Interactions among Natural Active Ingredients to Improve the Efficiency of Rumen Fermentation In Vitro
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment 1
2.1.1. Experimental Procedures
2.1.2. Sample Collection and Chemical Analyses
2.1.3. Statistical Analyses
2.2. Experiment 2
2.2.1. Experimental Procedures
2.2.2. Statistical Analyses
3. Results
3.1. Experiment 1 (Individual Essential Oils)
3.2. Experiment 2 (Mixtures of Essential Oils)
4. Discussion
4.1. Effect of Phenolic Compounds (Experiment 1)
4.2. Effect of Monoterpene Compounds (Experiment 1)
4.3. Interaction among Different Essential Oils (Experiment 2)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Benchaar, C.; Calsamiglia, S.; Chaves, A.V.; Fraser, G.R.; Colombatto, D.; Mcallister, T.A.; Beauchemin, K.A. A review of plant-derived essential oils in ruminant nutrition and production. Anim. Feed Sci. Technol. 2008, 145, 209–228. [Google Scholar] [CrossRef]
- Busquet, M.; Calsamiglia, S.; Ferret, A.; Carro, M.D.; Kamel, C. Effect of garlic oil and four of its compounds on rumen microbial fermentation. J. Dairy Sci. 2005, 88, 4393–4404. [Google Scholar] [CrossRef]
- Hristov, A.N.; Oh, J.; Firkins, J.L.; Dijkstra, J.; Kebreab, E.; Waghorn, G.; Makkar, H.P.S.; Adesogan, A.T.; Yang, W.; Lee, C.; et al. Special topics–Mitigation of methane and nitrous oxide emissions from animal operations: I. A review of enteric methane mitigation options. J. Ani. Sci. 2013, 91, 5045–5069. [Google Scholar] [CrossRef]
- Tedeschi, L.O.; Fox, D.G.; Tylutki, T.P. Potential environmental benefits of ionophores in ruminant diets. J. Environ. Qual. 2003, 32, 1591–1602. [Google Scholar] [CrossRef]
- Richardson, L.F.; Raun, A.P.; Potter, E.L.; Cooley, C.O.; Rathmacher, R.P. Effect of monensin on rumen fermentation in vivo and in vitro. J. Anim. Sci. 1976, 43, 3. [Google Scholar] [CrossRef]
- European Commision. Regulation (EC) No. 1931/2003 of the European parliament and of the council of 22 September 2003 on additives for use in animal nutrition. Off. J. Eur. Union. 2003, L268/229–L268/243. Available online: https://ec.europa.eu/jrc/sites/jrcsh/files/EC-1831-2003.pdf (accessed on 7 January 2021).
- Calsamiglia, S.; Busquet, M.; Cardozo, P.W.; Castillejos, L.; Ferret, A. Invited review: Essential oils as modifiers of rumen microbial fermentation. J. Dairy Sci. 2007, 90, 2580–2595. [Google Scholar] [CrossRef]
- Dorman, H.J.D.; Deans, S.G. Antimicrobial agents from plants: Antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2000, 88, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Castillejos, L.; Calsamiglia, S.; Ferret, A. Effect of essential oil active compounds on rumen microbial fermentation and nutrient flow in in vitro systems. J. Dairy Sci. 2006, 89, 2649–2658. [Google Scholar] [CrossRef]
