Chronic Pain in Dogs and Cats: Is There Place for Dietary Intervention with Micro-Palmitoylethanolamide?
Abstract
Simple Summary
Abstract
1. Introduction
2. Pain Classification
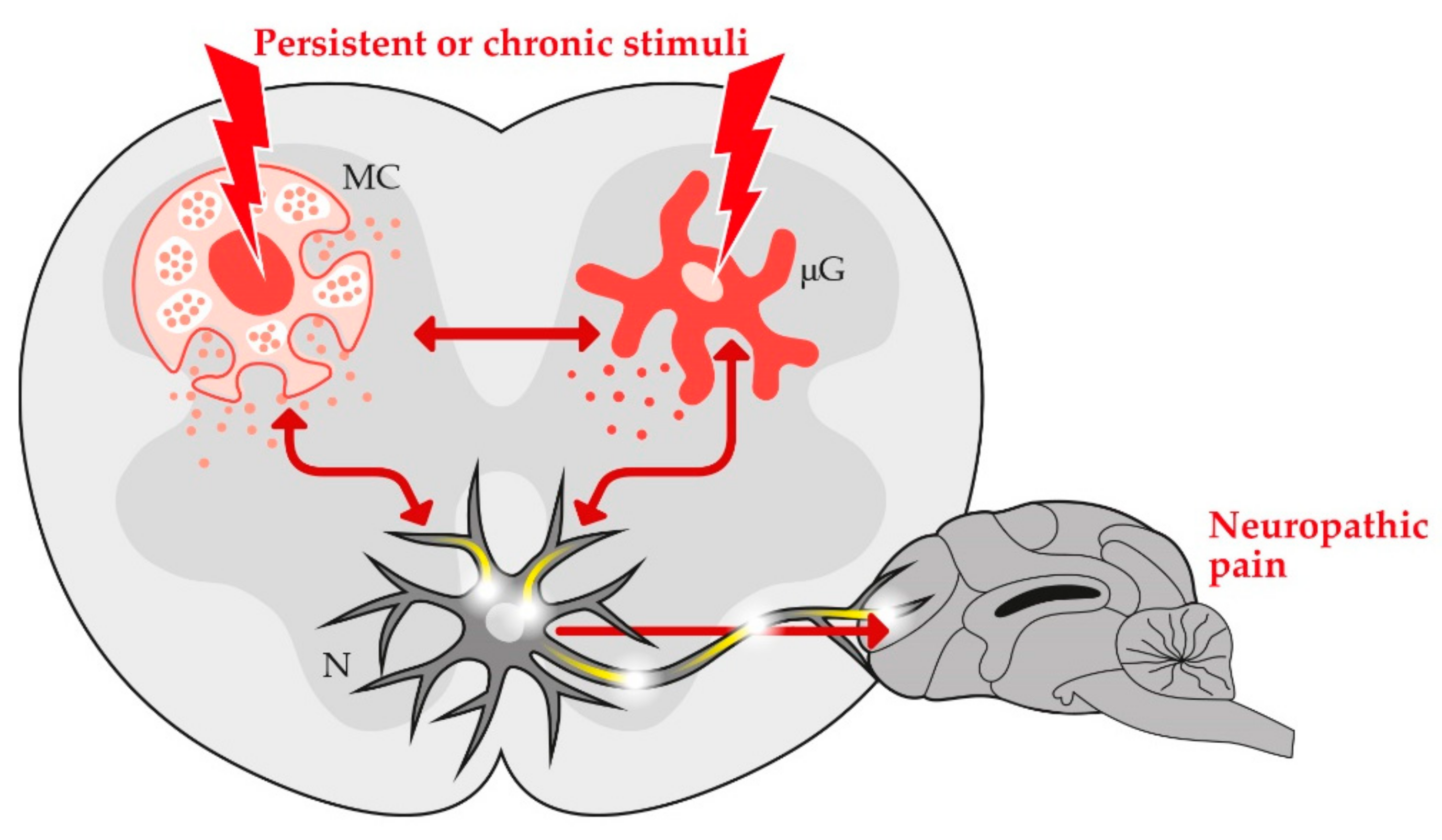
3. Role of Non-Neuronal Cells in the Development and Resolution of Chronic Pain
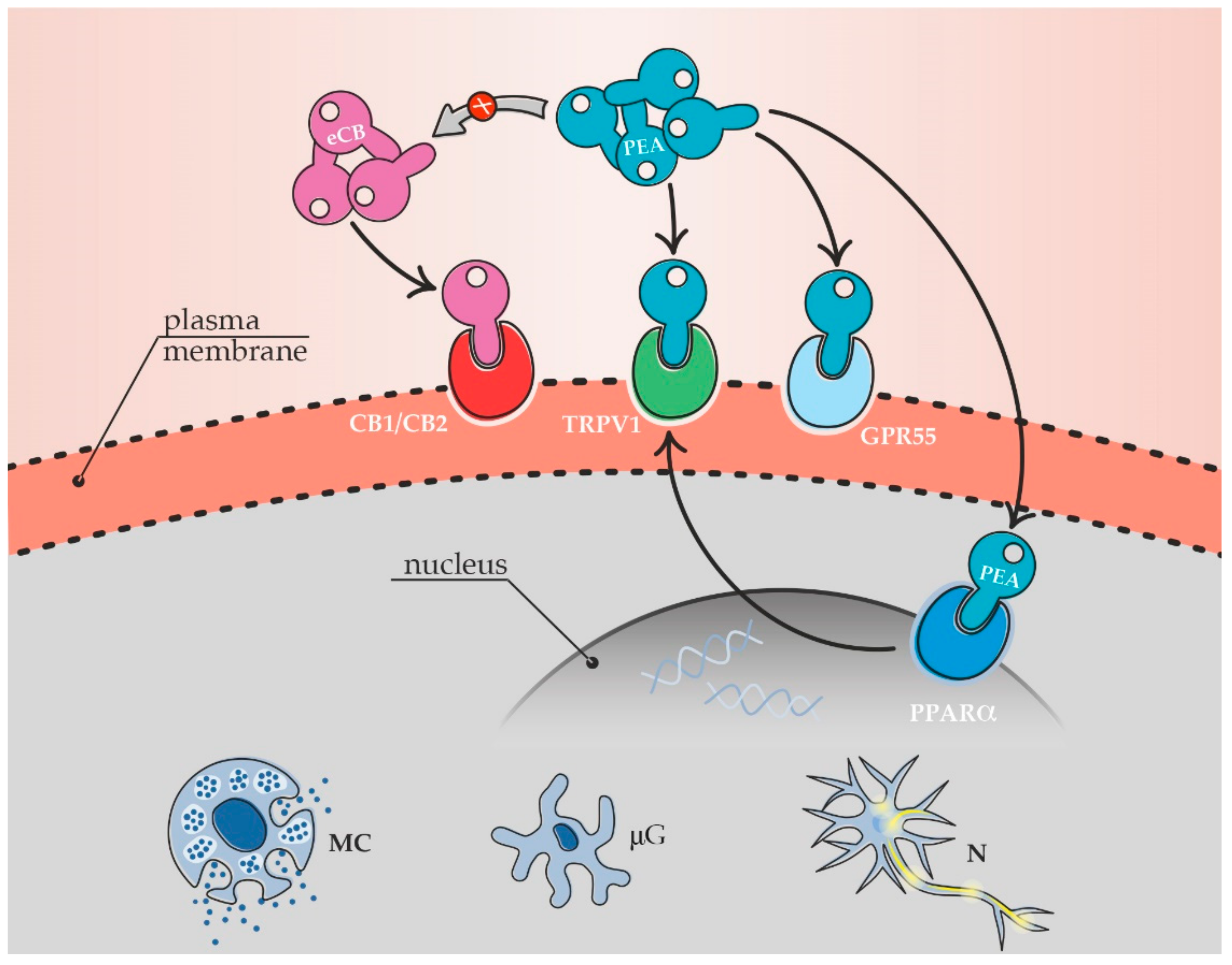
4. Endogenous PEA and Pain Modulation
5. Causes and Prevalence of Maladaptive Pain in Dogs and Cats
6. Management of Pain in Dogs and Cats
7. PEA and Formulation Challenges: A Size Issue
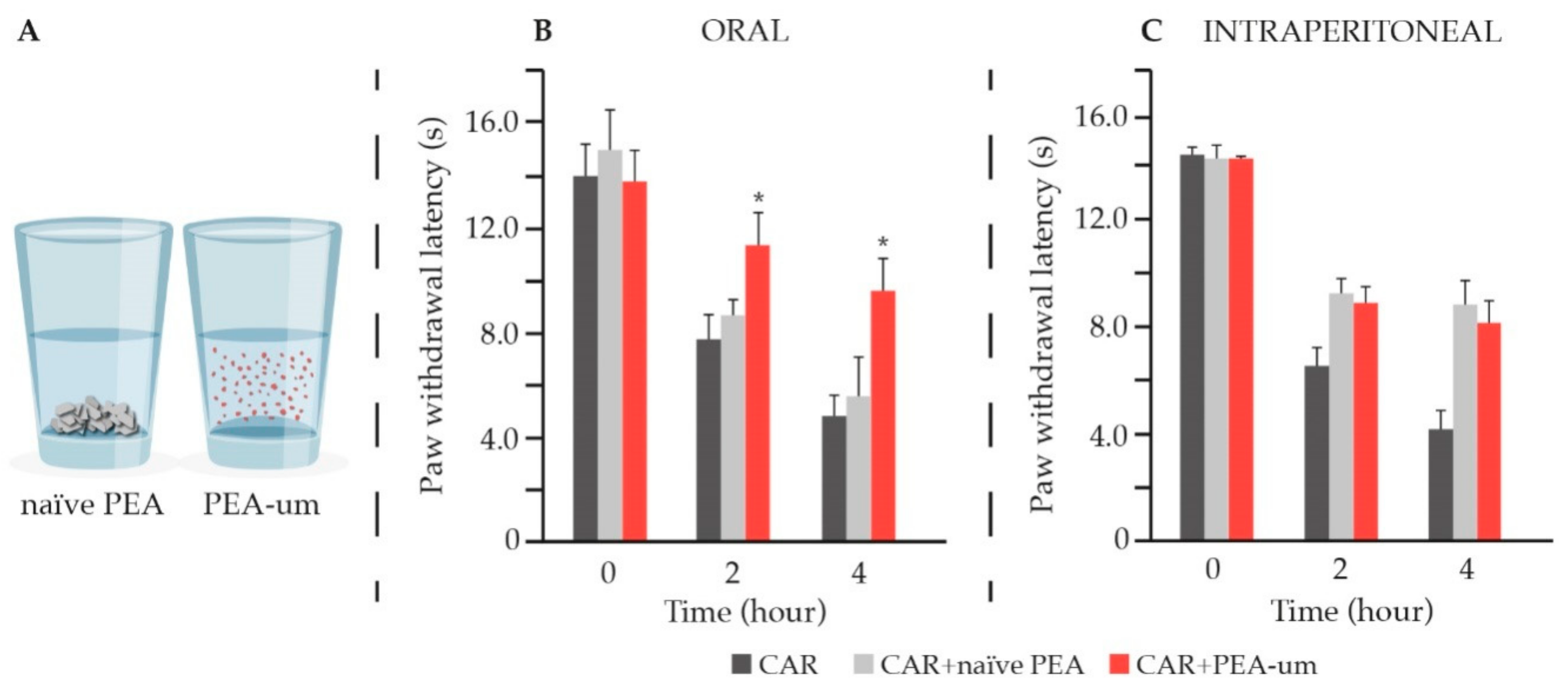
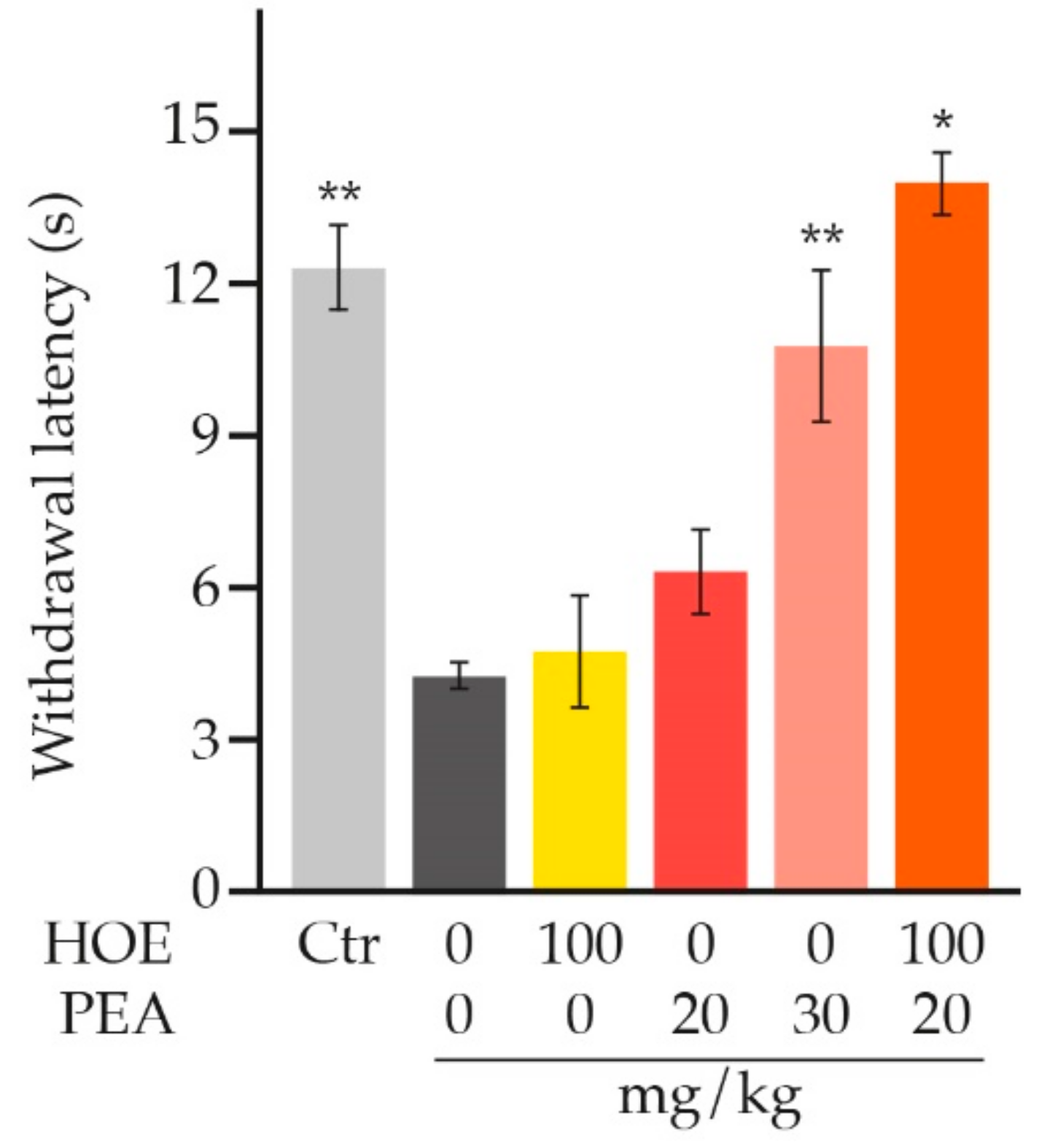
8. Preclinical Evidence for PEA in Pain Relief
| Animal Model | Main Behavioural Effect | Ref. |
|---|---|---|
| Somatic Inflammatory Pain | ||
| Carrageenan-induced hyperalgesia | Significant reduction of mechanical hyperalgesia | [179] |
| Formalin-induced persistent somatic pain | Significant inhibition of both early and late phases of formalin-evoked pain behaviour | [144] |
| Formalin-induced persistent somatic pain | Significant reduction of the second phase behavioural response (composite pain score) | [180] |
| Formalin-induced persistent somatic pain | Marked inhibition of pain behaviour | [174] |
| Carrageenan-induced hyperalgesia | Abolishment of hyperalgesic response | [181] |
| Intraplantar NGF-induced hyperalgesia | Significant reduction of hyperalgesia and neutrophil accumulation | [189] |
| Carrageenan-induced hyperalgesia | Marked time-dependent reduction of mechanical hyperalgesia | [183] |
| Carrageenan-induced hyperalgesia (s.c. sponge implant) | Significant reduction of new nerve formation and decrease of granuloma-associated hyperalgesia | [184] |
| Carrageenan-induced hyperalgesia | Significant increased mechanical and thermal thresholds (anti-hyperalgesic effect) | [202] |
| Formalin-induced nociception | Dose-dependent reduction of nocifensive behaviour in both early and late phases | [202] |
| Formalin-induced neuropathic-like behaviour | Significant and dose-dependent decrease of mechanical allodynia and thermal hyperalgesia | [185] |
| Oxaliplatin-induced neuropathic pain | Significant decrease of hyperalgesia and allodynia and improvement in motor coordination | [176] |
| Streptozotocin-induced diabetic neuropathy | Dose-dependent and significant relief of mechanical allodynia | [186] |
| Formalin-induced persistent somatic pain | Significant attenuation of the first and early second phases of nociceptive behaviour | [132] |
| Carrageenan-induced hyperalgesia | Significant reduction of thermal hyperalgesia by 57% (superior effect compared to meloxicam) | [187] |
| CFA-induced joint pain | Significant decrease of extravasation and mechanical allodynia | [175] |
| Formalin-evoked persistent somatic pain | Significant attenuation of mechanical allodynia and heat hyperalgesia (over 90%) | [201] |
| Visceral Inflammatory Pain | ||
| Turpentine inflammation of the urinary bladder | Significant attenuation of the vesical hyper-reflexic response | [180] |
| Acetic acid-evoked writhing | Dose-dependent attenuation of the writhing response | [174] |
| Turpentine inflammation of the urinary bladder | Dose-dependent attenuation of referred hyperalgesia | [188] |
| Kaolin-evoked writhing | Potent inhibition of the nocifensive response | [174] |
| Magnesium sulphate-evoked writhing | Dose-dependent inhibition of the nocifensive response | [174] |
| NGF-induced inflammation of the urinary bladder | Significant increase of micturition threshold | [182] |
| PPQ-induced persistent visceral pain | Dose dependent inhibition of visceral pain measured as stretching movement inhibition | [190] |
| Cyclophosphamide-induced cystitis | Significant decrease of the pain score | [191] |
| Animal Model | Main Behavioural Effect | Ref. |
|---|---|---|
| Neuropathic Pain | ||
| Partial sciatic nerve injury | Reduction of hyperalgesia (−79.4%) | [192] |
| Spinal cord injury | Significant reduction of the severity of spinal cord trauma | [193] |
| Chronic constriction injury | Significant relief of thermal hyperalgesia and mechanical allodynia | [194] |
| Chronic constriction injury | Significant and time-dependent relief of thermal hyperalgesia and mechanical allodynia (already after two administrations) | [120] |
| Partial sciatic nerve injury | Restored thermal and mechanical thresholds. Decrease of pain-induced memory deficits | [195] |
| Diabetic neuropathic pain | Significant antinociceptive effect. Significantly increased thresholds to mechanical stimuli | [196] |
| Sciatic nerve injury | Reduced nerve edema and inflammatory infiltrate (sub-optimal doses of PEA combined with acetaminophen) | [197] |
| Partial sciatic nerve injury | Restored cognitive behaviour and reduced cognitive decline associated with neuropathic pain | [198] |
| Chronic constriction injury | Strong dose-dependent suppression of mechanical allodynia and heat hyperalgesia upon single and repeated (7 consecutive days) administration | [201] |
| Chronic mixed pain | ||
| MIA-induced OA pain | Significant decrease of mechanical allodynia and improved locomotor function | [187] |
| MIA-induced OA pain | Significantly restored paw withdrawal threshold and weight-bearing compared to the vehicle-treated controls in a dose-dependent fashion | [199] |
| Vitamin D deficiency-induced chronic pain | Significant reduction of allodynia and neuronal sensitization | [200] |
9. Clinical Evidence for Micro-PEA Dietary Supplementation in Pain Relief
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raja, S.N.; Carr, D.B.; Cohen, M.; Finnerup, N.B.; Flor, H.; Gibson, S.; Keefe, F.J.; Mogil, J.S.; Ringkamp, M.; Sluka, K.A.; et al. The Revised International Association for the Study of Pain Definition of Pain: Concepts, Challenges, and Compromises. Pain 2020, 161, 1976–1982. [Google Scholar] [CrossRef]
- Wiseman-Orr, M.L.; Scott, E.M.; Reid, J.; Nolan, A.M. Validation of a Structured Questionnaire as an Instrument to Measure Chronic Pain in Dogs on the Basis of Effects on Health-Related Quality of Life. Am. J. Vet. Res. 2006, 67, 1826–1836. [Google Scholar] [CrossRef]
- Tomas, A.; Marcellin-Little, D.J.; Roe, S.C.; Motsinger-Reif, A.; Lascelles, B.D.X. Relationship between Mechanical Thresholds and Limb Use in Dogs with Coxofemoral Joint Oa-Associated Pain and the Modulating Effects of Pain Alleviation from Total Hip Replacement on Mechanical Thresholds. Vet. Surg. VS 2014, 43, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Steagall, P.V.; Monteiro, B.P.; Ruel, H.L.M.; Beauchamp, G.; Luca, G.; Berry, J.; Little, S.; Stiles, E.; Hamilton, S.; Pang, D. Perceptions and Opinions of Canadian Pet Owners about Anaesthesia, Pain and Surgery in Small Animals. J. Small Anim. Pract. 2017, 58, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Davis, K.N.; Hellyer, P.W.; Carr, E.C.J.; Wallace, J.E.; Kogan, L.R. Qualitative Study of Owner Perceptions of Chronic Pain in Their Dogs. J. Am. Vet. Med. Assoc. 2019, 254, 88–92. [Google Scholar] [CrossRef]
- Mills, D.S.; Demontigny-Bédard, I.; Gruen, M.; Klinck, M.P.; McPeake, K.J.; Barcelos, A.M.; Hewison, L.; Van Haevermaet, H.; Denenberg, S.; Hauser, H.; et al. Pain and Problem Behavior in Cats and Dogs. Amimals 2020, 10, 318. [Google Scholar] [CrossRef]
- Fox, S.M. Chronic Pain in Small Animal Medicine, 1st ed.; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Morton, C.M.; Reid, J.; Scott, E.M.; Holton, L.L.; Nolan, A.M. Application of a Scaling Model to Establish and Validate an Interval Level Pain Scale for Assessment of Acute Pain in Dogs. Am. J. Vet. Res. 2005, 66, 2154–2166. [Google Scholar] [CrossRef] [PubMed]
- Walton, M.B.; Cowderoy, E.; Lascelles, D.; Innes, J.F. Evaluation of Construct and Criterion Validity for the “Liverpool Osteoarthritis in Dogs” (LOAD) Clinical Metrology Instrument and Comparison to Two Other Instruments. PLoS ONE 2013, 8, e58125. [Google Scholar] [CrossRef]
- Freire, M.; Knazovicky, D.; Case, B.; Thomson, A.; Lascelles, B.D.X. Comparison of Thermal and Mechanical Quantitative Sensory Testing in Client-Owned Dogs with Chronic Naturally Occurring Pain and Normal Dogs. Vet. J. Lond. Engl. 1997 2016, 210, 95–97. [Google Scholar] [CrossRef]
- Harris, L.K.; Whay, H.R.; Murrell, J.C. An Investigation of Mechanical Nociceptive Thresholds in Dogs with Hind Limb Joint Pain Compared to Healthy Control Dogs. Vet. J. Lond. Engl. 1997 2018, 234, 85–90. [Google Scholar] [CrossRef]
- Hofmeister, E.H.; Barletta, M.; Shepard, M.; Brainard, B.M.; Trim, C.M.; Quandt, J. Agreement among Anesthesiologists Regarding Postoperative Pain Assessment in Dogs. Vet. Anaesth. Analg. 2018, 45, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Hunt, J.; Knazovicky, D.; Lascelles, B.D.X.; Murrell, J. Quantitative Sensory Testing in Dogs with Painful Disease: A Window to Pain Mechanisms? Vet. J. Lond. Engl. 1997 2019, 243, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Lascelles, B.D.X.; Brown, D.C.; Conzemius, M.; Gill, M.; Oshinsky, M.L.; Sharkey, M. Measurement of Chronic Pain in Companion Animals: Priorities for Future Research and Development Based on Discussions from the Pain in Animals Workshop (PAW) 2017. Vet. J. Lond. Engl. 1997 2019, 252, 105370. [Google Scholar] [CrossRef] [PubMed]
- Della Rocca, G.; Olivieri, E.; Di Salvo, A.; Malvisi, J.; Gogny, M. Attitude and Concern of Italian Veterinary Practitioners toward Management of Pain in Dogs and Cats. J. Vet. Pharmacol. Ther. 2009, 108, 154–163. [Google Scholar]
- Catanzaro, A.; Di Salvo, A.; Steagall, P.V.; Zampini, D.; Polisca, A.; Della Rocca, G. Preliminary Study on Attitudes, Opinions and Knowledge of Italian Veterinarians with Regard to Abdominal Visceral Pain in Dogs. Vet. Anaesth. Analg. 2016, 43, 361–370. [Google Scholar] [CrossRef]
- Bradbrook, C.; Clark, L. State of the Art Analgesia-Recent Developments Pharmacological Approaches to Acute Pain Management in Dogs and Cats: Part 2. Vet. J. Lond. Engl. 1997 2018, 236, 62–67. [Google Scholar] [CrossRef]
- Baker-Meuten, A.; Wendland, T.; Shamir, S.K.; Hess, A.M.; Duerr, F.M. Evaluation of Acupuncture for the Treatment of Pain Associated with Naturally-Occurring Osteoarthritis in Dogs: A Prospective, Randomized, Placebo-Controlled, Blinded Clinical Trial. BMC Vet. Res. 2020, 16, 357. [Google Scholar] [CrossRef]
- Brown, D.C.; Boston, R.C.; Farrar, J.T. Comparison of Force Plate Gait Analysis and Owner Assessment of Pain Using the Canine Brief Pain Inventory in Dogs with Osteoarthritis. J. Vet. Intern. Med. 2013, 27, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.C.; Boston, R.C.; Coyne, J.C.; Farrar, J.T. Ability of the Canine Brief Pain Inventory to Detect Response to Treatment in Dogs with Osteoarthritis. J. Am. Vet. Med. Assoc. 2008, 233, 1278–1283. [Google Scholar] [CrossRef]
- Hielm-Björkman, A.K.; Rita, H.; Tulamo, R.-M. Psychometric Testing of the Helsinki Chronic Pain Index by Completion of a Questionnaire in Finnish by Owners of Dogs with Chronic Signs of Pain Caused by Osteoarthritis. Am. J. Vet. Res. 2009, 70, 727–734. [Google Scholar] [CrossRef]
- Reid, J.; Nolan, A.M.; Scott, E.M. Measuring Pain in Dogs and Cats Using Structured Behavioural Observation. Vet. J. Lond. Engl. 1997 2018, 236, 72–79. [Google Scholar] [CrossRef]
- Belshaw, Z.; Yeates, J. Assessment of Quality of Life and Chronic Pain in Dogs. Vet. J. Lond. Engl. 1997 2018, 239, 59–64. [Google Scholar] [CrossRef]
