) | V1 | 23 | 1810 (952–2090) | 30 | 1670 (301–2230) a |
| V4 | 21 | 1650 (1240–2030) | 19 | 1780 (993–2730) a | |
| PON-1 (IU/mL) | V1 | 19 | 0.91 (0.14–3.10) | 23 | 0.67 (0.14–1.64) b |
| V4 | 16 | 0.14 (0.12–1.11) | 19 | 0.14 (0.13–1.87) b | |
| NO (µmol/L) | V1 | 17 | 8.47 (3.08–150) | 19 | 8.30 (1.96–81.80) |
| V4 | 17 | 6.26 (1.80–78.70) | 13 | 5.38 (2.61–97.50) | |
| HAP (g/L) | V1 | 22 | 0.15 (0.01–1.29) | 22 | 0.03 (0.01–1.66) |
| V4 | 21 | 0.02 (0.01–1.53) | 21 | 0.02 (0.01–1.50) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serra Aguado, C.I.; Ramos-Plá, J.J.; Soler, C.; Segarra, S.; Moratalla, V.; Redondo, J.I. Effects of Oral Hyaluronic Acid Administration in Dogs Following Tibial Tuberosity Advancement Surgery for Cranial Cruciate Ligament Injury. Animals 2021, 11, 1264. https://doi.org/10.3390/ani11051264
Serra Aguado CI, Ramos-Plá JJ, Soler C, Segarra S, Moratalla V, Redondo JI. Effects of Oral Hyaluronic Acid Administration in Dogs Following Tibial Tuberosity Advancement Surgery for Cranial Cruciate Ligament Injury. Animals. 2021; 11(5):1264. https://doi.org/10.3390/ani11051264
Chicago/Turabian StyleSerra Aguado, Claudio Iván, Juan José Ramos-Plá, Carme Soler, Sergi Segarra, Víctor Moratalla, and José Ignacio Redondo. 2021. "Effects of Oral Hyaluronic Acid Administration in Dogs Following Tibial Tuberosity Advancement Surgery for Cranial Cruciate Ligament Injury" Animals 11, no. 5: 1264. https://doi.org/10.3390/ani11051264
APA StyleSerra Aguado, C. I., Ramos-Plá, J. J., Soler, C., Segarra, S., Moratalla, V., & Redondo, J. I. (2021). Effects of Oral Hyaluronic Acid Administration in Dogs Following Tibial Tuberosity Advancement Surgery for Cranial Cruciate Ligament Injury. Animals, 11(5), 1264. https://doi.org/10.3390/ani11051264