- Rodríguez-Prado, M.; Ferret, A.; Zwieten, J.; Gonzalez, L.; Bravo, D.; Calsamiglia, S. Effects of dietary addition of capsicum extract on intake, water consumption, and rumen fermentation of fattening heifers fed a high-concentrate diet. J. Anim. Sci. 2012, 90, 1879–1884. [Google Scholar] [CrossRef] [PubMed]
- Foskolos, A.; Ferret, A.; Siurana, A.; Castillejos, L.; Calsamiglia, S. Effects of capsicum and propyl-propane thiosulfonate on rumen fermentation, digestion, and milk production and composition in dairy cows. Animals 2020, 10, 859. [Google Scholar] [CrossRef]
- Fandiño, I.; Fernandez-Turren, G.; Ferret, A.; Moya, D.; Castillejos, L.; Calsamiglia, S. Exploring additive, synergistic or antagonistic effects of natural plant extracts on in vitro beef feedlot-type rumen microbial fermentation conditions. Animals 2020, 10, 173. [Google Scholar] [CrossRef] [PubMed]
- Tserennadmid, R.; Takó, M.; Galgóczy, L.; Papp, T.; Pesti, M.; Vágvölgyi, C.; Almássy, K.; Krisch, J. Anti-yeast activities of some essential oils in growth medium, fruit juices and milk. Inter. J. Food Micro. 2011, 144, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Bassolé, I.H.N.; Lamien-Meda, A.; Bayala, B.; Tirogo, S.; Franz, C.; Novak, J.; Nebié, R.C.; Dicko, M.H. Composition and antimicrobial activities of Lippia Multiflora Moldenke, Mentha x piperita L and Ocimum basilicum L. essential oils and their major monoterpene alcohols alone and in combination. Molecules 2010, 15, 7825. [Google Scholar] [CrossRef] [PubMed]
- Gallucci, M.N.; Oliva, M.; Casero, C.; Dambolena, J.; Luna, A.; Zygadlo, J.; Demo, M. Antimicrobial combined action of terpenes against the food-borne microorganisms Escherichia coli, Staphylococcus aureus and Bacillus cereus. Flavour. Fragr. J. 2009, 24, 348–354. [Google Scholar] [CrossRef]
- Bassolé, I.H.N.; Juliani, H.R. Essential oils in combination and their antimicrobial properties. Molecules. 2012, 17, 3989. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods: A review. Int. J. Food Microbial. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Langeveld, W.T.; Veldhuizen, E.J.A.; Burt, S.A. Synergy between essential oil components and antibiotics: A review. Crit. Rev. Microbiol. 2013, 40, 76–94. [Google Scholar] [CrossRef] [PubMed]
- Chao, S.C.; Young, D.G. Screening for inhibitory activity of essential oils on selected bacteria, fungi and viruses. J. Essent. Oil Res. 2000, 12, 639–649. [Google Scholar] [CrossRef]