- Hernandez-Avalos, I.; Mota-Rojas, D.; Mora-Medina, P.; Martínez-Burnes, J.; Casas Alvarado, A.; Verduzco-Mendoza, A.; Lezama-García, K.; Olmos-Hernandez, A. Review of Different Methods Used for Clinical Recognition and Assessment of Pain in Dogs and Cats. Int. J. Vet. Sci. Med. 2019, 7, 43–54. [Google Scholar] [CrossRef]
- Enomoto, M.; Lascelles, B.D.X.; Gruen, M.E. Development of a Checklist for the Detection of Degenerative Joint Disease-Associated Pain in Cats. J. Feline Med. Surg. 2020, 22, 1137–1147. [Google Scholar] [CrossRef]
- Evangelista, M.C.; Watanabe, R.; Leung, V.S.Y.; Monteiro, B.P.; O’Toole, E.; Pang, D.S.J.; Steagall, P.V. Facial Expressions of Pain in Cats: The Development and Validation of a Feline Grimace Scale. Sci. Rep. 2019, 9, 19128. [Google Scholar] [CrossRef]
- Epstein, M.; Rodan, I.; Griffenhagen, G.; Kadrlik, J.; Petty, M.; Robertson, S.; Simpson, W. 2015 AAHA/AAFP Pain Management Guidelines for Dogs and Cats. J. Am. Anim. Hosp. Assoc. 2015, 51, 67–84. [Google Scholar] [CrossRef]
- Mathews, K.; Kronen, P.W.; Lascelles, D.; Nolan, A.; Robertson, S.; Steagall, P.V.; Wright, B.; Yamashita, K. Guidelines for Recognition, Assessment and Treatment of Pain: WSAVA Global Pain Council Members and Co-Authors of This Document. J. Small Anim. Pract. 2014, 55, E10–E68. [Google Scholar] [CrossRef] [PubMed]
- Della Rocca, G.; Bufalari, A. Terapia del Dolore Negli Animali da Compagnia, 1st ed.; Poletto Editore Srl: Milano, Italy, 2016. [Google Scholar]
- Gamba, D. Il Dolore nel Cane. In Valutazione, Diagnosi e Trattamento, 1st ed.; Edra ev (Edizioni Veterinarie): Milano, Italy, 2020. [Google Scholar]
- Fox, S.M. Multimodal Management of Canine Osteoarthritis, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Fry, L.M.; Neary, S.M.; Sharrock, J.; Rychel, J.K. Acupuncture for Analgesia in Veterinary Medicine. Top. Companion Anim. Med. 2014, 29, 35–42. [Google Scholar] [CrossRef]
- Lane, D.M.; Hill, S.A. Effectiveness of Combined Acupuncture and Manual Therapy Relative to No Treatment for Canine Musculoskeletal Pain. Can. Vet. J. 2016, 57, 407–414. [Google Scholar] [PubMed]
- Gupta, R.; Srivastava, A.; Lall, R. Nutraceuticals in Veterinary Medicine; Springer Nature: Cham, Switzerland, 2019. [Google Scholar]
- Vandeweerd, J.-M.; Coisnon, C.; Clegg, P.; Cambier, C.; Pierson, A.; Hontoir, F.; Saegerman, C.; Gustin, P.; Buczinski, S. Systematic Review of Efficacy of Nutraceuticals to Alleviate Clinical Signs of Osteoarthritis. J. Vet. Intern. Med. 2012, 26, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Kilaru, A.; Blancaflor, E.B.; Venables, B.J.; Tripathy, S.; Mysore, K.S.; Chapman, K.D. The N-Acylethanolamine-Mediated Regulatory Pathway in Plants. Chem. Biodivers. 2007, 4, 1933–1955. [Google Scholar] [CrossRef]
- Venables, B.J.; Waggoner, C.A.; Chapman, K.D. N-Acylethanolamines in Seeds of Selected Legumes. Phytochemistry 2005, 66, 1913–1918. [Google Scholar] [CrossRef]
- Chapman, K.D. Occurrence, Metabolism, and Prospective Functions of N-Acylethanolamines in Plants. Prog. Lipid Res. 2004, 43, 302–327. [Google Scholar] [CrossRef] [PubMed]
- De Luca, L.; Ferracane, R.; Vitaglione, P. Food Database of N-Acyl-Phosphatidylethanolamines, N-Acylethanolamines and Endocannabinoids and Daily Intake from a Western, a Mediterranean and a Vegetarian Diet. Food Chem. 2019, 300, 125218. [Google Scholar] [CrossRef]
- Diep, T.A.; Madsen, A.N.; Holst, B.; Kristiansen, M.M.; Wellner, N.; Hansen, S.H.; Hansen, H.S. Dietary Fat Decreases Intestinal Levels of the Anorectic Lipids through a Fat Sensor. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2011, 25, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, S.; Pechereau, F.; Leblanc, N.; Boubertakh, B.; Houde, A.; Martin, C.; Flamand, N.; Silvestri, C.; Raymond, F.; Di Marzo, V.; et al. Rapid and Concomitant Gut Microbiota and Endocannabinoidome Response to Diet-Induced Obesity in Mice. mSystems 2019, 4. [Google Scholar] [CrossRef]
- Lin, L.; Rideout, T.; Yurkova, N.; Yang, H.; Eck, P.; Jones, P.J.H. Fatty Acid Ethanolamides Modulate CD36-MRNA through Dietary Fatty Acid Manipulation in Syrian Golden Hamsters. Appl. Physiol. Nutr. Metab. Physiol. Appl. Nutr. Metab. 2013, 38, 870–878. [Google Scholar] [CrossRef]
- Kleberg, K.; Hassing, H.A.; Hansen, H.S. Classical Endocannabinoid-like Compounds and Their Regulation by Nutrients. BioFactors Oxf. Engl. 2014, 40, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Carta, G.; Murru, E.; Vargiu, R.; Collu, M.; Carta, M.; Banni, S.; Stancampiano, R. Essential Fatty Acids Deficient Diet Modulates N-Acylethanolamide Profile in Rat’s Tissues. Prostaglandins Leukot. Essent. Fatty Acids 2020, 153, 102053. [Google Scholar] [CrossRef] [PubMed]
- Artmann, A.; Petersen, G.; Hellgren, L.I.; Boberg, J.; Skonberg, C.; Nellemann, C.; Hansen, S.H.; Hansen, H.S. Influence of Dietary Fatty Acids on Endocannabinoid and N-Acylethanolamine Levels in Rat Brain, Liver and Small Intestine. Biochim. Biophys. Acta 2008, 1781, 200–212. [Google Scholar] [CrossRef]
- Gugliandolo, E.; Peritore, A.F.; Piras, C.; Cuzzocrea, S.; Crupi, R. Palmitoylethanolamide and Related ALIAmides: Prohomeostatic Lipid Compounds for Animal Health and Wellbeing. Vet. Sci. 2020, 7, 78. [Google Scholar] [CrossRef]
- Epps, D.E.; Schmid, P.C.; Natarajan, V.; Schmid, H.H. N-Acylethanolamine Accumulation in Infarcted Myocardium. Biochem. Biophys. Res. Commun. 1979, 90, 628–633. [Google Scholar] [CrossRef]
- Natarajan, V.; Schmid, P.C.; Reddy, P.V.; Zuzarte-Augustin, M.L.; Schmid, H.H. Biosynthesis of N-Acylethanolamine Phospholipids by Dog Brain Preparations. J. Neurochem. 1983, 41, 1303–1312. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, V.; Schmid, P.C.; Reddy, P.V.; Schmid, H.H. Catabolism of N-Acylethanolamine Phospholipids by Dog Brain Preparations. J. Neurochem. 1984, 42, 1613–1619. [Google Scholar] [CrossRef] [PubMed]
- Re, G.; Barbero, R.; Miolo, A.; Di Marzo, V. Palmitoylethanolamide, Endocannabinoids and Related Cannabimimetic Compounds in Protection against Tissue Inflammation and Pain: Potential Use in Companion Animals. Vet. J. Lond. Engl. 1997 2007, 173, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Petrosino, S.; Schiano Moriello, A. Palmitoylethanolamide: A Nutritional Approach to Keep Neuroinflammation within Physiological Boundaries-A Systematic Review. Int. J. Mol. Sci. 2020, 21, 9526. [Google Scholar] [CrossRef] [PubMed]
- Petrosino, S.; Di Marzo, V. The Pharmacology of Palmitoylethanolamide and First Data on the Therapeutic Efficacy of Some of Its New Formulations. Br. J. Pharmacol. 2017, 174, 1349–1365. [Google Scholar] [CrossRef]
- Peritore, A.F.; Siracusa, R.; Crupi, R.; Cuzzocrea, S. Therapeutic Efficacy of Palmitoylethanolamide and Its New Formulations in Synergy with Different Antioxidant Molecules Present in Diets. Nutrients 2019, 11, 2175. [Google Scholar] [CrossRef]
- Woolf, C.J. American College of Physicians; American Physiological Society. Pain: Moving from Symptom Control toward Mechanism-Specific Pharmacologic Management. Ann. Intern. Med. 2004, 140, 441–451. [Google Scholar] [CrossRef]
- Monteiro, B.P.; Otis, C.; Del Castillo, J.R.E.; Nitulescu, R.; Brown, K.; Arendt-Nielsen, L.; Troncy, E. Quantitative Sensory Testing in Feline Osteoarthritic Pain—A Systematic Review and Meta-Analysis. Osteoarthr. Cartil. 2020, 28, 885–896. [Google Scholar] [CrossRef]
- Orlandini, G. Orlandini, La Semeiotica Del Dolore, 2nd ed.; Delfino, A., Ed.; Medicina Scienze: Bologna, Italy, 2016. [Google Scholar]
- Elieh Ali Komi, D.; Wöhrl, S.; Bielory, L. Mast Cell Biology at Molecular Level: A Comprehensive Review. Clin. Rev. Allergy Immunol. 2020, 58, 342–365. [Google Scholar] [CrossRef]
- Galli, S.J.; Gaudenzio, N.; Tsai, M. Mast Cells in Inflammation and Disease: Recent Progress and Ongoing Concerns. Annu. Rev. Immunol. 2020, 38, 49–77. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zhang, Y.-Q.; Qadri, Y.J.; Serhan, C.N.; Ji, R.-R. Microglia in Pain: Detrimental and Protective Roles in Pathogenesis and Resolution of Pain. Neuron 2018, 100, 1292–1311. [Google Scholar] [CrossRef]
- Gosselin, R.-D.; Suter, M.R.; Ji, R.-R.; Decosterd, I. Glial Cells and Chronic Pain. Neurosci. Rev. J. Bringing Neurobiol. Neurol. Psychiatry 2010, 16, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Bienenstock, J.; MacQueen, G.; Sestini, P.; Marshall, J.S.; Stead, R.H.; Perdue, M.H. Mast Cell/Nerve Interactions in Vitro and in Vivo. Am. Rev. Respir. Dis. 1991, 143, S55–S58. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Perkins, N.M.; Tracey, D.J.; Geczy, C.L. Inflammation and Hyperalgesia Induced by Nerve Injury in the Rat: A Key Role of Mast Cells. Pain 2003, 105, 467–479. [Google Scholar] [CrossRef]
- Xu, H.; Shi, X.; Li, X.; Zou, J.; Zhou, C.; Liu, W.; Shao, H.; Chen, H.; Shi, L. Neurotransmitter and Neuropeptide Regulation of Mast Cell Function: A Systematic Review. J. Neuroinflamm. 2020, 17, 356. [Google Scholar] [CrossRef]
- Skaper, S.D.; Facci, L.; Zusso, M.; Giusti, P. An Inflammation-Centric View of Neurological Disease: Beyond the Neuron. Front. Cell. Neurosci. 2018, 12, 72. [Google Scholar] [CrossRef]
- Marinelli, S.; Basilico, B.; Marrone, M.C.; Ragozzino, D. Microglia-Neuron Crosstalk: Signaling Mechanism and Control of Synaptic Transmission. Semin. Cell Dev. Biol. 2019, 94, 138–151. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, I.; Inoue, Y.; Shimada, T.; Aikawa, T. Brain Mast Cells Act as an Immune Gate to the Hypothalamic-Pituitary-Adrenal Axis in Dogs. J. Exp. Med. 2001, 194, 71–78. [Google Scholar] [CrossRef]
- Yaksh, T.L.; Allen, J.W.; Veesart, S.L.; Horais, K.A.; Malkmus, S.A.; Scadeng, M.; Steinauer, J.J.; Rossi, S.S. Role of Meningeal Mast Cells in Intrathecal Morphine-Evoked Granuloma Formation. Anesthesiology 2013, 118, 664–678. [Google Scholar] [CrossRef]
- Sandhu, J.K.; Kulka, M. Decoding Mast Cell-Microglia Communication in Neurodegenerative Diseases. Int. J. Mol. Sci. 2021, 22, 1093. [Google Scholar] [CrossRef] [PubMed]
- Green, D.P.; Limjunyawong, N.; Gour, N.; Pundir, P.; Dong, X. A Mast-Cell-Specific Receptor Mediates Neurogenic Inflammation and Pain. Neuron 2019, 101, 412–420.e3. [Google Scholar] [CrossRef] [PubMed]
- Kalesnikoff, J.; Galli, S.J. New Developments in Mast Cell Biology. Nat. Immunol. 2008, 9, 1215–1223. [Google Scholar] [CrossRef] [PubMed]
- Silver, R.; Curley, J.P. Mast Cells on the Mind: New Insights and Opportunities. Trends Neurosci. 2013, 36, 513–521. [Google Scholar] [CrossRef]
- Pastwińska, J.; Żelechowska, P.; Walczak-Drzewiecka, A.; Brzezińska-Błaszczyk, E.; Dastych, J. The Art of Mast Cell Adhesion. Cells 2020, 9, 2664. [Google Scholar] [CrossRef]
- Coraggio, V.; Guida, F.; Boccella, S.; Scafuro, M.; Paino, S.; Romano, D.; Maione, S.; Luongo, L. Neuroimmune-Driven Neuropathic Pain Establishment: A Focus on Gender Differences. Int. J. Mol. Sci. 2018, 19, 281. [Google Scholar] [CrossRef]
- Zhao, H.; Alam, A.; Chen, Q.; Eusman, M.A.; Pal, A.; Eguchi, S.; Wu, L.; Ma, D. The Role of Microglia in the Pathobiology of Neuropathic Pain Development: What Do We Know? Br. J. Anaesth. 2017, 118, 504–516. [Google Scholar] [CrossRef] [PubMed]
- Héron, A.; Dubayle, D. A Focus on Mast Cells and Pain. J. Neuroimmunol. 2013, 264, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Iuvone, T.; Affaitati, G.; De Filippis, D.; Lopopolo, M.; Grassia, G.; Lapenna, D.; Negro, L.; Costantini, R.; Vaia, M.; Cipollone, F.; et al. Ultramicronized Palmitoylethanolamide Reduces Viscerovisceral Hyperalgesia in a Rat Model of Endometriosis plus Ureteral Calculosis: Role of Mast Cells. Pain 2016, 157, 80–91. [Google Scholar] [CrossRef]
