Effect of Leukoreduction on Hematobiochemical Parameters and Storage Hemolysis in Canine Whole Blood Units
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Blood Donors
2.2. Blood Collection
2.3. Sample Analysis
- A: CBC, % of WBC and PLT depletion to evaluate leukoreduction effectiveness, % RBC recovery after filtration (only at T0), LDH, electrolytes concentrations, and blood smear evaluation.
- B: % of storage hemolysis.
- C: aerobic and anaerobic bacterial culture.
2.3.1. Complete Blood Count
2.3.2. Percentages of WBC and PLT Depletion (Leukoreduction Effectiveness)
2.3.3. Percentage of RBC Recovery
2.3.4. LDH and Electrolytes
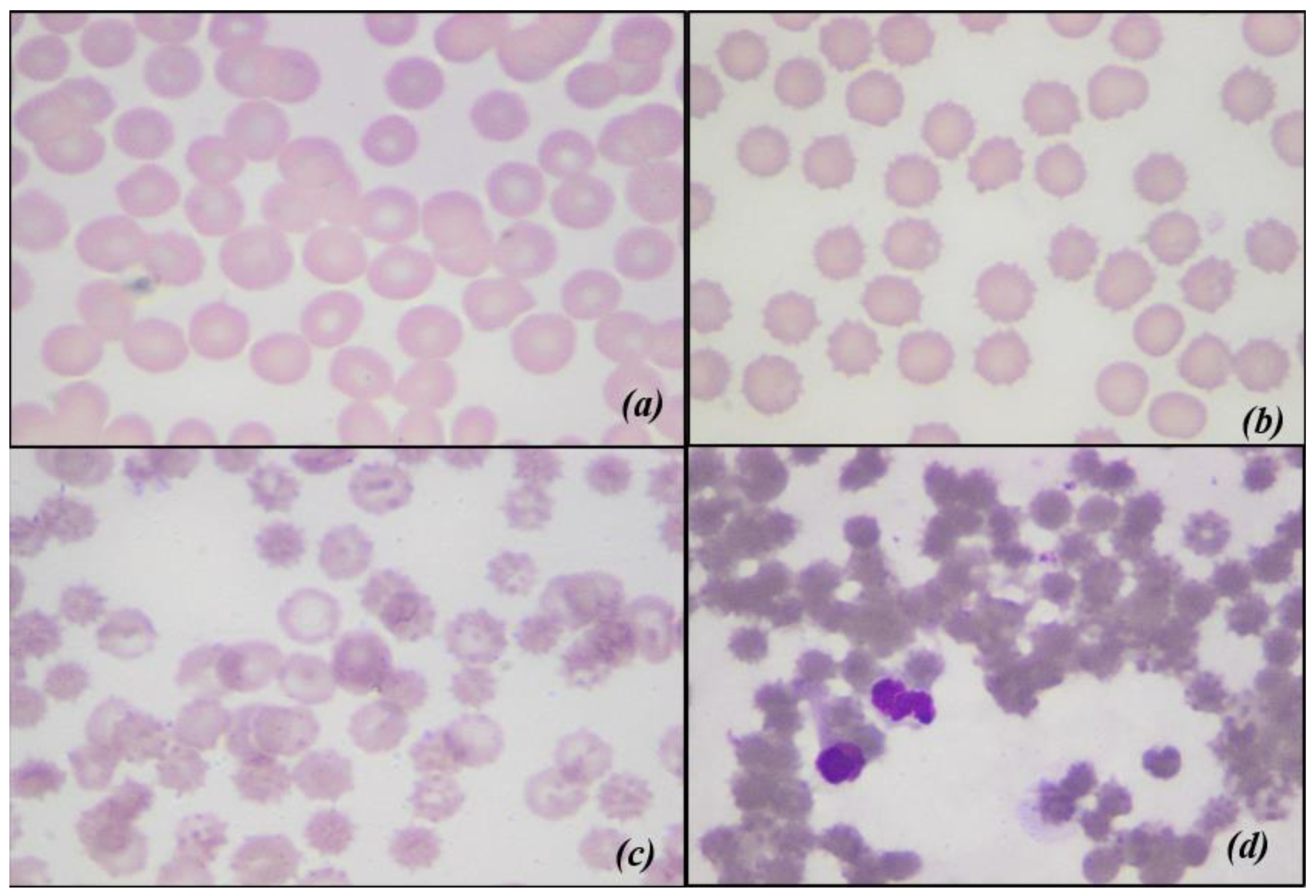
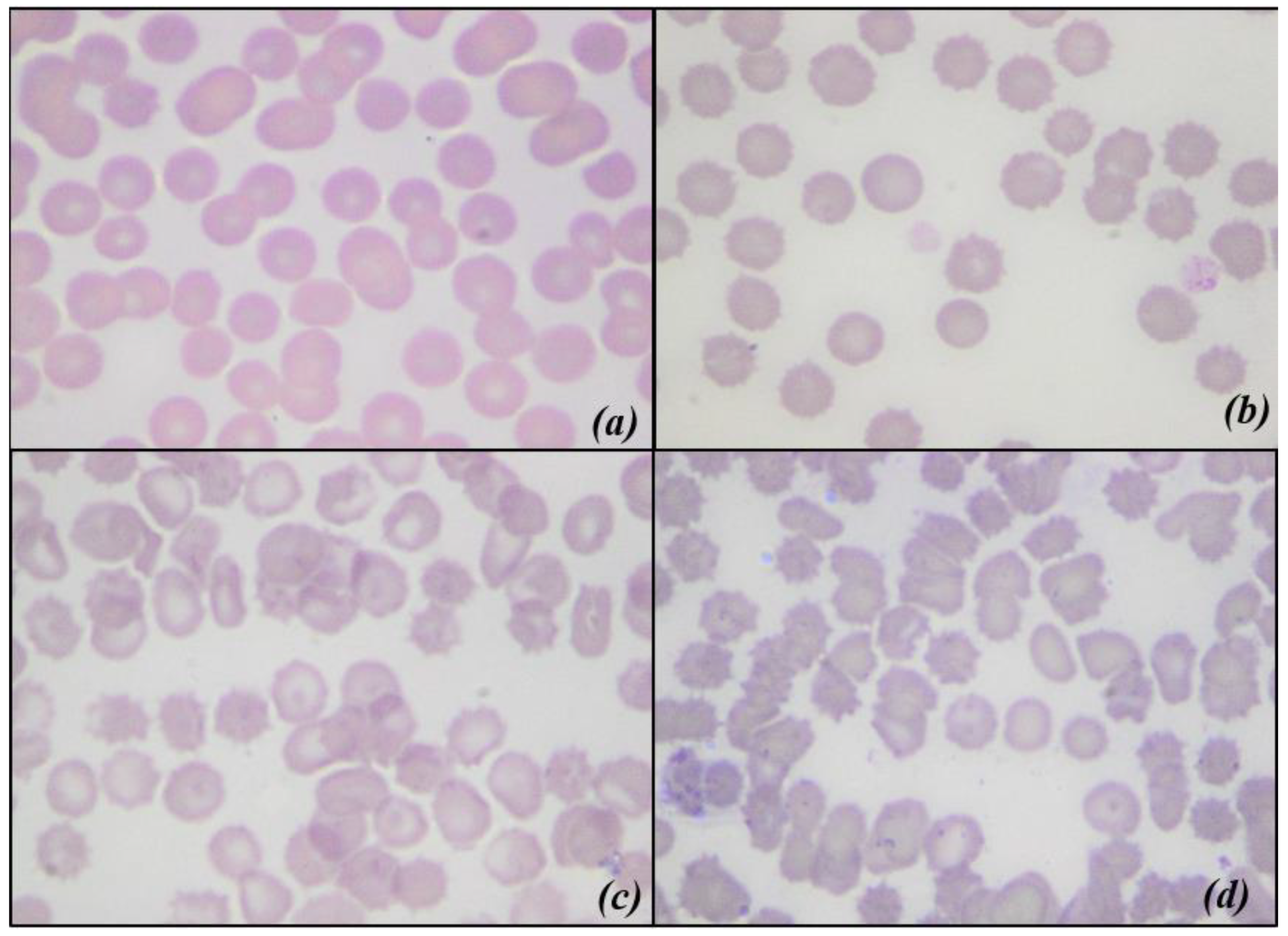