- Hristov, A.N.; Ropp, J.K.; Zaman, S.; Melgar, A. Effects of essential oils on in vitro ruminal fermentation and ammonia release. Anim. Feed Sci. Technol. 2008, 144, 55–64. [Google Scholar] [CrossRef]
- Tilley, J.M.A.; Terry, R.A. A two-stage technique for the in vitro digestion of forage crops. J. Brit. Grassland Soc. 1963, 18, 104–111. [Google Scholar] [CrossRef]
- McDougall, E.I. Studies on ruminant saliva: The composition and output of sheep’s saliva. Biochem. J. 1948, 43, 99–109. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Dairy Cattle, 7th ed.; The National Academies Press: Washington, DC, USA, 2001. [Google Scholar]
- Chaney, A.L.; Marbach, E.P. Modified reagents for determination of urea and ammonia. Clin. Chem. 1962, 8, 130–132. [Google Scholar] [CrossRef]
- Miller, R.G. Simultaneous Statistical Inference, 2nd ed.; Springer: New York, NY, USA, 1981. [Google Scholar]
- Cornell, J.A. Experiments with Mixtures: Designs, Models, and the Analysis of 348 Mixture Data, 2nd ed.; Wiley: New York, NY, USA, 1990. [Google Scholar]
- Benchaar, C.; Chaves, A.V.; Fraser, G.R.; Wang, Y.; Beauchemin, K.A.; McAllister, T.A. Effects of essential oils and their components on in vitro rumen microbial fermentation. Can. J. Anim. Sci. 2008, 87, 413–419. [Google Scholar] [CrossRef]
- Lambert, R.J.W.; Skandamis, P.N.; Coote, P.J.; Nychas, G.J.E. A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J. Appl. Microbiol. 2001, 91, 453–462. [Google Scholar] [CrossRef]
- Busquet, M.; Calsamiglia, S.; Ferret, A.; Kamel, C. Plant extracts affect in vitro rumen microbial fermentation. J. Dairy Sci. 2006, 89, 761–771. [Google Scholar] [CrossRef]
- Chaves, A.V.; He, M.L.; Yang, W.Z.; Hristov, A.N.; McAllister, T.A.; Benchaar, C. Effects of essential oils on proteolytic, deaminative and methanogenic activities of mixed ruminal bacteria. Can. J. Anim. Sci. 2008, 88, 117–122. [Google Scholar] [CrossRef]
- Nanon, A.; Suksombat, W.; Yang, W. Use of essential oils for manipulation of rumen microbial fermentation using batch culture. Thai. J. Vet. Med. 2015, 45, 167–180. [Google Scholar]
- Cardozo, P.W.; Calsamiglia, S.; Ferret, A.; Kamel, C. Screening for the effects of natural plant extracts at different pH on in vitro rumen microbial fermentation of a high-concentrate diet for beef cattle. J. Anim. Sci. 2005, 83, 2572–2579. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, F.M.; Newbold, C.J.; Losa, R.; Williams, P.; Wallace, R.J. Effects of essential oils on rumen fermentation. Reprod. Nutr. Dev. 2000, 40, 221–222. [Google Scholar]