- Giancola, F.; Volta, U.; Repossi, R.; Latorre, R.; Beeckmans, D.; Carbone, F.; Van den Houte, K.; Bianco, F.; Bonora, E.; Gori, A.; et al. Mast Cell-Nerve Interactions Correlate with Bloating and Abdominal Pain Severity in Patients with Non-Celiac Gluten/Wheat Sensitivity. Neurogastroenterol. Motil. Off. J. Eur. Gastrointest. Motil. Soc. 2020, 32, e13814. [Google Scholar] [CrossRef] [PubMed]
- Frossi, B.; De Carli, M.; Calabrò, A. Coeliac Disease and Mast Cells. Int. J. Mol. Sci. 2019, 20, 3400. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.J.; Malfait, A.-M.; Miller, R.E. The Innate Immune Response as a Mediator of Osteoarthritis Pain. Osteoarthr. Cartil. 2020, 28, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Richards, J.; Tang, S.; Gunsch, G.; Sul, P.; Wiet, M.; Flanigan, D.C.; Khan, S.N.; Moore, S.; Walter, B.; Purmessur, D. Mast Cell/Proteinase Activated Receptor 2 (PAR2) Mediated Interactions in the Pathogenesis of Discogenic Back Pain. Front. Cell. Neurosci. 2019, 13, 294. [Google Scholar] [CrossRef] [PubMed]
- Kempuraj, D.; Mentor, S.; Thangavel, R.; Ahmed, M.E.; Selvakumar, G.P.; Raikwar, S.P.; Dubova, I.; Zaheer, S.; Iyer, S.S.; Zaheer, A. Mast Cells in Stress, Pain, Blood-Brain Barrier, Neuroinflammation and Alzheimer’s Disease. Front. Cell. Neurosci. 2019, 13, 54. [Google Scholar] [CrossRef] [PubMed]
- Forsythe, P. Mast Cells in Neuroimmune Interactions. Trends Neurosci. 2019, 42, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Song, J.; Bai, T.; Wang, R.; Hou, X. Sustained Pain Hypersensitivity in the Stressed Colon: Role of Mast Cell-Derived Nerve Growth Factor-Mediated Enteric Synaptic Plasticity. Neurogastroenterol. Motil. Off. J. Eur. Gastrointest. Motil. Soc. 2018, 30, e13430. [Google Scholar] [CrossRef]
- Gupta, K.; Harvima, I.T. Mast Cell-Neural Interactions Contribute to Pain and Itch. Immunol. Rev. 2018, 282, 168–187. [Google Scholar] [CrossRef]
- Ioan-Facsinay, A. Initiating Pain in Osteoarthritis (OA): Is It the Mast Cell? Osteoarthr. Cartil. 2018, 26, 1–3. [Google Scholar] [CrossRef]
- Wiet, M.G.; Piscioneri, A.; Khan, S.N.; Ballinger, M.N.; Hoyland, J.A.; Purmessur, D. Mast Cell-Intervertebral Disc Cell Interactions Regulate Inflammation, Catabolism and Angiogenesis in Discogenic Back Pain. Sci. Rep. 2017, 7, 12492. [Google Scholar] [CrossRef]
- Kaur, G.; Singh, N.; Jaggi, A.S. Mast Cells in Neuropathic Pain: An Increasing Spectrum of Their Involvement in Pathophysiology. Rev. Neurosci. 2017, 28, 759–766. [Google Scholar] [CrossRef]
- Fusco, M.; Skaper, S.D.; Coaccioli, S.; Varrassi, G.; Paladini, A. Degenerative Joint Diseases and Neuroinflammation. Pain Pract. Off. J. World Inst. Pain 2017, 17, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.A. Managing Neuropathic Pain in Dogs. Front. Vet. Sci. 2016, 3, 12. [Google Scholar] [CrossRef]
- Cao, L.; Palmer, C.D.; Malon, J.T.; De Leo, J.A. Critical Role of Microglial CD40 in the Maintenance of Mechanical Hypersensitivity in a Murine Model of Neuropathic Pain. Eur. J. Immunol. 2009, 39, 3562–3569. [Google Scholar] [CrossRef] [PubMed]
- Costigan, M.; Scholz, J.; Woolf, C.J. Neuropathic Pain: A Maladaptive Response of the Nervous System to Damage. Annu. Rev. Neurosci. 2009, 32, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Spitzbarth, I.; Bock, P.; Haist, V.; Stein, V.M.; Tipold, A.; Wewetzer, K.; Baumgärtner, W.; Beineke, A. Prominent Microglial Activation in the Early Proinflammatory Immune Response in Naturally Occurring Canine Spinal Cord Injury. J. Neuropathol. Exp. Neurol. 2011, 70, 703–714. [Google Scholar] [CrossRef]
- Yamasaki, R.; Fujii, T.; Wang, B.; Masaki, K.; Kido, M.A.; Yoshida, M.; Matsushita, T.; Kira, J.-I. Allergic Inflammation Leads to Neuropathic Pain via Glial Cell Activation. J. Neurosci. Off. J. Soc. Neurosci. 2016, 36, 11929–11945. [Google Scholar] [CrossRef]
- Fields, R.D. New Culprits in Chronic Pain. Sci. Am. 2009, 301, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Skaper, S.D.; Facci, L.; Giusti, P. Mast Cells, Glia and Neuroinflammation: Partners in Crime? Immunology 2014, 141, 314–327. [Google Scholar] [CrossRef]
- Marchand, F.; Perretti, M.; McMahon, S.B. Role of the Immune System in Chronic Pain. Nat. Rev. Neurosci. 2005, 6, 521–532. [Google Scholar] [CrossRef]
- Ferreira, R.; Bernardino, L. Dual Role of Microglia in Health and Disease: Pushing the Balance toward Repair. Front. Cell. Neurosci. 2015, 9, 51. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yuan, H.; Zhu, X.; Zhou, S.; Chen, Q.; Zhu, X.; Ma, X.; He, X.; Tian, M.; Shi, X. Role of Mast Cell Activation in Inducing Microglial Cells to Release Neurotrophin. J. Neurosci. Res. 2010, 88, 1348–1354. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Tsilioni, I.; Bawazeer, M. Mast Cells, Neuroinflammation and Pain in Fibromyalgia Syndrome. Front. Cell. Neurosci. 2019, 13, 353. [Google Scholar] [CrossRef]
- Ellis, A.; Bennett, D.L.H. Neuroinflammation and the Generation of Neuropathic Pain. Br. J. Anaesth. 2013, 111, 26–37. [Google Scholar] [CrossRef]
- Ji, R.-R.; Xu, Z.-Z.; Gao, Y.-J. Emerging Targets in Neuroinflammation-Driven Chronic Pain. Nat. Rev. Drug Discov. 2014, 13, 533–548. [Google Scholar] [CrossRef]
- Matsuda, M.; Huh, Y.; Ji, R.-R. Roles of Inflammation, Neurogenic Inflammation, and Neuroinflammation in Pain. J. Anesth. 2019, 33, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Chiurchiù, V.; Leuti, A.; Maccarrone, M. Bioactive Lipids and Chronic Inflammation: Managing the Fire Within. Front. Immunol. 2018, 9, 38. [Google Scholar] [CrossRef]
- Ren, K.; Dubner, R. Interactions between the Immune and Nervous Systems in Pain. Nat. Med. 2010, 16, 1267–1276. [Google Scholar] [CrossRef] [PubMed]
- Skaper, S.D.; Facci, L.; Barbierato, M.; Zusso, M.; Bruschetta, G.; Impellizzeri, D.; Cuzzocrea, S.; Giusti, P. N-Palmitoylethanolamine and Neuroinflammation: A Novel Therapeutic Strategy of Resolution. Mol. Neurobiol. 2015, 52, 1034–1042. [Google Scholar] [CrossRef]
- Lerner, R.; Pascual Cuadrado, D.; Post, J.M.; Lutz, B.; Bindila, L. Broad Lipidomic and Transcriptional Changes of Prophylactic PEA Administration in Adult Mice. Front. Neurosci. 2019, 13, 527. [Google Scholar] [CrossRef]
- Paladini, A.; Fusco, M.; Cenacchi, T.; Schievano, C.; Piroli, A.; Varrassi, G. Palmitoylethanolamide, a Special Food for Medical Purposes, in the Treatment of Chronic Pain: A Pooled Data Meta-Analysis. Pain Physician 2016, 19, 11–24. [Google Scholar]
- Skaper, S.D.; Facci, L.; Fusco, M.; Della Valle, M.F.; Zusso, M.; Costa, B.; Giusti, P. Palmitoylethanolamide, a Naturally Occurring Disease-Modifying Agent in Neuropathic Pain. Inflammopharmacology 2014, 22, 79–94. [Google Scholar] [CrossRef]
- Piomelli, D.; Scalvini, L.; Fotio, Y.; Lodola, A.; Spadoni, G.; Tarzia, G.; Mor, M. N-Acylethanolamine Acid Amidase (NAAA): Structure, Function, and Inhibition. J. Med. Chem. 2020, 63, 7475–7490. [Google Scholar] [CrossRef]
- Piomelli, D. A Thickening Network of Lipids. Pain 2012, 153, 3–4. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Piomelli, D.; Hohmann, A.G.; Seybold, V.; Hammock, B.D. A Lipid Gate for the Peripheral Control of Pain. J. Neurosci. Off. J. Soc. Neurosci. 2014, 34, 15184–15191. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, R.; Impellizzeri, D.; Cuzzocrea, S.; Di Paola, R. ALIAmides Update: Palmitoylethanolamide and Its Formulations on Management of Peripheral Neuropathic Pain. Int. J. Mol. Sci. 2020, 21, 5330. [Google Scholar] [CrossRef] [PubMed]
- Piscitelli, F. Chapter 9—Endocannabinoidomics: “Omics” Approaches Applied to Endocannabinoids and Endocannabinoid-Like Mediators. In The Endocannabinoidome; Di Marzo, V., Wang, J., Eds.; Academic Press: Boston, MA, USA, 2015; pp. 137–152. [Google Scholar] [CrossRef]
- Petrosino, S.; Marzo, V.D. Anandamide and Other Acylethanolamides. In Handbook of Neurochemistry and Molecular Neurobiology: Neural Lipids; Lajtha, A., Tettamanti, G., Goracci, G., Eds.; Springer US: Boston, MA, USA, 2009; pp. 75–98. [Google Scholar] [CrossRef]
- Kallendrusch, S.; Hobusch, C.; Ehrlich, A.; Ziebell, S.; Ueda, N.; Geisslinger, G.; Koch, M.; Dehghani, F. Site-Specific and Time-Dependent Activation of the Endocannabinoid System after Transection of Long-Range Projections. PLoS ONE 2012, 7, e33537. [Google Scholar] [CrossRef]
- Aloe, L.; Leon, A.; Levi-Montalcini, R. A Proposed Autacoid Mechanism Controlling Mastocyte Behaviour. Agents Actions 1993, 39, C145–C147. [Google Scholar] [CrossRef] [PubMed]
- Levi-Montalcini, R.; Skaper, S.D.; Dal Toso, R.; Petrelli, L.; Leon, A. Nerve Growth Factor: From Neurotrophin to Neurokine. Trends Neurosci. 1996, 19, 514–520. [Google Scholar] [CrossRef]
- Bisogno, T.; Maurelli, S.; Melck, D.; De Petrocellis, L.; Di Marzo, V. Biosynthesis, Uptake, and Degradation of Anandamide and Palmitoylethanolamide in Leukocytes. J. Biol. Chem. 1997, 272, 3315–3323. [Google Scholar] [CrossRef]
- Muccioli, G.G.; Stella, N. Microglia Produce and Hydrolyze Palmitoylethanolamide. Neuropharmacology 2008, 54, 16–22. [Google Scholar] [CrossRef]
- Bettoni, I.; Comelli, F.; Colombo, A.; Bonfanti, P.; Costa, B. Non-Neuronal Cell Modulation Relieves Neuropathic Pain: Efficacy of the Endogenous Lipid Palmitoylethanolamide. CNS Neurol. Disord. Drug Targets 2013, 12, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Skaper, S.D.; Facci, L.; Giusti, P. Glia and Mast Cells as Targets for Palmitoylethanolamide, an Anti-Inflammatory and Neuroprotective Lipid Mediator. Mol. Neurobiol. 2013, 48, 340–352. [Google Scholar] [CrossRef]
- Sayd, A.; Antón, M.; Alén, F.; Caso, J.R.; Pavón, J.; Leza, J.C.; Rodríguez de Fonseca, F.; García-Bueno, B.; Orio, L. Systemic Administration of Oleoylethanolamide Protects from Neuroinflammation and Anhedonia Induced by LPS in Rats. Int. J. Neuropsychopharmacol. 2014, 18. [Google Scholar] [CrossRef] [PubMed]
- Skaper, S.D.; Buriani, A.; Dal Toso, R.; Petrelli, L.; Romanello, S.; Facci, L.; Leon, A. The ALIAmide Palmitoylethanolamide and Cannabinoids, but Not Anandamide, Are Protective in a Delayed Postglutamate Paradigm of Excitotoxic Death in Cerebellar Granule Neurons. Proc. Natl. Acad. Sci. USA 1996, 93, 3984–3989. [Google Scholar] [CrossRef] [PubMed]
- Portavella, M.; Rodriguez-Espinosa, N.; Galeano, P.; Blanco, E.; Romero, J.I.; Holubiec, M.I.; Rodriguez de Fonseca, F.; Fernández-Espejo, E. Oleoylethanolamide and Palmitoylethanolamide Protect Cultured Cortical Neurons Against Hypoxia. Cannabis Cannabinoid Res. 2018, 3, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Musella, A.; Fresegna, D.; Rizzo, F.R.; Gentile, A.; Bullitta, S.; De Vito, F.; Guadalupi, L.; Centonze, D.; Mandolesi, G. A Novel Crosstalk within the Endocannabinoid System Controls GABA Transmission in the Striatum. Sci. Rep. 2017, 7, 7363. [Google Scholar] [CrossRef]
- Ambrosino, P.; Soldovieri, M.V.; Russo, C.; Taglialatela, M. Activation and Desensitization of TRPV1 Channels in Sensory Neurons by the PPARα Agonist Palmitoylethanolamide. Br. J. Pharmacol. 2013, 168, 1430–1444. [Google Scholar] [CrossRef] [PubMed]
- Khasabova, I.A.; Xiong, Y.; Coicou, L.G.; Piomelli, D.; Seybold, V. Peroxisome Proliferator-Activated Receptor α Mediates Acute Effects of Palmitoylethanolamide on Sensory Neurons. J. Neurosci. Off. J. Soc. Neurosci. 2012, 32, 12735–12743. [Google Scholar] [CrossRef]
- Koch, M.; Kreutz, S.; Böttger, C.; Benz, A.; Maronde, E.; Ghadban, C.; Korf, H.-W.; Dehghani, F. Palmitoylethanolamide Protects Dentate Gyrus Granule Cells via Peroxisome Proliferator-Activated Receptor-α. Neurotox. Res. 2011, 19, 330–340. [Google Scholar] [CrossRef]
- LoVerme, J.; Russo, R.; La Rana, G.; Fu, J.; Farthing, J.; Mattace-Raso, G.; Meli, R.; Hohmann, A.; Calignano, A.; Piomelli, D. Rapid Broad-Spectrum Analgesia through Activation of Peroxisome Proliferator-Activated Receptor-Alpha. J. Pharmacol. Exp. Ther. 2006, 319, 1051–1061. [Google Scholar] [CrossRef]
- Im, D.-S. GPR119 and GPR55 as Receptors for Fatty Acid Ethanolamides, Oleoylethanolamide and Palmitoylethanolamide. Int. J. Mol. Sci. 2021, 22, 1034. [Google Scholar] [CrossRef]
- Herrera, M.I.; Kölliker-Frers, R.; Barreto, G.; Blanco, E.; Capani, F. Glial Modulation by N-Acylethanolamides in Brain Injury and Neurodegeneration. Front. Aging Neurosci. 2016, 8. [Google Scholar] [CrossRef]
- Okine, B.N.; Madasu, M.K.; McGowan, F.; Prendergast, C.; Gaspar, J.C.; Harhen, B.; Roche, M.; Finn, D.P. N-Palmitoylethanolamide in the Anterior Cingulate Cortex Attenuates Inflammatory Pain Behaviour Indirectly via a CB1 Receptor-Mediated Mechanism. Pain 2016, 157, 2687–2696. [Google Scholar] [CrossRef]
- Campora, L.; Miragliotta, V.; Ricci, E.; Cristino, L.; Di Marzo, V.; Albanese, F.; Federica Della Valle, M.; Abramo, F. Cannabinoid Receptor Type 1 and 2 Expression in the Skin of Healthy Dogs and Dogs with Atopic Dermatitis. Am. J. Vet. Res. 2012, 73, 988–995. [Google Scholar] [CrossRef]
- Miragliotta, V.; Ricci, P.L.; Albanese, F.; Pirone, A.; Tognotti, D.; Abramo, F. Cannabinoid Receptor Types 1 and 2 and Peroxisome Proliferator-Activated Receptor-α: Distribution in the Skin of Clinically Healthy Cats and Cats with Hypersensitivity Dermatitis. Vet. Dermatol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Mercati, F.; Dall’Aglio, C.; Pascucci, L.; Boiti, C.; Ceccarelli, P. Identification of Cannabinoid Type 1 Receptor in Dog Hair Follicles. Acta Histochem. 2012, 114, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Polidoro, G.; Galiazzo, G.; Giancola, F.; Papadimitriou, S.; Kouki, M.; Sabattini, S.; Rigillo, A.; Chiocchetti, R. Expression of Cannabinoid and Cannabinoid-Related Receptors in the Oral Mucosa of Healthy Cats and Cats with Chronic Gingivostomatitis. J. Feline Med. Surg. 2020. [Google Scholar] [CrossRef] [PubMed]
- Stanzani, A.; Galiazzo, G.; Giancola, F.; Tagliavia, C.; De Silva, M.; Pietra, M.; Fracassi, F.; Chiocchetti, R. Localization of Cannabinoid and Cannabinoid Related Receptors in the Cat Gastrointestinal Tract. Histochem. Cell Biol. 2020, 153, 339–356. [Google Scholar] [CrossRef]
- Rossi, G.; Gioacchini, G.; Pengo, G.; Suchodolski, J.S.; Jergens, A.E.; Allenspach, K.; Gavazza, A.; Scarpona, S.; Berardi, S.; Galosi, L.; et al. Enterocolic Increase of Cannabinoid Receptor Type 1 and Type 2 and Clinical Improvement after Probiotic Administration in Dogs with Chronic Signs of Colonic Dysmotility without Mucosal Inflammatory Changes. Neurogastroenterol. Motil. Off. J. Eur. Gastrointest. Motil. Soc. 2020, 32, e13717. [Google Scholar] [CrossRef]
- Pirone, A.; Cantile, C.; Miragliotta, V.; Lenzi, C.; Giannessi, E.; Cozzi, B. Immunohistochemical Distribution of the Cannabinoid Receptor 1 and Fatty Acid Amide Hydrolase in the Dog Claustrum. J. Chem. Neuroanat. 2016, 74, 21–27. [Google Scholar] [CrossRef]
- Freundt-Revilla, J.; Heinrich, F.; Zoerner, A.; Gesell, F.; Beyerbach, M.; Shamir, M.; Oevermann, A.; Baumgärtner, W.; Tipold, A. The Endocannabinoid System in Canine Steroid-Responsive Meningitis-Arteritis and Intraspinal Spirocercosis. PLoS ONE 2018, 13, e0187197. [Google Scholar] [CrossRef]
- Freundt-Revilla, J.; Kegler, K.; Baumgärtner, W.; Tipold, A. Spatial Distribution of Cannabinoid Receptor Type 1 (CB1) in Normal Canine Central and Peripheral Nervous System. PLoS ONE 2017, 12, e0181064. [Google Scholar] [CrossRef]
- Fernández-Trapero, M.; Espejo-Porras, F.; Rodríguez-Cueto, C.; Coates, J.R.; Pérez-Díaz, C.; de Lago, E.; Fernández-Ruiz, J. Upregulation of CB2 Receptors in Reactive Astrocytes in Canine Degenerative Myelopathy, a Disease Model of Amyotrophic Lateral Sclerosis. Dis. Model. Mech. 2017, 10, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Chiocchetti, R.; Galiazzo, G.; Tagliavia, C.; Stanzani, A.; Giancola, F.; Menchetti, M.; Militerno, G.; Bernardini, C.; Forni, M.; Mandrioli, L. Cellular Distribution of Canonical and Putative Cannabinoid Receptors in Canine Cervical Dorsal Root Ganglia. Front. Vet. Sci. 2019, 6, 313. [Google Scholar] [CrossRef]
- Calignano, A.; La Rana, G.; Giuffrida, A.; Piomelli, D. Control of Pain Initiation by Endogenous Cannabinoids. Nature 1998, 394, 277–281. [Google Scholar] [CrossRef]
- Piomelli, D.; Sasso, O. Peripheral Gating of Pain Signals by Endogenous Lipid Mediators. Nat. Neurosci. 2014, 17, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Habib, A.M.; Okorokov, A.L.; Hill, M.N.; Bras, J.T.; Lee, M.-C.; Li, S.; Gossage, S.J.; van Drimmelen, M.; Morena, M.; Houlden, H.; et al. Microdeletion in a FAAH Pseudogene Identified in a Patient with High Anandamide Concentrations and Pain Insensitivity. Br. J. Anaesth. 2019, 123, e249–e253. [Google Scholar] [CrossRef] [PubMed]
- Adrian, D.; Papich, M.; Baynes, R.; Murrell, J.; Lascelles, B.D.X. Chronic Maladaptive Pain in Cats: A Review of Current and Future Drug Treatment Options. Vet. J. 2017, 230, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Della Rocca, G.; Conti, M.B. Terapie Palliative E Cure Di Fine Vita In Medicina Veterinaria, 1st ed.; Poletto Editore—Casa Editrice: Milano, Italy, 2018. [Google Scholar]
- Wiese, A.J.; Muir, W.W.; Wittum, T.E. Characteristics of Pain and Response to Analgesic Treatment in Dogs and Cats Examined at a Veterinary Teaching Hospital Emergency Service. J. Am. Vet. Med. Assoc. 2005, 226, 2004–2009. [Google Scholar] [CrossRef] [PubMed]
- Muir, W.W.; Wiese, A.J.; Wittum, T.E. Prevalence and Characteristics of Pain in Dogs and Cats Examined as Outpatients at a Veterinary Teaching Hospital. J. Am. Vet. Med. Assoc. 2004, 224, 1459–1463. [Google Scholar] [CrossRef] [PubMed]
- Epstein, M.E. Feline Neuropathic Pain. Vet. Clin. N. Am. Small Anim. Pract. 2020, 50, 789–809. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, D.G.; Church, D.B.; McGreevy, P.D.; Thomson, P.C.; Brodbelt, D.C. Prevalence of Disorders Recorded in Dogs Attending Primary-Care Veterinary Practices in England. PLoS ONE 2014, 9, e90501. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.L.; O’Neill, D.G.; Brodbelt, D.C.; Church, D.B.; Meeson, R.L.; Sargan, D.; Summers, J.F.; Zulch, H.; Collins, L.M. Prevalence, Duration and Risk Factors for Appendicular Osteoarthritis in a UK Dog Population under Primary Veterinary Care. Sci. Rep. 2018, 8, 5641. [Google Scholar] [CrossRef]
- Pettitt, R.A.; German, A.J. Investigation and Management of Canine Osteoarthritis. Practice 2015, 37, 1–8. [Google Scholar] [CrossRef]
- Johnston, S.A. Osteoarthritis: Joint Anatomy, Physiology, and Pathobiology. Vet. Clin. N. Am. Small Anim. Pract. 1997, 27, 699–723. [Google Scholar] [CrossRef]
- Lascelles, B.D.X.; Dong, Y.-H.; Marcellin-Little, D.J.; Thomson, A.; Wheeler, S.; Correa, M. Relationship of Orthopedic Examination, Goniometric Measurements, and Radiographic Signs of Degenerative Joint Disease in Cats. BMC Vet. Res. 2012, 8, 10. [Google Scholar] [CrossRef]
- Lascelles, B.D.X.; Henry, J.B.; Brown, J.; Robertson, I.; Sumrell, A.T.; Simpson, W.; Wheeler, S.; Hansen, B.D.; Zamprogno, H.; Freire, M.; et al. Cross-Sectional Study of the Prevalence of Radiographic Degenerative Joint Disease in Domesticated Cats. Vet. Surg. VS 2010, 39, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Gruen, M.E.; White, P.; Hare, B. Do Dog Breeds Differ in Pain Sensitivity? Veterinarians and the Public Believe They Do. PLoS ONE 2020, 15, e0230315. [Google Scholar] [CrossRef]
- Della Rocca, G.; Catanzaro, A.; Salvo, A.D.; Goldberg, M.E. Diagnosis of Pain in Small Companion Animals. Am. J. Anim. Vet. Sci. 2015, 10, 57–66. [Google Scholar] [CrossRef]
- Monteiro, B.P.; Steagall, P.V. Chronic Pain in Cats: Recent Advances in Clinical Assessment. J. Feline Med. Surg. 2019, 21, 601–614. [Google Scholar] [CrossRef]
- MacFarlane, P.D.; Tute, A.S.; Alderson, B. Therapeutic Options for the Treatment of Chronic Pain in Dogs. J. Small Anim. Pract. 2014, 55, 127–134. [Google Scholar] [CrossRef]
- Grubb, T. Where Do We Go from Here? Future Treatment Strategies for Chronic Pain. Top. Companion Anim. Med. 2010, 25, 59–63. [Google Scholar] [CrossRef]
- Monteiro-Steagall, B.P.; Steagall, P.V.M.; Lascelles, B.D.X. Systematic Review of Nonsteroidal Anti-Inflammatory Drug-Induced Adverse Effects in Dogs. J. Vet. Intern. Med. 2013, 27, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- KuKanich, B. Outpatient Oral Analgesics in Dogs and Cats beyond Nonsteroidal Antiinflammatory Drugs: An Evidence-Based Approach. Vet. Clin. N. Am. Small Anim. Pract. 2013, 43, 1109–1125. [Google Scholar] [CrossRef] [PubMed]
- Grubb, T.; Lobprise, H. Local and Regional Anaesthesia in Dogs and Cats: Overview of Concepts and Drugs (Part 1). Vet. Med. Sci. 2020, 6, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Goich, M.; Bascuñán, A.; Faúndez, P.; Valdés, A. Multimodal Analgesia for Treatment of Allodynia and Hyperalgesia after Major Trauma in a Cat. JFMS Open Rep. 2019, 5. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.M. Pain Management in Veterinary Patients with Cancer. Vet. Clin. N. Am. Small Anim. Pract. 2014, 44, 989–1001. [Google Scholar] [CrossRef] [PubMed]
- Petrosino, S.; Cordaro, M.; Verde, R.; Schiano Moriello, A.; Marcolongo, G.; Schievano, C.; Siracusa, R.; Piscitelli, F.; Peritore, A.F.; Crupi, R.; et al. Oral Ultramicronized Palmitoylethanolamide: Plasma and Tissue Levels and Spinal Anti-Hyperalgesic Effect. Front. Pharmacol. 2018, 9, 249. [Google Scholar] [CrossRef]
- Sareen, S.; Mathew, G.; Joseph, L. Improvement in Solubility of Poor Water-Soluble Drugs by Solid Dispersion. Int. J. Pharm. Investig. 2012, 2, 12–17. [Google Scholar] [CrossRef]
- Impellizzeri, D.; Campolo, M.; Di Paola, R.; Bruschetta, G.; de Stefano, D.; Esposito, E.; Cuzzocrea, S. Ultramicronized Palmitoylethanolamide Reduces Inflammation an a Th1-Mediated Model of Colitis. Eur. J. Inflamm. 2015, 13, 14–31. [Google Scholar] [CrossRef]
- Nestmann, E.R. Safety of Micronized Palmitoylethanolamide (MicroPEA): Lack of Toxicity and Genotoxic Potential. Food Sci. Nutr. 2017, 5, 292–309. [Google Scholar] [CrossRef]
- Impellizzeri, D.; Bruschetta, G.; Cordaro, M.; Crupi, R.; Siracusa, R.; Esposito, E.; Cuzzocrea, S. Micronized/Ultramicronized Palmitoylethanolamide Displays Superior Oral Efficacy Compared to Nonmicronized Palmitoylethanolamide in a Rat Model of Inflammatory Pain. J. Neuroinflamm. 2014, 11, 136. [Google Scholar] [CrossRef]
- Al Shoyaib, A.; Archie, S.R.; Karamyan, V.T. Intraperitoneal Route of Drug Administration: Should It Be Used in Experimental Animal Studies? Pharm. Res. 2019, 37, 12. [Google Scholar] [CrossRef]
- Calignano, A.; La Rana, G.; Piomelli, D. Antinociceptive Activity of the Endogenous Fatty Acid Amide, Palmitylethanolamide. Eur. J. Pharmacol. 2001, 419, 191–198. [Google Scholar] [CrossRef]
- Bartolucci, M.L.; Marini, I.; Bortolotti, F.; Impellizzeri, D.; Di Paola, R.; Bruschetta, G.; Crupi, R.; Portelli, M.; Militi, A.; Oteri, G.; et al. Micronized Palmitoylethanolamide Reduces Joint Pain and Glial Cell Activation. Inflamm. Res. Off. J. Eur. Histamine Res. Soc. Al 2018, 67, 891–901. [Google Scholar] [CrossRef]