- Ferme, D.; Banjac, M.; Calsamiglia, S.; Busquet, M.; Kamel, C.; Avgustin, G. The effects of plant extracts on microbial community structure in a rumen-simulating continuous-culture system as revealed by molecular profiling. Folia Microbiol. 2004, 49, 151–155. [Google Scholar] [CrossRef]
- Kebreab, E.; France, J.; Mills, J.A.N.; Allison, R.; Dijkstra, J. A dynamic model of N metabolism in the lactating dairy cow and an assessment of impact of N excretion on the environment. J. Anim. Sci. 2002, 80, 248–259. [Google Scholar] [CrossRef]
- Cobellis, G.; Trabalza-Marinucci, M.; Marcotullio, M.C.; Yu, Z. Evaluation of different essential oils in modulating methane and ammonia production, rumen fermentation, and rumen bacteria in vitro. Anim. Feed Sci. Technol. 2016, 215, 25–36. [Google Scholar] [CrossRef]
- Benchaar, C.; Greathead, H. Essential oils and opportunities to mitigate enteric methane emissions from ruminants. Anim. Feed Sci. Technol. 2011, 166, 338–355. [Google Scholar] [CrossRef]
- Herman, A.; Tambor, K.; Herman, A. Linalool affects the antimicrobial efficacy of essential oils. Curr. Microbiol. 2016, 72, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Joch, M.; Cermak, L.; Hakl, J.; Hucko, B.; Duskova, D.; Marounek, M. In vitro screening of essential oil active compounds for manipulation of rumen fermentation and methane mitigation. Asian Australas. J. Anim. Sci. 2016, 29, 952–959. [Google Scholar] [CrossRef] [PubMed]
- Joch, M.; Kudrna, V.; Hucko, B.; Marounek, M. Effects of geraniol and camphene on in vitro rumen fermentation and methane production. Anim. Sci. 2017, 48, 63–69. [Google Scholar] [CrossRef]
| Product | Active Compound and Purity (%) | Dose * (mg/L) | ||
|---|---|---|---|---|
| Low | Medium | High | ||
| Monensin | Monensin, 93 | 10 | ||
| Anise star | Trans-Anethol, 99 | 80 | 300 | 750 |
| Black pepper | Piperine, 95 | 0.4 | 3 | 7.5 |
| Capsicum | Capsaicin, 6 | 0.4 | 3 | 7.5 |
| Cassia | Cinnamaldehyde, 75 | 80 | 300 | 750 |
| Coriander | Linalool, 65 | 40 | 150 | 375 |
| Geraniol | Geraniol, 84 | 80 | 300 | 750 |
| Ginger | Gingerols, 63.2 | 10 | 40 | 150 |
| Lemongrass | Citral, 75 | 80 | 300 | 750 |
| Limonene | Limonene, 93 | 80 | 300 | 750 |
| Tea tree | Terpinen-4-ol, 38.9 | 40 | 150 | 375 |
| Thyme | Thymol, 47 | 80 | 300 | 750 |
| Turmeric | Curcumin, 40 | 10 | 40 | 150 |
| Treatments | Composition 1 | % | Low Dose (mg/L) | High Dose (mg/L) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| LEM | COR | GIN | lem | cor | gin | LEM | COR | GIN | ||
| T1 | LEM | 1.00 | 75.0 | 150.0 | ||||||
| T2 | COR | 1.00 | 75.0 | 150.0 | ||||||
| T3 | GIN | 1.00 | 75.0 | 150.0 | ||||||
| T4 | LEM + COR | 0.50 | 0.50 | 37.5 | 37.5 | 75.0 | 75.0 | |||