- Di Cesare Mannelli, L.; Corti, F.; Micheli, L.; Zanardelli, M.; Ghelardini, C. Delay of Morphine Tolerance by Palmitoylethanolamide. BioMed Res. Int. 2015, 2015, 894732. [Google Scholar] [CrossRef] [PubMed]
- Di Cesare Mannelli, L.; Micheli, L.; Lucarini, E.; Ghelardini, C. Ultramicronized N-Palmitoylethanolamine Supplementation for Long-Lasting, Low-Dosed Morphine Antinociception. Front. Pharmacol. 2018, 9, 473. [Google Scholar] [CrossRef] [PubMed]
- González-Hernández, A.; Martínez-Lorenzana, G.; Rodríguez-Jiménez, J.; Rojas-Piloni, G.; Condés-Lara, M. Intracisternal Injection of Palmitoylethanolamide Inhibits the Peripheral Nociceptive Evoked Responses of Dorsal Horn Wide Dynamic Range Neurons. J. Neural Transm. Vienna Austria 1996 2015, 122, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Mazzari, S.; Canella, R.; Petrelli, L.; Marcolongo, G.; Leon, A. N-(2-Hydroxyethyl)Hexadecanamide Is Orally Active in Reducing Edema Formation and Inflammatory Hyperalgesia by down-Modulating Mast Cell Activation. Eur. J. Pharmacol. 1996, 300, 227–236. [Google Scholar] [CrossRef]
- Jaggar, S.I.; Hasnie, F.S.; Sellaturay, S.; Rice, A.S. The Anti-Hyperalgesic Actions of the Cannabinoid Anandamide and the Putative CB2 Receptor Agonist Palmitoylethanolamide in Visceral and Somatic Inflammatory Pain. Pain 1998, 76, 189–199. [Google Scholar] [CrossRef]
- Conti, S.; Costa, B.; Colleoni, M.; Parolaro, D.; Giagnoni, G. Antiinflammatory Action of Endocannabinoid Palmitoylethanolamide and the Synthetic Cannabinoid Nabilone in a Model of Acute Inflammation in the Rat. Br. J. Pharmacol. 2002, 135, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Farquhar-Smith, W.P.; Jaggar, S.I.; Rice, A.S.C. Attenuation of Nerve Growth Factor-Induced Visceral Hyperalgesia via Cannabinoid CB(1) and CB(2)-like Receptors. Pain 2002, 97, 11–21. [Google Scholar] [CrossRef]
- D’Agostino, G.; La Rana, G.; Russo, R.; Sasso, O.; Iacono, A.; Esposito, E.; Mattace Raso, G.; Cuzzocrea, S.; Loverme, J.; Piomelli, D.; et al. Central Administration of Palmitoylethanolamide Reduces Hyperalgesia in Mice via Inhibition of NF-KappaB Nuclear Signalling in Dorsal Root Ganglia. Eur. J. Pharmacol. 2009, 613, 54–59. [Google Scholar] [CrossRef] [PubMed]
- De Filippis, D.; Luongo, L.; Cipriano, M.; Palazzo, E.; Cinelli, M.P.; de Novellis, V.; Maione, S.; Iuvone, T. Palmitoylethanolamide Reduces Granuloma-Induced Hyperalgesia by Modulation of Mast Cell Activation in Rats. Mol. Pain 2011, 7, 3. [Google Scholar] [CrossRef]
- Luongo, L.; Guida, F.; Boccella, S.; Bellini, G.; Gatta, L.; Rossi, F.; de Novellis, V.; Maione, S. Palmitoylethanolamide Reduces Formalin-Induced Neuropathic-like Behaviour through Spinal Glial/Microglial Phenotypical Changes in Mice. CNS Neurol. Disord. Drug Targets 2013, 12, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Donvito, G.; Bettoni, I.; Comelli, F.; Colombo, A.; Costa, B. Palmitoylethanolamide Relieves Pain and Preserves Pancreatic Islet Cells in a Murine Model of Diabetes. CNS Neurol. Disord. Drug Targets 2015, 14, 452–462. [Google Scholar] [CrossRef]
- Britti, D.; Crupi, R.; Impellizzeri, D.; Gugliandolo, E.; Fusco, R.; Schievano, C.; Morittu, V.M.; Evangelista, M.; Di Paola, R.; Cuzzocrea, S. A Novel Composite Formulation of Palmitoylethanolamide and Quercetin Decreases Inflammation and Relieves Pain in Inflammatory and Osteoarthritic Pain Models. BMC Vet. Res. 2017, 13, 229. [Google Scholar] [CrossRef]
- Farquhar-Smith, W.P.; Rice, A.S. Administration of Endocannabinoids Prevents a Referred Hyperalgesia Associated with Inflammation of the Urinary Bladder. Anesthesiology 2001, 94, 507–513. [Google Scholar] [CrossRef]
- Farquhar-Smith, W.P.; Rice, A.S.C. A Novel Neuroimmune Mechanism in Cannabinoid-Mediated Attenuation of Nerve Growth Factor-Induced Hyperalgesia. Anesthesiology 2003, 99, 1391–1401. [Google Scholar] [CrossRef]
- Haller, V.L.; Cichewicz, D.L.; Welch, S.P. Non-Cannabinoid CB1, Non-Cannabinoid CB2 Antinociceptive Effects of Several Novel Compounds in the PPQ Stretch Test in Mice. Eur. J. Pharmacol. 2006, 546, 60–68. [Google Scholar] [CrossRef]
- Pessina, F.; Capasso, R.; Borrelli, F.; Aveta, T.; Buono, L.; Valacchi, G.; Fiorenzani, P.; Di Marzo, V.; Orlando, P.; Izzo, A.A. Protective Effect of Palmitoylethanolamide in a Rat Model of Cystitis. J. Urol. 2015, 193, 1401–1408. [Google Scholar] [CrossRef]
- Helyes, Z.; Németh, J.; Thán, M.; Bölcskei, K.; Pintér, E.; Szolcsányi, J. Inhibitory Effect of Anandamide on Resiniferatoxin-Induced Sensory Neuropeptide Release in Vivo and Neuropathic Hyperalgesia in the Rat. Life Sci. 2003, 73, 2345–2353. [Google Scholar] [CrossRef]
- Genovese, T.; Esposito, E.; Mazzon, E.; Di Paola, R.; Meli, R.; Bramanti, P.; Piomelli, D.; Calignano, A.; Cuzzocrea, S. Effects of Palmitoylethanolamide on Signaling Pathways Implicated in the Development of Spinal Cord Injury. J. Pharmacol. Exp. Ther. 2008, 326, 12–23. [Google Scholar] [CrossRef]
- Costa, B.; Comelli, F.; Bettoni, I.; Colleoni, M.; Giagnoni, G. The Endogenous Fatty Acid Amide, Palmitoylethanolamide, Has Anti-Allodynic and Anti-Hyperalgesic Effects in a Murine Model of Neuropathic Pain: Involvement of CB(1), TRPV1 and PPARgamma Receptors and Neurotrophic Factors. Pain 2008, 139, 541–550. [Google Scholar] [CrossRef]
- Guida, F.; Luongo, L.; Marmo, F.; Romano, R.; Iannotta, M.; Napolitano, F.; Belardo, C.; Marabese, I.; D’Aniello, A.; De Gregorio, D.; et al. Palmitoylethanolamide Reduces Pain-Related Behaviors and Restores Glutamatergic Synapses Homeostasis in the Medial Prefrontal Cortex of Neuropathic Mice. Mol. Brain 2015, 8, 47. [Google Scholar] [CrossRef] [PubMed]
- Impellizzeri, D.; Peritore, A.F.; Cordaro, M.; Gugliandolo, E.; Siracusa, R.; Crupi, R.; D’Amico, R.; Fusco, R.; Evangelista, M.; Cuzzocrea, S.; et al. The Neuroprotective Effects of Micronized PEA (PEA-m) Formulation on Diabetic Peripheral Neuropathy in Mice. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2019, 33, 11364–11380. [Google Scholar] [CrossRef] [PubMed]
- Peritore, A.F.; Siracusa, R.; Fusco, R.; Gugliandolo, E.; D’Amico, R.; Cordaro, M.; Crupi, R.; Genovese, T.; Impellizzeri, D.; Cuzzocrea, S.; et al. Ultramicronized Palmitoylethanolamide and Paracetamol, a New Association to Relieve Hyperalgesia and Pain in a Sciatic Nerve Injury Model in Rat. Int. J. Mol. Sci. 2020, 21, 3509. [Google Scholar] [CrossRef] [PubMed]
- Boccella, S.; Marabese, I.; Iannotta, M.; Belardo, C.; Neugebauer, V.; Mazzitelli, M.; Pieretti, G.; Maione, S.; Palazzo, E. Metabotropic Glutamate Receptor 5 and 8 Modulate the Ameliorative Effect of Ultramicronized Palmitoylethanolamide on Cognitive Decline Associated with Neuropathic Pain. Int. J. Mol. Sci. 2019, 20, 1757. [Google Scholar] [CrossRef] [PubMed]
- Alsalem, M.; Haddad, M.; Aldossary, S.A.; Kalbouneh, H.; Altarifi, A.; Jaffal, S.M.; Abbas, M.A.; Aldaoud, N.; El-Salem, K. Role of Cannabinoid Receptor 1 and the Peroxisome Proliferator-Activated Receptor α in Mediating Anti-Nociceptive Effects of Synthetic Cannabinoids and a Cannabinoid-like Compound. Inflammopharmacology 2019, 27, 1131–1142. [Google Scholar] [CrossRef] [PubMed]
- Guida, F.; Boccella, S.; Belardo, C.; Iannotta, M.; Piscitelli, F.; De Filippis, F.; Paino, S.; Ricciardi, F.; Siniscalco, D.; Marabese, I.; et al. Altered Gut Microbiota and Endocannabinoid System Tone in Vitamin D Deficiency-Mediated Chronic Pain. Brain. Behav. Immun. 2020, 85, 128–141. [Google Scholar] [CrossRef] [PubMed]
- Tagne, A.M.; Fotio, Y.; Lin, L.; Squire, E.; Ahmed, F.; Rashid, T.I.; Azari, E.K.; Piomelli, D. Palmitoylethanolamide and Hemp Oil Extract Exert Synergistic Anti-Nociceptive Effects in Mouse Models of Acute and Chronic Pain. Pharmacol. Res. 2021, 105545. [Google Scholar] [CrossRef] [PubMed]
- Sasso, O.; Russo, R.; Vitiello, S.; Raso, G.M.; D’Agostino, G.; Iacono, A.; La Rana, G.; Vallée, M.; Cuzzocrea, S.; Piazza, P.V.; et al. Implication of Allopregnanolone in the Antinociceptive Effect of N-Palmitoylethanolamide in Acute or Persistent Pain. Pain 2012, 153, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Di Cesare Mannelli, L.; D’Agostino, G.; Pacini, A.; Russo, R.; Zanardelli, M.; Ghelardini, C.; Calignano, A. Palmitoylethanolamide Is a Disease-Modifying Agent in Peripheral Neuropathy: Pain Relief and Neuroprotection Share a PPAR-Alpha-Mediated Mechanism. Mediat. Inflamm. 2013, 2013, 328797. [Google Scholar] [CrossRef]
- Guida, F.; Boccella, S.; Iannotta, M.; De Gregorio, D.; Giordano, C.; Belardo, C.; Romano, R.; Palazzo, E.; Scafuro, M.A.; Serra, N.; et al. Palmitoylethanolamide Reduces Neuropsychiatric Behaviors by Restoring Cortical Electrophysiological Activity in a Mouse Model of Mild Traumatic Brain Injury. Front. Pharmacol. 2017, 8, 95. [Google Scholar] [CrossRef]
- Soliman, N.; Haroutounian, S.; Hohmann, A.G.; Krane, E.; Liao, J.; Macleod, M.; Segelcke, D.; Sena, C.; Thomas, J.; Vollert, J.; et al. A Systematic Review and Meta-Analysis of Cannabis-Based Medicines, Cannabinoids and Endocannabinoid System Modulators Tested for Antinociceptive Effects in Animal Models of Injury-Related or Pathological Persistent Pain. Pain 2021. [Google Scholar] [CrossRef] [PubMed]
- Guida, G.; de Martino, M.; de Fabiani, A.; Canteri, L.; Alexandre, A.; Vassallo, G.; Rogai, M.; Lanaia, F.; Petrosino, S. La Palmitoiletanolamida (Normast®) En El Dolor Neuropático Crónico Por Lumbociatalgia de Tipo Compresivo: Estudio Clínico Multicéntrico. Dolor 2010, 25, 35–42. [Google Scholar]
- Assini, A.; Laricchia, D.; Pizzo, R.; Pandolfini, L.; Belletti, M.; Colucci, M.; Ratto, S. The Carpal Tunnel Syndrome in Diabetes: Clinical and Electrophysiological Improvement after Treatment with Palmitoylethanolamide. Eur. J. Neurol. 2010, 17, 295. [Google Scholar] [CrossRef]
- Biasiotta, A.; Cesa, S.L.; Leone, C.; Stefano, G.D.; Truini, A.; Cruccu, G. 265 Efficacy of Palmitoylethanolamide in Patients with Painful Neuropathy. A Clinical and Neurophysiological Open Study. Preliminary Results. Eur. J. Pain Suppl. 2010, 4, 77. [Google Scholar] [CrossRef]
- Schifilliti, C.; Cucinotta, L.; Fedele, V.; Ingegnosi, C.; Savoca, G.; Leotta, C. Palmitoylethanolamide Reduces the Symptoms of Neuropathic Pain in Diabetic Patients. Shock 2011, 36 (Suppl. 1), 30. [Google Scholar]
- Assini, A.; Laricchia, D.; Pizzo, R. Tunnel Carpale Nel Paziente Diabetico Migliloramento Clinico Ed Elettrofisiologico Dopo Trattamento Con Palmitoiletanolamide. Carpal Tunnel Syndrome in the Diabetic Patient. Clinical and Electrophysiologic Improvement after Treatment with Palmitoylethanolamide. In Proceedings of the 34th National Congress AISD—New Frontiers in Pain Medicine, Riccione, Italy, 29–31 May 2011. [Google Scholar]
- Conigliaro, R.; Drago, V.; Foster, P.S.; Schievano, C.; Di Marzo, V. Use of Palmitoylethanolamide in the Entrapment Neuropathy of the Median in the Wrist. Minerva Med. 2011, 102, 141–147. [Google Scholar]
- Truini, A.; Biasiotta, A.; Di Stefano, G.; La Cesa, S.; Leone, C.; Cartoni, C.; Federico, V.; Petrucci, M.T.; Cruccu, G. Palmitoylethanolamide Restores Myelinated-Fibre Function in Patients with Chemotherapy-Induced Painful Neuropathy. CNS Neurol. Disord. Drug Targets 2011, 10, 916–920. [Google Scholar] [CrossRef]
- Schifilliti, C.; Cucinotta, L.; Fedele, V.; Ingegnosi, C.; Luca, S.; Leotta, C. Micronized Palmitoylethanolamide Reduces the Symptoms of Neuropathic Pain in Diabetic Patients. Pain Res. Treat. 2014, 2014, 849623. [Google Scholar] [CrossRef]
- Alshelh, Z.; Mills, E.P.; Kosanovic, D.; Di Pietro, F.; Macey, P.M.; Vickers, E.R.; Henderson, L.A. Effects of the Glial Modulator Palmitoylethanolamide on Chronic Pain Intensity and Brain Function. J. Pain Res. 2019, 12, 2427–2439. [Google Scholar] [CrossRef]
- Ottaviani, G.; Rupel, K.; Gobbo, M.; Poropat, A.; Zoi, V.; Faraon, M.; Di Lenarda, R.; Biasotto, M. Efficacy of Ultramicronized Palmitoylethanolamide in Burning Mouth Syndrome-Affected Patients: A Preliminary Randomized Double-Blind Controlled Trial. Clin. Oral Investig. 2019, 23, 2743–2750. [Google Scholar] [CrossRef]
- Cruccu, G.; Stefano, G.D.; Marchettini, P.; Truini, A. Micronized Palmitoylethanolamide: A Post Hoc Analysis of a Controlled Study in Patients with Low Back Pain—Sciatica. CNS Neurol. Disord. Drug Targets 2019, 18, 491–495. [Google Scholar] [CrossRef]
- Mancardi, G.; Infante, M.; Capello, E.; Sormani, M.; Uccelli, A. La Palmitoiletanolamide Allevia Il Dolore Neuropatico Associato Alla Sclerosi Multipla. Palmitoylethanolamide Relieves Neuropathic Pain Associated with Multiple Sclerosis. In Proceedings of the XL Congress of the Italian Society of Neurology, Padova, Italy, 21–25 November 2009. [Google Scholar]
- Dalla Volta, G.; Zavarize, P.; Ngonga, G.F.; Carli, D. Ultramicronized Palmitoylethanolamide Reduces Frequency and Pain Intensity in Migraine. A Pilot Study. Int. J. Neurol. Brain Disord. 2016, 3, 1–5. [Google Scholar] [CrossRef]
- Chirchiglia, D.; Della Torre, A.; Signorelli, F.; Volpentesta, G.; Guzzi, G.; Stroscio, C.A.; Deodato, F.; Gabriele, D.; Lavano, A. Administration of Palmitoylethanolamide in Combination with Topiramate in the Preventive Treatment of Nummular Headache. Int. Med. Case Rep. J. 2016, 9, 193–195. [Google Scholar] [CrossRef] [PubMed]
- Calabrò, R.S.; Bramanti, P. Occipital Neuralgia Responding to Palmitoylethanolamide. Headache 2017, 57, E23–E24. [Google Scholar] [CrossRef]
- Chirchiglia, D.; Cione, E.; Caroleo, M.C.; Wang, M.; Di Mizio, G.; Faedda, N.; Giacolini, T.; Siviglia, S.; Guidetti, V.; Gallelli, L. Effects of Add-On Ultramicronized N-Palmitol Ethanol Amide in Patients Suffering of Migraine With Aura: A Pilot Study. Front. Neurol. 2018, 9, 674. [Google Scholar] [CrossRef] [PubMed]
- Papetti, L.; Sforza, G.; Tullo, G.; Alaimo di Loro, P.; Moavero, R.; Ursitti, F.; Ferilli, M.A.N.; Tarantino, S.; Vigevano, F.; Valeriani, M. Tolerability of Palmitoylethanolamide in a Pediatric Population Suffering from Migraine: A Pilot Study. Pain Res. Manag. 2020, 2020, 3938640. [Google Scholar] [CrossRef]
- Marini, I.; Bartolucci, M.L.; Bortolotti, F.; Gatto, M.R.; Bonetti, G.A. Palmitoylethanolamide versus a Nonsteroidal Anti-Inflammatory Drug in the Treatment of Temporomandibular Joint Inflammatory Pain. J. Orofac. Pain 2012, 26, 99–104. [Google Scholar]
- Steels, E.; Venkatesh, R.; Steels, E.; Vitetta, G.; Vitetta, L. A Double-Blind Randomized Placebo Controlled Study Assessing Safety, Tolerability and Efficacy of Palmitoylethanolamide for Symptoms of Knee Osteoarthritis. Inflammopharmacology 2019, 27, 475–485. [Google Scholar] [CrossRef]
- Sani, I.; Hamza, Y. A Systematic Review on the Effectiveness of Palmitoylethanolamide for the Treatment of Pain in Arthrogenic Temporomandibular Joint Dysfunction and Related Disorders. J. Dent. Maxillofac. Res. 2019, 2, 1–8. [Google Scholar] [CrossRef]
- Canteri, L.; Petrosino, S.; Guida, G. Reducción Del Consumo de Antiinflamatorios y Analgésicos En El Tratamiento Del Dolor Neuropático Crónico En Pacientes Afectados Por Lumbociatalgia de Tipo Compresivo y En Tratamiento Con Normast® 300 Mg. Dolor 2010, 25, 227–234. [Google Scholar]
- Desio, P. Associazione tra pregabalin e palmitoiletanolamide (PEA) per il trattamento del dolore neuropatico. Pathos 2010, 17, 9–14. [Google Scholar]
- Palomba, R.A.; Adiletta, S.; Candiello, A.; Penimpede, M.; Bonaccia, P.; De Martino, C.J. Analgesia multimodale per il dolore cronico: Direttive future e razionale. Multimodal analgesia for chronic pain: Rationale and future directions. In Proceedings of the 33rd National Congress of the Italian Association for the Study of Pain (AISD), Firenze, Italy, 23–25 May 2010. [Google Scholar]
- Dominguez, C.M.; Diaz Martin, A.A.; Ferrer, F.G. Palmitoiletanolamida (PEA) En Lumbociatica En Asociacion al Tratamiento Habitual. Palmitoylethanolamide in Lumbosciatic Pain in Association with Standard Therapy. In Proceedings of the 8th National Congress of the Sociedad Española Del Dolor, Madrid, Spain, 26–29 May 2010. [Google Scholar]
- Desio, P. Associazione Dell’ossicodone a Lenta Titolazione Con Palmitoiletanolamide per Il Trattamento Del Low Back Pain. Anest. E Med. Crit. AMC 2011, 1, 63–71. [Google Scholar]
- Di Paolo, A.; Gianfelice, V.; Silvestri, C. La Palmitoiletanolamide Nel Trattamento Del Dolore Attivato Dal Sistema Gliale: Nostra Esperienza. Palmitoylethanolamide in the Management of Glia-Activated Pain. Our Experience. In Proceedings of the 34th National Congress of the Italian Association for the Study of Pain (AISD), Riccione, Italy, 29–31 May 2011. [Google Scholar]
- Adiletta, S.; Candiello, A.; Arminio, D.; Porcaro, S.; Saporito, M.M.; Palomba, R. Sinergismo Tra Pregabalin e Palmitoiletanolamide (PEA) Nel Trattamento Della Neuropatia Diabetica. In Proceedings of the 34th National Congress of the Italian Association for the Study of Pain (AISD), Riccione, Italy, 29–31 May 2011. [Google Scholar]
- Domínguez, C.M.; Martín, A.D.; Ferrer, F.G.; Puertas, M.I.; Muro, A.L.; González, J.M.; Prieto, J.P.; Taberna, I.R. N-Palmitoylethanolamide in the Treatment of Neuropathic Pain Associated with Lumbosciatica. Pain Manag. 2012, 2, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Gatti, A.; Lazzari, M.; Gianfelice, V.; Di Paolo, A.; Sabato, E.; Sabato, A.F. Palmitoylethanolamide in the Treatment of Chronic Pain Caused by Different Etiopathogenesis. Pain Med. Malden Mass 2012, 13, 1121–1130. [Google Scholar] [CrossRef]
- Cocito, D.; Peci, E.; Ciaramitaro, P.; Merola, A.; Lopiano, L. Short-Term Efficacy of Ultramicronized Palmitoylethanolamide in Peripheral Neuropathic Pain. Pain Res. Treat. 2014, 2014, 854560. [Google Scholar] [CrossRef] [PubMed]
- Del Giorno, R.; Skaper, S.; Paladini, A.; Varrassi, G.; Coaccioli, S. Palmitoylethanolamide in Fibromyalgia: Results from Prospective and Retrospective Observational Studies. Pain Ther. 2015, 4, 169–178. [Google Scholar] [CrossRef]
- Desio, P. Aliamidi e duloxetina nel trattamento del low back pain Aliamides and duloxetina in the treatment of low back pain. Pathos 2016, 23, 1. [Google Scholar]
- Paladini, A.; Varrassi, G.; Bentivegna, G.; Carletti, S.; Piroli, A.; Coaccioli, S. Palmitoylethanolamide in the Treatment of Failed Back Surgery Syndrome. Pain Res. Treat. 2017, 2017, 1486010. [Google Scholar] [CrossRef]
- Germini, F.; Coerezza, A.; Andreinetti, L.; Nobili, A.; Rossi, P.D.; Mari, D.; Guyatt, G.; Marcucci, M. N-of-1 Randomized Trials of Ultra-Micronized Palmitoylethanolamide in Older Patients with Chronic Pain. Drugs Aging 2017, 34, 941–952. [Google Scholar] [CrossRef]
- Passavanti, M.B.; Fiore, M.; Sansone, P.; Aurilio, C.; Pota, V.; Barbarisi, M.; Fierro, D.; Pace, M.C. The Beneficial Use of Ultramicronized Palmitoylethanolamide as Add-on Therapy to Tapentadol in the Treatment of Low Back Pain: A Pilot Study Comparing Prospective and Retrospective Observational Arms. BMC Anesthesiol. 2017, 17, 171. [Google Scholar] [CrossRef] [PubMed]
- Chirchiglia, D.; Chirchiglia, P.; Signorelli, F. Nonsurgical Lumbar Radiculopathies Treated with Ultramicronized Palmitoylethanolamide (UmPEA): A Series of 100 Cases. Neurol. Neurochir. Pol. 2018, 52, 44–47. [Google Scholar] [CrossRef] [PubMed]
- Chirchiglia, D.; Paventi, S.; Seminara, P.; Cione, E.; Gallelli, L. N-Palmitoyl Ethanol Amide Pharmacological Treatment in Patients With Nonsurgical Lumbar Radiculopathy. J. Clin. Pharmacol. 2018, 58, 733–739. [Google Scholar] [CrossRef]
- Evangelista, M.; Cilli, V.; De Vitis, R.; Militerno, A.; Fanfani, F. Ultra-Micronized Palmitoylethanolamide Effects on Sleep-Wake Rhythm and Neuropathic Pain Phenotypes in Patients with Carpal Tunnel Syndrome: An Open-Label, Randomized Controlled Study. CNS Neurol. Disord. Drug Targets 2018, 17, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Chirchiglia, D.; Chirchiglia, P.; Marotta, R.; Gallelli, L. Add-on Administration of Ultramicronized Palmitoylethanolamide in the Treatment of New-Onset Burning Mouth Syndrome. Int. Med. Case Rep. J. 2019, 12, 39–42. [Google Scholar] [CrossRef]
- Scaturro, D.; Asaro, C.; Lauricella, L.; Tomasello, S.; Varrassi, G.; Letizia Mauro, G. Combination of Rehabilitative Therapy with Ultramicronized Palmitoylethanolamide for Chronic Low Back Pain: An Observational Study. Pain Ther. 2020, 9, 319–326. [Google Scholar] [CrossRef]
- Schweiger, V.; Martini, A.; Bellamoli, P.; Donadello, K.; Schievano, C.; Balzo, G.D.; Sarzi-Puttini, P.; Parolini, M.; Polati, E. Ultramicronized Palmitoylethanolamide (Um-PEA) as Add-on Treatment in Fibromyalgia Syndrome (FMS): Retrospective Observational Study on 407 Patients. CNS Neurol. Disord. Drug Targets 2019, 18, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Parabita, M.; Amore, R.M.D.; Marinosci, F.; Matera, M.G.; Izzo, A.; Russo, G.A. Reduction of Post Stroke Spasticity with Palmitoylethanolamide Treatment. Shock 2011, 36 (Suppl. 1), 27. [Google Scholar]
- Marini, I.; Cavallaro, M.; Bartolucci, M.; Alessandri-Bonetti, A.; Gatto, M.; Cordaro, M.; Checchi, L. Can Celecoxib Enhance Palmitoylethanolamide’s Effect in the Treatment of Temporo-Mandibular Arthralgia in Osteoarthritis Patients? J. Transl. Sci. 2018, 5. [Google Scholar] [CrossRef][Green Version]
- Caltagirone, C.; Cisari, C.; Schievano, C.; Di Paola, R.; Cordaro, M.; Bruschetta, G.; Esposito, E.; Cuzzocrea, S.; Stroke Study Group. Co-Ultramicronized Palmitoylethanolamide/Luteolin in the Treatment of Cerebral Ischemia: From Rodent to Man. Transl. Stroke Res. 2016, 7, 54–69. [Google Scholar] [CrossRef] [PubMed]
- Palomba, R.; De Simone, M.; Giovannini, A.; Bonaccia, P.; Pinto, D. Use of Palmitoylethanolamide (PEA) + Polydatin in the Chronic Pelvic Pain. In Proceedings of the 64th National Congress of the Italian Society of Anesthesia, Analgesia and Intensive Care (SIAARTI), Parma, Italy, 13–16 October 2010. [Google Scholar]
- Fulghesu, A.; Magnini, R.; Mazzella, S.; Cappai, A.; Orrù, A.; Pisu, M. Treatment of Adolescent Dysmenorrhea by a New Inhibitor of Mast Cells-Induced Infiammation (Palmitoylethanolamide + Trans Polidatin). In Proceedings of the 16th World Congress of Pediatric and Adolescent Gynecology, Montpellier, Le Corum, France, 22–25 May 2010. [Google Scholar]
- Indraccolo, U.; Barbieri, F. Effect of Palmitoylethanolamide-Polydatin Combination on Chronic Pelvic Pain Associated with Endometriosis: Preliminary Observations. Eur. J. Obstet. Gynecol. Reprod. Biol. 2010, 150, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Calabrò, R.S.; Gervasi, G.; Marino, S.; Mondo, P.N.; Bramanti, P. Misdiagnosed Chronic Pelvic Pain: Pudendal Neuralgia Responding to a Novel Use of Palmitoylethanolamide. Pain Med. Malden Mass 2010, 11, 781–784. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cobellis, L.; Castaldi, M.A.; Giordano, V.; Trabucco, E.; De Franciscis, P.; Torella, M.; Colacurci, N. Effectiveness of the Association Micronized N-Palmitoylethanolamine (PEA)-Transpolydatin in the Treatment of Chronic Pelvic Pain Related to Endometriosis after Laparoscopic Assessment: A Pilot Study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011, 158, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Lo Monte, G.; Soave, I.; Marci, R. Administration of micronized palmitoylethanolamide (PEA)-transpolydatin in the treatment of chronic pelvic pain in women affected by endometriosis: Preliminary results. Minerva Ginecol. 2013, 65, 453–463. [Google Scholar] [PubMed]