| T5 | LEM + GIN | 0.50 | 0.50 | 37.5 | 37.5 | 75.0 | 75.0 | |||
| T6 | COR + GIN | 0.50 | 0.50 | 37.5 | 37.5 | 75.0 | 75.0 | |||
| T7 | LEM + cor + gin | 0.50 | 0.25 | 0.25 | 37.5 | 18.8 | 18.8 | 75.0 | 37.5 | 37.5 |
| T8 | lem + COR + gin | 0.25 | 0.50 | 0.25 | 18.8 | 37.5 | 18.8 | 37.5 | 75.0 | 37.5 |
| T9 | Lem + cor + GIN | 0.25 | 0.25 | 0.50 | 18.8 | 18.8 | 37.5 | 37.5 | 37.5 | 75.0 |
| T10 | LEM + COR + GIN | 0.33 | 0.33 | 0.33 | 25.0 | 25.0 | 25.0 | 50.0 | 50.0 | 50.0 |
| Treatment | Control | Dose 1 | SEM 2 | p-Value | ||
|---|---|---|---|---|---|---|
| Low | Medium | High | ||||
| Monensin | 28.1 | 28.2 | 1.31 | 0.80 | ||
| Anise star | 28.1 | 29.2 | 28.5 | 27.5 | 2.60 | 0.56 |
| Black pepper | 28.1 | 26.4 | 26.7 | 26.3 | 0.96 | 0.48 |
| Capsicum | 28.1 | 25.2 * | 24.3 * | 25.5 * | 1.56 | 0.02 |
| Cassia | 28.1 | 29.4 + | 30.8 + | 24.8 + | 1.74 | 0.09 |
| Coriander | 28.1 | 24.3 * | 23.2 * | 23.4 * | 0.99 | 0.01 |
| Geraniol | 28.1 | 33.1 * | 33.8 * | 27.8 | 1.06 | 0.03 |
| Ginger | 28.1 | 29.7 | 31.0 | 29.9 | 1.50 | 0.14 |
| Lemongrass | 28.1 | 32.5 | 31.9 | 31.1 | 1.68 | 0.21 |
| Limonene | 28.1 | 28.9 | 27.6+ | 25.7 + | 2.20 | 0.07 |
| Tea tree | 28.1 | 25.9 | 27.7 | 28.8 | 1.75 | 0.12 |
| Thyme | 28.1 | 26.4 * | 25.9 * | 17.2 * | 1.69 | 0.01 |
| Turmeric | 28.1 | 28.4 | 26.5 | 28.0 | 1.10 | 0.63 |
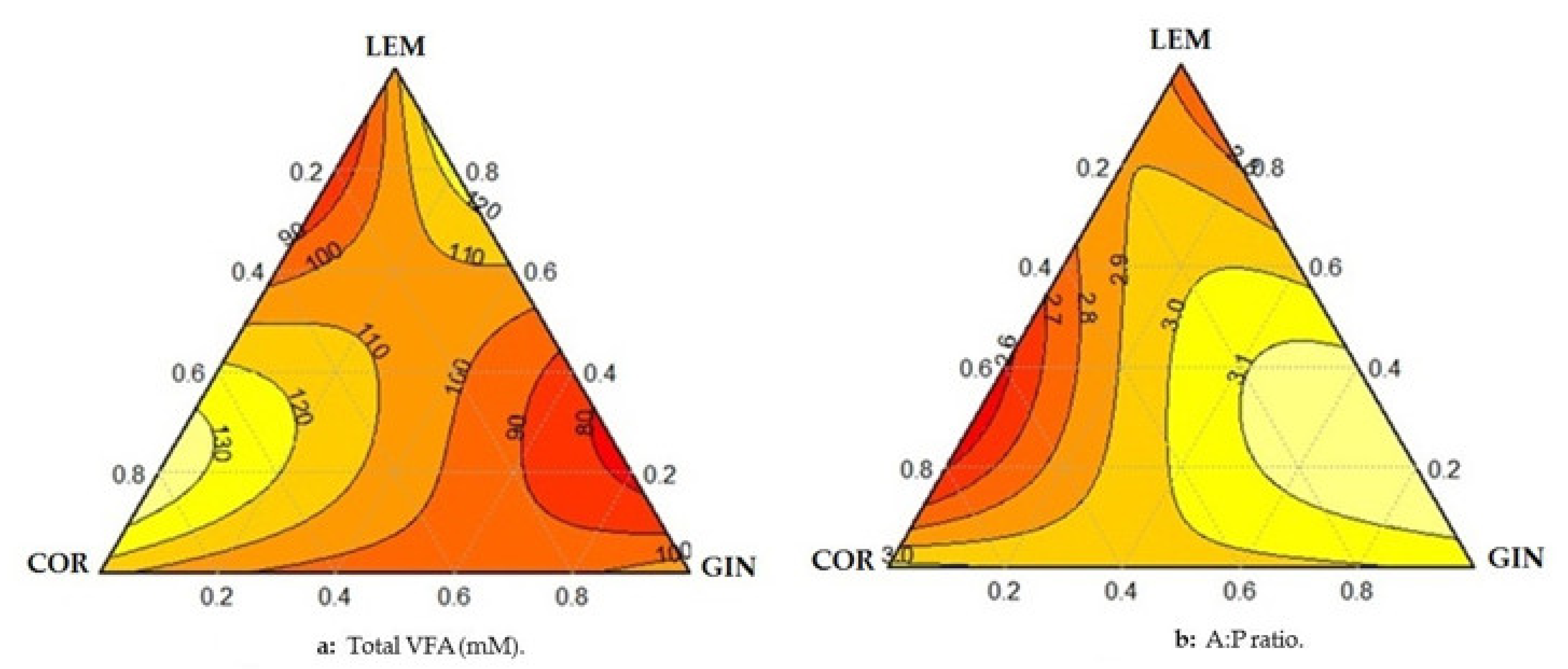
| Treatment | Dose (mg/L) | TVFA 1 | Acetate | Propionate | Butyrate | A:P 2 | N-NH3 | ||
|---|---|---|---|---|---|---|---|---|---|
| LEM | COR | GIN | (mol/100 mol) | (%) | (%) | (%) | mg/100 mL | ||
| T1 | 150 | 109.0 * | 52.4 * | 19.0 * | 24.6 * | 2.77 + | 18.1 | ||
| T2 | 150 | 114.0 * | 54.1 * | 18.1 | 24.1 * | 3.02 | 17.9 * | ||
| T3 | 150 | 107.0 * | 53.7 * | 18.7 | 23.9 * | 2.88 + | 18.7 | ||
| T4 | 75 | 75 | 108.0 * | 50.2 * | 19.4 * | 24.8 * | 2.70 + | 18.7 | |
| T5 | 75 | 75 | 93.0 | 55.1 * | 18.4 | 21.8 * | 3.02 | 19.1 * | |
| T6 | 75 | 75 | 91.0 | 56.8 | 18.2 | 21.9 * | 2.97 + | 18.6 | |
| T7 | 75 | 38 | 38 | 100.0 * | 54.8 | 17.6 | 18.0 | 3.06 | 20.4 * |
| T8 | 38 | 75 | 38 | 97.0 | 57.9 | 17.5 | 17.6 | 2.83 + | 20.0 |
| T9 | 38 | 38 | 75 | 104.0 * | 57.3 | 18.5 | 24.5 * | 2.83 + | 19.8 * |
| T10 | 50 | 50 | 50 | 96.0 | 58.3 | 17.5 | 18.8 | 2.98 + | 18.8 |
| Control | 0 | 0 | 0 | 88.8 | 57.6 | 17.9 | 17.9 | 3.24 | 18.5 |
| p‒Value | <0.01 | <0.01 | <0.01 | <0.01 | 0.080 | 0.03 | |||
| SEM 3 | 0.84 | 0.21 | 0.12 | 0.15 | 0.100 | 0.44 | |||
| Treatment | TVFA 1 | Acetate | Propionate | Butyrate | A:P 2 | NNH3 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coef | SEM | p-Value | Coef | SEM | p-Value | Coef | SEM | p-Value | Coef | SEM | p-Value | Coef | SEM | p-Value | Coef | SEM | p-Value | |