- Giugliano, E.; Cagnazzo, E.; Soave, I.; Lo Monte, G.; Wenger, J.M.; Marci, R. The Adjuvant Use of N-Palmitoylethanolamine and Transpolydatin in the Treatment of Endometriotic Pain. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 168, 209–213. [Google Scholar] [CrossRef]
- Murina, F.; Graziottin, A.; Felice, R.; Radici, G.; Tognocchi, C. Vestibulodynia: Synergy between Palmitoylethanolamide + Transpolydatin and Transcutaneous Electrical Nerve Stimulation. J. Low. Genit. Tract Dis. 2013, 17, 111–116. [Google Scholar] [CrossRef]
- Tartaglia, E.; Armentano, M.; Giugliano, B.; Sena, T.; Giuliano, P.; Loffredo, C.; Mastrantonio, P. Effectiveness of the Association N-Palmitoylethanolamine and Transpolydatin in the Treatment of Primary Dysmenorrhea. J. Pediatr. Adolesc. Gynecol. 2015, 28, 447–450. [Google Scholar] [CrossRef] [PubMed]
- Cremon, C.; Stanghellini, V.; Barbaro, M.R.; Cogliandro, R.F.; Bellacosa, L.; Santos, J.; Vicario, M.; Pigrau, M.; Alonso Cotoner, C.; Lobo, B.; et al. Randomised Clinical Trial: The Analgesic Properties of Dietary Supplementation with Palmitoylethanolamide and Polydatin in Irritable Bowel Syndrome. Aliment. Pharmacol. Ther. 2017, 45, 909–922. [Google Scholar] [CrossRef] [PubMed]
- Stochino Loi, E.; Pontis, A.; Cofelice, V.; Pirarba, S.; Fais, M.F.; Daniilidis, A.; Melis, I.; Paoletti, A.M.; Angioni, S. Effect of Ultramicronized-Palmitoylethanolamide and Co-Micronized Palmitoylethanolamide/Polydatin on Chronic Pelvic Pain and Quality of Life in Endometriosis Patients: An Open-Label Pilot Study. Int. J. Womens Health 2019, 11, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Cervigni, M.; Nasta, L.; Schievano, C.; Lampropoulou, N.; Ostardo, E. Micronized Palmitoylethanolamide-Polydatin Reduces the Painful Symptomatology in Patients with Interstitial Cystitis/Bladder Pain Syndrome. BioMed Res. Int. 2019, 2019, 9828397. [Google Scholar] [CrossRef]
- Artukoglu, B.B.; Beyer, C.; Zuloff-Shani, A.; Brener, E.; Bloch, M.H. Efficacy of Palmitoylethanolamide for Pain: A Meta-Analysis. Pain Physician 2017, 20, 353–362. [Google Scholar]
- Finnerup, N.B.; Attal, N.; Haroutounian, S.; McNicol, E.; Baron, R.; Dworkin, R.H.; Gilron, I.; Haanpää, M.; Hansson, P.; Jensen, T.S.; et al. Pharmacotherapy for Neuropathic Pain in Adults: A Systematic Review and Meta-Analysis. Lancet Neurol. 2015, 14, 162–173. [Google Scholar] [CrossRef]
- Gugliandolo, E.; Barbagallo, A.; Peritore, A.F.; Cuzzocrea, S.; Crupi, R. Oral Supplementation with Ultramicronized Palmitoylethanolamide for Joint Disease and Lameness Management in Four Jumping Horses: A Case Report. Amimals 2020, 10, 1469. [Google Scholar] [CrossRef]
- Vezzoni, A.; Crupi, F.; Boiocchi, S.; Boano, S. Effect of Palmitoylethanolamide Co-Ultra Micronized with Quercetin in Dogs with Osteoarthritis by Means of Dynamic Gate Analysis and Canine Brief Pain Inventory Questionnaire. In Proceedings of the 5th World Veterinary Orthopaedic Congress ESVOT-VOS, Barcelona, Spain, 12–15 September 2018; pp. 771–772. [Google Scholar]
- Alkaitis, M.S.; Solorzano, C.; Landry, R.P.; Piomelli, D.; DeLeo, J.A.; Romero-Sandoval, E.A. Evidence for a Role of Endocannabinoids, Astrocytes and P38 Phosphorylation in the Resolution of Postoperative Pain. PLoS ONE 2010, 5, e10891. [Google Scholar] [CrossRef]
- Guerrero-Alba, R.; Barragán-Iglesias, P.; González-Hernández, A.; Valdez-Moráles, E.E.; Granados-Soto, V.; Condés-Lara, M.; Rodríguez, M.G.; Marichal-Cancino, B.A. Some Prospective Alternatives for Treating Pain: The Endocannabinoid System and Its Putative Receptors GPR18 and GPR55. Front. Pharmacol. 2018, 9, 1496. [Google Scholar] [CrossRef]
- Luongo, L.; Maione, S.; Di Marzo, V. Endocannabinoids and Neuropathic Pain: Focus on Neuron-Glia and Endocannabinoid-Neurotrophin Interactions. Eur. J. Neurosci. 2014, 39, 401–408. [Google Scholar] [CrossRef]
- Maione, S.; Costa, B.; Di Marzo, V. Endocannabinoids: A Unique Opportunity to Develop Multitarget Analgesics. Pain 2013, 154 (Suppl. 1), S87–S93. [Google Scholar] [CrossRef]
- Kaye, A.D.; Cornett, E.M.; Hart, B.; Patil, S.; Pham, A.; Spalitta, M.; Mancuso, K.F. Novel Pharmacological Nonopioid Therapies in Chronic Pain. Curr. Pain Headache Rep. 2018, 22, 31. [Google Scholar] [CrossRef] [PubMed]


| Mast Cells | Microglia | |
|---|---|---|
| Cell type | Resident long-lived immune-inflammatory cells [57,58] | Resident long-lived immune-inflammatory cells [59,60] |
| Location | Periphery In association with sensory nerves, forming synapse-like structures, in virtually any tissue, especially those exposed to the environment PNS Within nerves (endoneural mast cells) [61,62,63,64] | CNS Throughout the brain and spinal cord (largely outnumbering neurons), where they provide nourishment to neurons, regulate neural activity and generate innate immune responses [65] |
| CNS Spinal meninges; different brain parenchymal sites (e.g., hippocampus and thalamic, hypothalamic region); blood brain barrier (brain side), generally located near microglia [66,67,68] | ||
| Activation kinetics | Rapid release of prestored mediator in response to stimuli (e.g., sensory nerve activation), thanks to a wide range of receptors Release more than 50 mediators with vasoactive, neurosensitizing and pro-inflammatory effects [69,70,71,72] | Become activated in response to local stress (e.g., nerve injury), shifting their phenotype from a quiescent to an activated state Release pro-inflammatory cytokines and chemokines in the brain and spinal cord [73,74] |
| Type of pain involved in | Inflammatory and neuropathic pain, either visceral and somatic, e.g., osteoarthritis pain, discogenic pain, viscerovisceral hyperalgesia [75,76,77,78,79,80,81,82,83,84,85,86,87,88] | Neuropathic pain (e.g., canine intervertebral disk disease); also involved in allergic-induced neuropathic pain, acute inflammatory pain, paradoxical pain associated with long-term opioid administration [59,89,90,91,92,93] |
| Main Causes of Inflammatory Pain |
| Chronic lesions/inflammations affecting superficial tissues (skin, mucous membranes, teeth, some portions of the eye) and deep somatic tissues (bones, muscles, joints) |
| Chronic ulcers at skin, mucous, or corneal sites |
| Chronic inflammatory diseases |
| Gingivostomatitis Periodontitis Pulpits Otitis Conjunctivitis Keratitis Osteoarthritis |
| Myofascial trigger points |
| Discs herniation |
| Somatic cancers (skin, breast, osteosarcoma) |
| Chronic injury/inflammation affecting deep visceral tissues |
| Chronic inflammatory diseases Inflammatory bowel disease (IBD) Pancreatitis Cystitis (i.e., feline idiopathic cystitis) Prostatitis |
| Gastrointestinal ulcers |
| Cancers affecting visceral districts Primary visceral cancer Metastatic invasion of viscera |
| Main Causes of Neuropathic Pain |
| Peripheral and central nervous system disorders |
| Poliradiculoneuritis |
| Diabetic neuropathy |
| Disk compression radiculopathy with nerve damage |
| Tumor infiltration neuropathy |
| Paraneoplastic neuropathies |
| Myelin sheath cancer |
| Central nervous system (CNS) cancers |
| Chronic visceral pathologies with neuropathic component |
| Chronic pancreatitis |
| IBD |
| Feline interstitial cystitis |
| Visceral cancers |
| Diagnosis (Trial Design) | No. of Pts | Dose | Main Result | Ref. |
|---|---|---|---|---|
| Peripheral neuropathic pain | ||||
| Sciatic pain due to radicular and/or core compression of the sciatic nerve and discopathy (Double-blind, randomized, two doses of micro-PEA vs. placebo) | 636 | 300 mg/die or 300 mg/bid for three weeks | Significant decrease of pain on VAS (from 7 to 2) | [206] |
| Diabetic neuropathy pain associated with carpal tunnel syndrome (Group-controlled, randomized, micro-PEA treatment vs. standard care) | 50 | 600 mg/bid for two months | Significant relief of pain. Significant improvement of neurophysiologic parameters | [207] |
| Painful neuropathies (Open-label study) | 27 | 300 mg/bid for three weeks, followed by 300 mg/die for four weeks | Significant reduction of pain and improvement of electrophysiological parameters | [208] |
| Sciatic pain (Double-blind, randomized, two doses of micro-PEA vs. placebo (as an add-on therapy)) | 111 | 300 mg/die or 300 mg/bid for three weeks | Significant decrease in pain severity and duration of treatment with anti-inflammatory and analgesic drugs | [226] |
| Neuropathic chronic pain (diabetic neuropathy and postherpetic neuralgia) (Open, combination therapy with GBPs) | 30 | 600 mg/bid for 45 days | Significant decrease of pain on VAS (from 7.6 to 1.8) | [227] |
| Low back pain (Group-controlled (add-on therapy to standard analgesics)) | 81 | 600 mg/bid for three weeks followed by 600 mg/die for four weeks | Significant reduction of pain intensity compared to control group | [228] |
| Sciatic pain (Group-controlled, randomized, add-on therapy to standard analgesics) | 85 | 300 mg/bid for 30 days | Significant relief of pain (scored both on VAS and Oswestry Low Back Pain Scale) compared to the analgesic-only group | [229] |
| Diabetic neuropathic pain (Open-label study) | 30 | 300 mg/bid for two months | Significant reduction of pain, burning, paraesthesia and numbness | [209] |
| Carpal tunnel syndrome in diabetic pts (Group-controlled, randomized vs. non-treated pts) | 40 | 600 mg/bid for two months | Significant reduction of pain and improvement of functional status and neurophysiologic parameters | [210] |
| Pain associated with carpal tunnel syndrome (Group-controlled, randomized, two doses of micro-PEA vs. non-treated pts) | 26 | 1st arm: 300 mg/bid for 30 days 2nd arm: 600 mg/bid for 30 days | Significant dose-dependent reduction of pain and improvement of neurophysiologic parameters compared with control group | [211] |
| Chemotherapy-induced painful neuropathy (Open-label study) | 20 | 300 mg/bid for two months | Significant pain reduction on NRS and significantly increased conduction velocity of myelinated fibers on neurophysiological assessment | [212] |
| Low back pain (Open, combination therapywith OPI) | 20 | 600 mg/bid for 30 days | Significant decrease of pain on VAS (from 7 to 2.5) | [230] |
| Various chronic pain-associated disorders (Open, combination therapy with GBPs and OPI) | 517 | 600 mg/bid for three weeks followed by 600 mg/die for four weeks | 61% decrease of mean pain score on NRS | [231] |
| Diabetic neuropathic pain (Group-controlled (micro-PEA + GBPs vs. GBPs)) | 74 | 600 mg/bid for the first 10 days, then 600 mg/die for 20 days, followed by 300 mg/die for 30 days | Significantly higher rate of responders (i.e., >60% decrease in pain score) compared to GBP group | [232] |
| Chronic neuropathic pain from lumbosciatica (Multicentral prospective, group-controlled study (add-on therapy to standard analgesics vs. standard analgesics)) | 118 | 300 mg/bid for 30 days | Significantly larger improvements in VAS and QoL compared to standard therapy alone | [233] |
| Chronic pain associated to different pathological conditions (Observational study (add-on to poorly effective standard analgesics)) | 610 | 600 mg/bid for three weeks + 600 mg/die for the following four weeks | Significant decrease of the mean score of pain on NRS (even in pts without concomitant analgesics) | [234] |
| Diabetic neuropathy (Open-label study) | 30 | 300 mg/bid for two months | Significant decrease of pain severity and related symptoms evaluated by Michigan Neuropathy Screening instrument and NPSI | [213] |
| Diabetic or traumatic chronic neuropathic pain, with VAS greater than 6 in spite of the best therapeutic regimen with GBPs and/or OPI (Open-label study (add-on)) | 30 | 1200 mg/die for 40 days | Significant and time dependent decrease of pain on VAS and NPSI, as well as QoL on EQ-5D | [235] |
| Pain associated to fibromyalgia syndrome (Retrospective + prospective study (SNRI + GBPs vs. SNRI + GBPs + micro-PEA)) | 80 | 600 mg/bid in the first month and 300 mg/bid in the next two months | Further reduction in the number of positive tender points and significant reduction in pain, compared to SNRI + GBPs only | [236] |
| Low back pain (Case report (combined to low dose SNRI)) | 2 | 600–1200 mg/bid for two months | Significant decrease of pain on NRS | [237] |
| Failed back surgery syndrome (caused by laminectomy, discectomy, or vertebral stabilization) (Observational study (add-on to 1-month standard analgesic treatment, i.e., OPI + GBPs)) | 35 | 1200 mg/die for the first month and 600 mg/die for the second month | Further and significant decrease in pain intensity compared to the first month of standard analgesics | [238] |
| Chronic, non-cancer, non-ischemic pain in the back, joints or limbs in elderly pts (≥ 65 years) (Series of N-of-1 randomized trials) | 10 | 600 mg/bid | Statistically significant favorable impact on either pain intensity or function impairment in some of the three of the pts | [239] |
| Chronic low back pain (Two arm (prospective and retrospective), pilot observational study (add-on to OPI compared to OPI only)) | 55 | 600 mg/bid for six months | Significantly higher reduction in:
| [240] |
| Neuropathic pain associated with nonsurgical lumbar radiculopathies (Retrospective study (add-on to 4-day treatment with ACT + OPI)) | 100 | 600 mg/bid for 30 days followed by 600 mg/die for 30 days | Significant pain relief in pts with mild, moderate and severe baseline painful symptoms | [241] |
| Neuropathic pain associated with nonsurgical lumbar radiculopathies with X-ray signs of spondylosis and CT/MRI signs of IVD protrusion or dehydration (Prospective single-blind (add-on to 7-day treatment with fixed combination ACT + OPI)) | 155 | 1200 mg/die for 30 days. If unsuccessful, further 30 days with 600 mg/die followed, if needed, by a second cycle of ACT + OPI for 30 days | Significant improvement of pain and disability after 30 or 60 days depending on the baseline pain severity (VAS 3–8). In pts with baseline VAS ≥9 the second ACT + OPI cycle was needed. | [242] |
| Carpal tunnel syndrome (Open, controlled study (PEA-um + surgery vs. surgery only)) | 42 | 600 mg/bid for 2 months before and 2 months after surgery + 600 mg/die for 30 days | Significant improvement in painful symptoms and overall sleep quality on PSQI | [243] |
| Burning mouth syndrome (Case report (add-on to poorly effective GBPs)) | 1 | 600 mg/bid for three months | Significant decrease of pain on VAS (from 9 to 5). Great reduction of the frequency of episodes | [244] |
| Chronic orofacial neuropathic pain (post-traumatic neuropathy) (Open-label clinical trial) | 22 | 300 mg/tidfor six weeks | Overall reduction in ongoing pain on VAS. Normalized activity patterns in the ascending pain pathway | [214] |
| Burning mouth syndrome (Preliminary randomized double-blind controlled trial) | 35 | 600 mg/bid for two months | Statistically significant higher reduction of burning mouth sensation on NRS compared to placebo | [215] |
| Fibromyalgia Syndrome (Retrospective observational study (add-on to concomitant pharmacological therapy, i.e., SSRI (n = 71), SNRI (n = 66), GBPs (n = 41), TCA (n = 40), BZD (n = 94),OPI (n = 78), NSAIDs (n = 87), MR (n = 35), ACT (n = 45)) | 407 | 600 mg/tid for 10 days followed by 600 mg/bid for 20 days followed by 600 mg/die for 125 months | Statistically significant decrease of pain on VAS and statistically significant improved QoL on FIQ | [246] |
| Low back pain—sciatica (Post-hoc analysis of a placebo-controlled study) | 600 | 600 mg/die | NNT of 1.7 (1.4–2) for the effect on pain and 1.5 (1.4–1.7) for the effect on function | [216] |
| Chronic low back pain (i.e., lumbo-sciatica and lumbo-cruralgia due to multiple herniated discs in the lumbar spine) (Open, add-on to standard analgesics + functional rehabilitation session) | 120 | 600 mg/bid for 20 days, followed by 600 mg/die for 40 days | Significant decrease of pain intensity scores (from 6.3 ± 0.1 at baseline to 3.7 ± 0.09 and 2 ± 0.09 at 30 and 60 days, respectively) | [245] |
| Central neuropathic pain | ||||
| Neuropathic pain associated with multiple sclerosis (Open-label study) | 20 | 300 mg/bid for two months | Significant decrease of neuropathic pain | [217] |
| Neuropathic pain and spasticity in post-stroke pts (Open, controlled micro-PEA + P vs. PT only) | 20 | 600 mg/bid for two months followed by 600 mg/die for 30 days | Significant decrease of pain and spasticity | [247] |
| Pain associated with stroke (Observational study (co-um PEA-Lut in association with the stroke therapy, e.g., thrombolytics)) | 250 | 700 mg + 70 mg for two months | Pain on NRS halved after 30 days | [249] |
| Migraine without aura—at least 6 months’ duration (Open-label study) | 50 | 600 mg/bid for three months | Significant decrease of
| [218] |
| Nummular headache (Case report (add-on to decreasing topiramate dose)) | 1 | 600 mg/die | Improvement in pain symptoms and on pain measuring scales | [219] |
| Occipital Neuralgia (Case report]) | 1 | 1200 mg/die | Significant improvement of pain, after around 2 weeks of treatment | [220] |
| Migraine with Aura (Single blind study (add-on to acute NSAIDs, i.e., ibuprofen, diclofenac sodium, or nimesuilde for about 2 days during acute migraine attack)) | 20 | 1200 mg/die for three months | Statistically significant and time-dependent pain relief, already evident at 60 days and lasting until the end of the study | [221] |
| Migraine without aura in a pediatric population (Open-label pilot study) | 70 | 600 mg/die for three months | Significant decrease of
| [222] |
| Diagnosis [Trial Design] | No. of Pts | Dose | Main Result | Ref. |
|---|---|---|---|---|
| TMJ pain caused by OA (Double-blind randomized vs. NSAIDs) | 24 | 300 mg in the morning + 600 mg in the eveningfor 7 days; followed by 300 mg/bid for 7 days | Significant decrease of pain on VAS (from 7 to 0.7) and significantly improved maximum mouth opening compared to NSAIDs | [223] |
| OA-induced TMJ arthralgia (Case series (initially combined with NSAIDs)) | 12 | 600 mg/die (together with NSAIDs for the first 4 days, then singly for the following 10 days) | Significant pain reduction after 4 days. Significant improvement of maximum mouth opening | [248] |
| Knee OA pain (Double-blind randomized placebo-controlled study (two doses)) | 111 | 300 mg/die or 600 mg/die | Significant reduction of WOMAC, pain on NRS and anxiety | [224] |
| Pain in arthrogenic TMJ dysfunction and similar disorders (Systematic review of 5 studies (4 RCTs + 1 retrospective cohort study)) | 227 | 300 mg/die and over | Effective in arthrogenic TMJ dysfunction and related disorders, with a superior analgesic effect to some NSAIDs and a low rate of adverse events | [225] |
| Diagnosis [Trial Design] | No. of Pts | Dose | Main Result | Ref. |
|---|---|---|---|---|
| Chronic pelvic pain associated with endometriosis/dysmenorrhea/ interstitial cystitis (Open-label study) | 25 | (200 + 20) mg/tid for 40 days | Significant reduction of pain on VAS (from 6.8 to 1.7); significant decrease in the use of NSAIDs. | [250] |
| Adolescent primary dysmenorrhea (Open-label study) | 20 | (400 + 40) mg/bid for six months | 70% decrease in pelvic pain | [251] |
| Chronic pelvic pain and dyspareunia associated with endometriosis (Open (case series)) | 4 | (200 + 20) mg/bid for three months | Significant decrease of pelvic pain and dyspareunia; significant reduction in the use of analgesics. | [252] |
| Pudendal neuralgia (Case report) | 1 | PEA-um 300 mg/tid gradually decreased to 300 mg/die for one year | Resolution of chronic pelvic pain | [253] |
| Chronic pelvic pain associated with endometriosis (Double-blind, randomized parallel-group (celecoxib), placebo-controlled) | 61 | (400 + 40) mg/tid for three months | Significant decrease of chronic pelvic pain, dysmenorrhea and dyspareunia | [254] |
| Endometriosis associated with severe pelvic pain (Open-label study) | 24 | (400 + 40) mg/bid for three months | Statistically significant decrease of pain, dysmenorrhea and dyspareunia and improved QoL, as well as decreased assumption of NSAIDs | [255] |
| Pain related to endometriosis (Prospective study) | 47 | (400 + 40) mg/bid for three months | Significant decrease of chronic pelvic pain, dyspareunia and dysmenorrhea on VAS since the first visit (day 30) | [256] |
| Vestibulodynia (Randomized, placebo-controlled, combined with TENS) | 20 | (400 + 40) mg/bid for two months | Significant decrease of pain on VAS in both groups. Superior decrease of current perception threshold for C fibers in treated (40%) compared to placebo group (4.6%) | [257] |
| Primary dysmenorrhea (Randomized placebo-controlled with follow-up) | 220 | (400 + 40) mg/die for 10 days (from the 24th day of cycle) | Improvement of pelvic pain in 98% of cases in the treated group vs. 56% in the placebo group. Statistically superior effect compared to placebo | [258] |
| Irritable bowel syndrome (Randomized double-blind placebo-controlled) | 54 | (200 + 20) mg/bid for 12 weeks | Reduction of abdominal pain and discomfort | [259] |
| Symptomatic women with laparoscopic diagnosis of endometriosis (Single-arm, open-label) | 30 | (400 + 40) mg/bid for 80 days, after 10 days PEA-um 600 mg/bid | Significant decrease of symptoms (pain on VAS, dysmenorrhea, dyspareunia, and dyschezia, dysuria); increased QoL and psychological well-being; significant reduction in the use of the analgesics | [260] |
| Interstitial cystitis/bladder pain syndrome (IC/BPS) (Pilot, open-label bicentric study) | 32 | (400 + 40) mg/tid for three months followed by (400 + 40) mg/die for three months | Significant decrease of pelvic pain intensity on VAS from 6.9 ± 0.4 to 4.6 ± 0.4 (the effect persisting up to two months after treatment withdrawal); PUF significantly and progressively decreased; significant reduction in urinary frequency | [261] |
| Intervention | NNT |
|---|---|
| Micro-PEA | 1.7 |
| TTAs | 3.6 |
| SNRIs | 6.4 |
| Gabapentin | 6.3 |
| Pregabalin | 7.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
della Rocca, G.; Gamba, D. Chronic Pain in Dogs and Cats: Is There Place for Dietary Intervention with Micro-Palmitoylethanolamide? Animals 2021, 11, 952. https://doi.org/10.3390/ani11040952
della Rocca G, Gamba D. Chronic Pain in Dogs and Cats: Is There Place for Dietary Intervention with Micro-Palmitoylethanolamide? Animals. 2021; 11(4):952. https://doi.org/10.3390/ani11040952
Chicago/Turabian Styledella Rocca, Giorgia, and Davide Gamba. 2021. "Chronic Pain in Dogs and Cats: Is There Place for Dietary Intervention with Micro-Palmitoylethanolamide?" Animals 11, no. 4: 952. https://doi.org/10.3390/ani11040952
APA Styledella Rocca, G., & Gamba, D. (2021). Chronic Pain in Dogs and Cats: Is There Place for Dietary Intervention with Micro-Palmitoylethanolamide? Animals, 11(4), 952. https://doi.org/10.3390/ani11040952