| LEM | 111 | 4.54 | <0.01 | 53.6 | 1.66 | <0.01 | 18.9 | 1.62 | <0.01 | 25.1 | 1.12 | <0.01 | 2.74 | 0.31 | <0.01 | 18.5 | 4.62 | 0.02 |
| COR | 114 | 4.54 | <0.01 | 51.3 | 1.66 | <0.01 | 18.8 | 1.62 | <0.01 | 24.6 | 1.12 | <0.01 | 3.04 | 0.31 | <0.01 | 17.7 | 4.62 | 0.02 |
| GIN | 105 | 4.54 | <0.01 | 55.0 | 1.66 | <0.01 | 17.2 | 1.62 | <0.01 | 23.3 | 1.12 | <0.01 | 3.03 | 0.31 | <0.01 | 18.6 | 4.62 | 0.02 |
| LEM + COR | 120 | 3.62 | 0.04 | 51.0 | 2.15 | 0.12 | 19.1 | 7.92 | 0.03 | 23.8 | 5.49 | 0.75 | 2.70 | 1.51 | 0.02 | 16.8 | 2.26 | 0.95 |
| LEM + GIN | 93 | 3.62 | 0.10 | 55.0 | 1.15 | 0.30 | 17.5 | 7.92 | 0.96 | 22.5 | 5.49 | 0.07 | 3.06 | 1.51 | 0.67 | 21.8 | 2.26 | 0.95 |
| COR + GIN | 97 | 3.62 | 0.14 | 54.1 | 2.15 | 0.78 | 17.0 | 7.92 | 0.91 | 23.0 | 5.49 | 0.20 | 2.83 | 1.51 | 0.87 | 22.3 | 2.26 | 0.89 |
| LEM + COR + GIN | 90 | 1.33 | 0.48 | 53.4 | 4.59 | 0.61 | 18.4 | 4.46 | 0.57 | 23.0 | 3.09 | 0.67 | 3.88 | 1.53 | 0.66 | 23.4 | 1.28 | 0.05 |
| Statistical value | ||||||||||||||||||
| Linear | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | ||||||||||||
| Quadratic | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | ||||||||||||
| RSD 3 | 1.21 | 2.35 | 2.29 | 1.58 | 0.44 | 6.54 | ||||||||||||
| R2 | 0.99 | 0.94 | 0.99 | 1.00 | 0.99 | 1.00 | ||||||||||||
| Adjusted R2 | 0.99 | 1.00 | 0.98 | 1.00 | 0.98 | 0.89 | ||||||||||||
| Predicted R2 | 0.97 | 1.00 | 0.97 | 0.99 | 0.96 | 0.80 | ||||||||||||
| PRESS | 0.07 | 0.07 | 0.03 | 1.23 | 0.03 | 0.09 | ||||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Temmar, R.; Rodríguez-Prado, M.; Forgeard, G.; Rougier, C.; Calsamiglia, S. Interactions among Natural Active Ingredients to Improve the Efficiency of Rumen Fermentation In Vitro. Animals 2021, 11, 1205. https://doi.org/10.3390/ani11051205
Temmar R, Rodríguez-Prado M, Forgeard G, Rougier C, Calsamiglia S. Interactions among Natural Active Ingredients to Improve the Efficiency of Rumen Fermentation In Vitro. Animals. 2021; 11(5):1205. https://doi.org/10.3390/ani11051205
Chicago/Turabian StyleTemmar, Rokia, María Rodríguez-Prado, Gwenael Forgeard, Cécile Rougier, and Sergio Calsamiglia. 2021. "Interactions among Natural Active Ingredients to Improve the Efficiency of Rumen Fermentation In Vitro" Animals 11, no. 5: 1205. https://doi.org/10.3390/ani11051205
APA StyleTemmar, R., Rodríguez-Prado, M., Forgeard, G., Rougier, C., & Calsamiglia, S. (2021). Interactions among Natural Active Ingredients to Improve the Efficiency of Rumen Fermentation In Vitro. Animals, 11(5), 1205. https://doi.org/10.3390/ani11051205