2.3.5. Blood Smear Evaluation and Morphological Index
- 0: Discocyte (normal form erythrocyte)
- 1: Echinocyte I (irregularly shaped erythrocyte, with a maximum of five membrane spicules)
- 2: Echinocyte II (flat erythrocyte with numerous membranous spicules)
- 3: Echinocyte III (ovoid or spherical erythrocyte with numerous membranous spicules)
2.3.6. Storage Hemolysis
2.3.7. Bacterial Culture
2.4. Statistical Analysis
3. Results
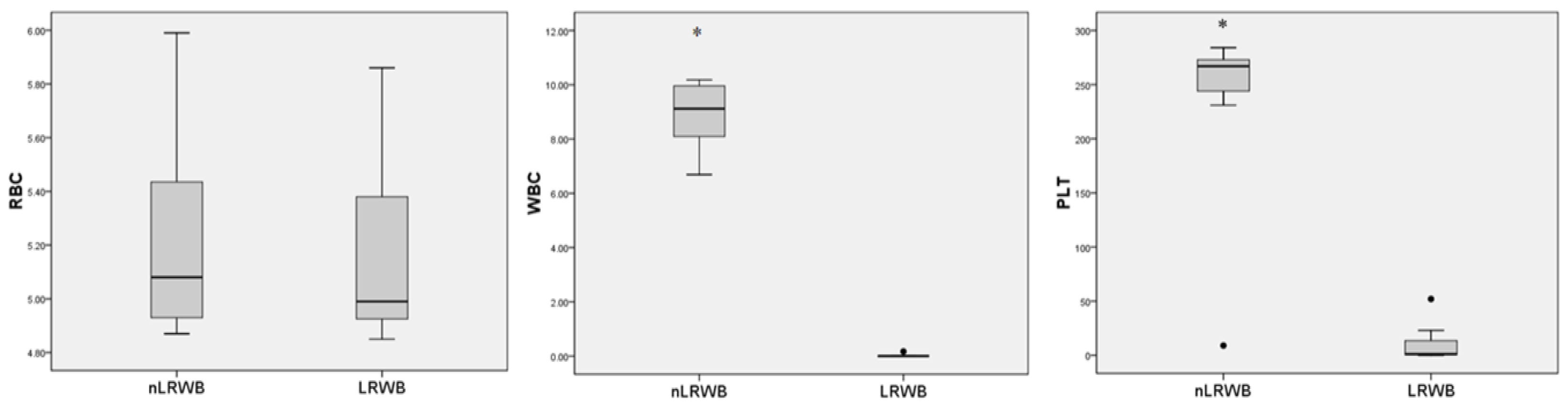
3.1. Complete Blood Count
3.2. Percentages of WBC and PLT Depletion, and Percentage of RBC Recovery (Leukoreduction Effectiveness)
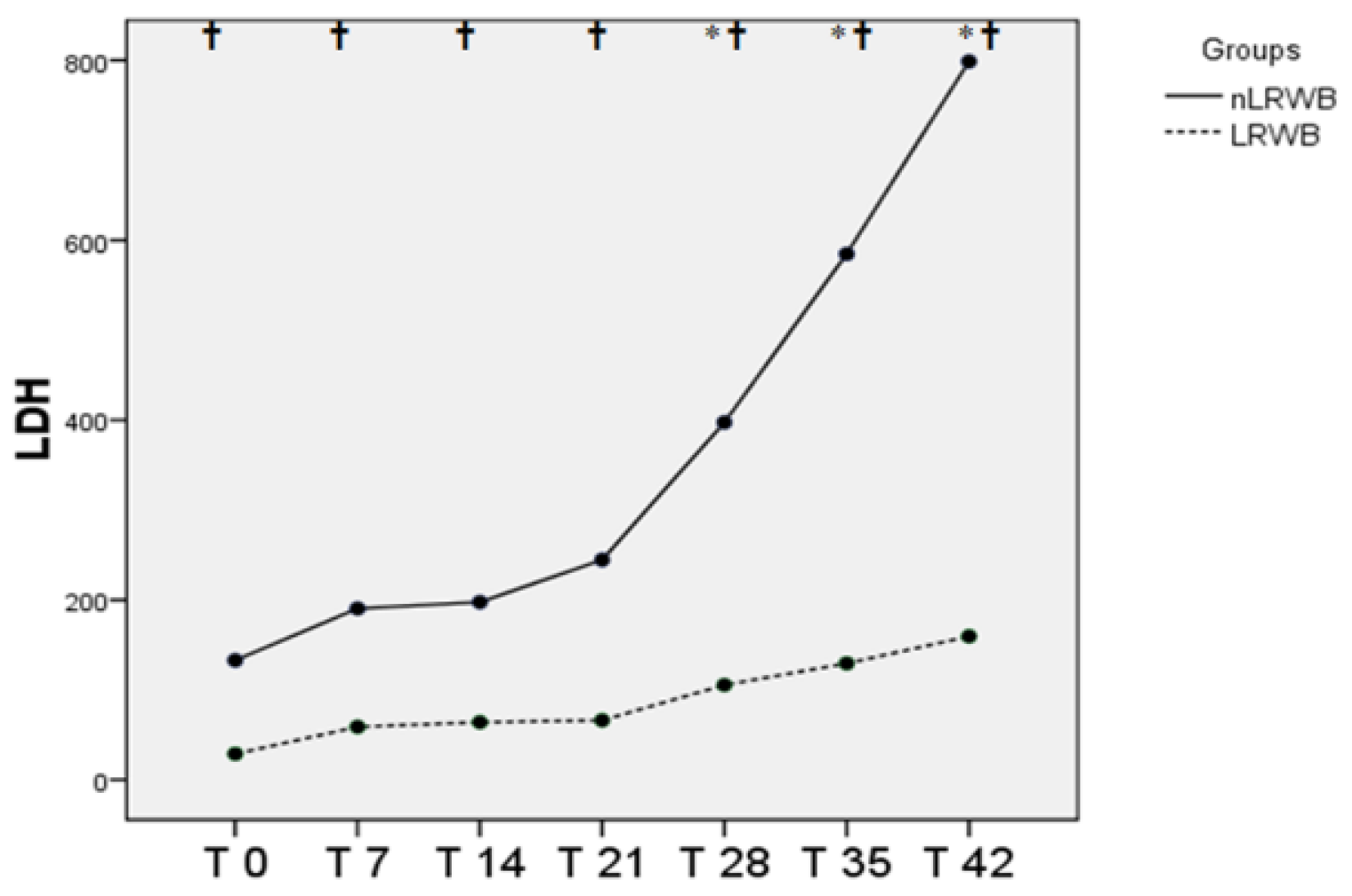
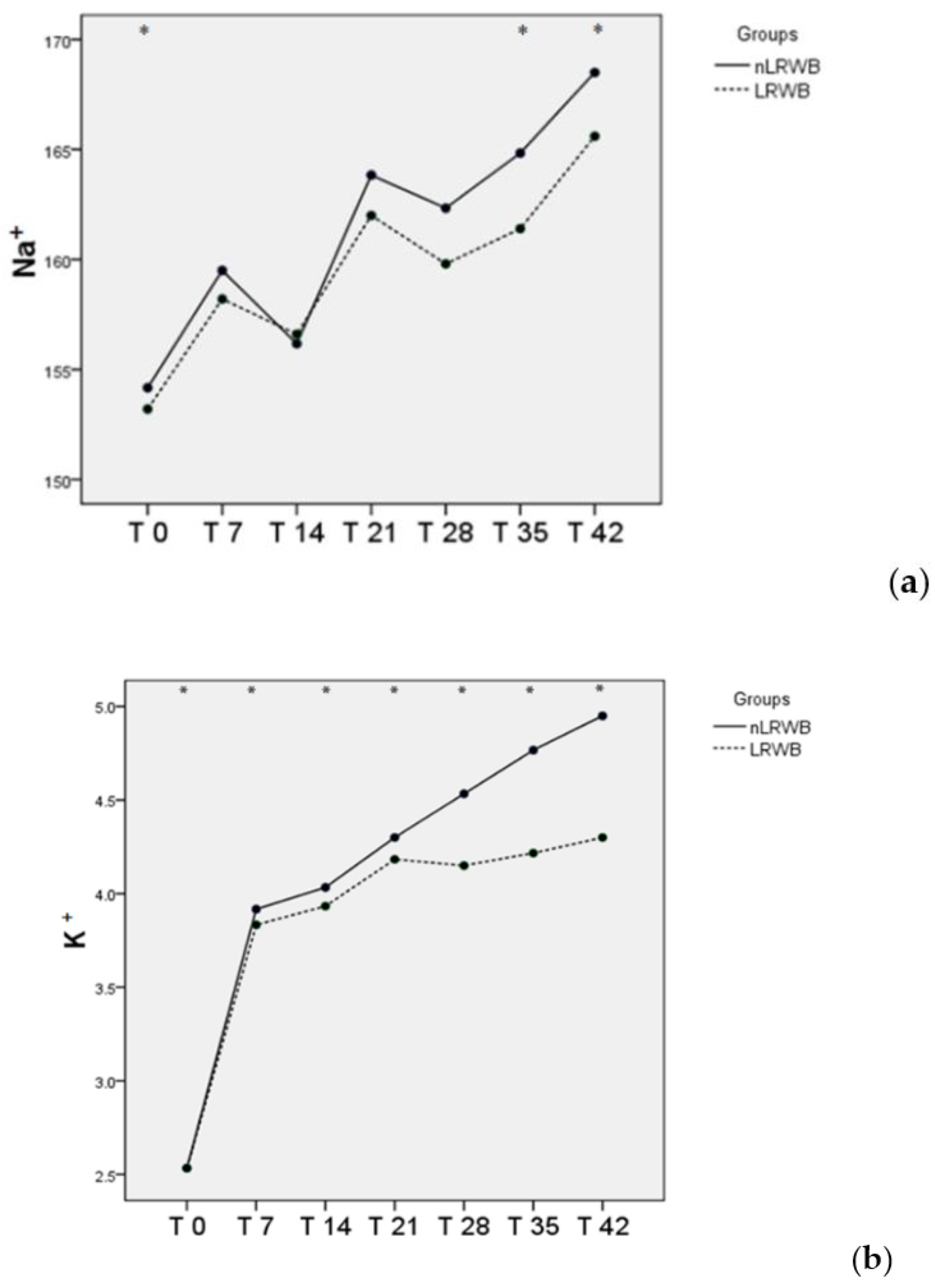
3.3. LDH and Electrolytes
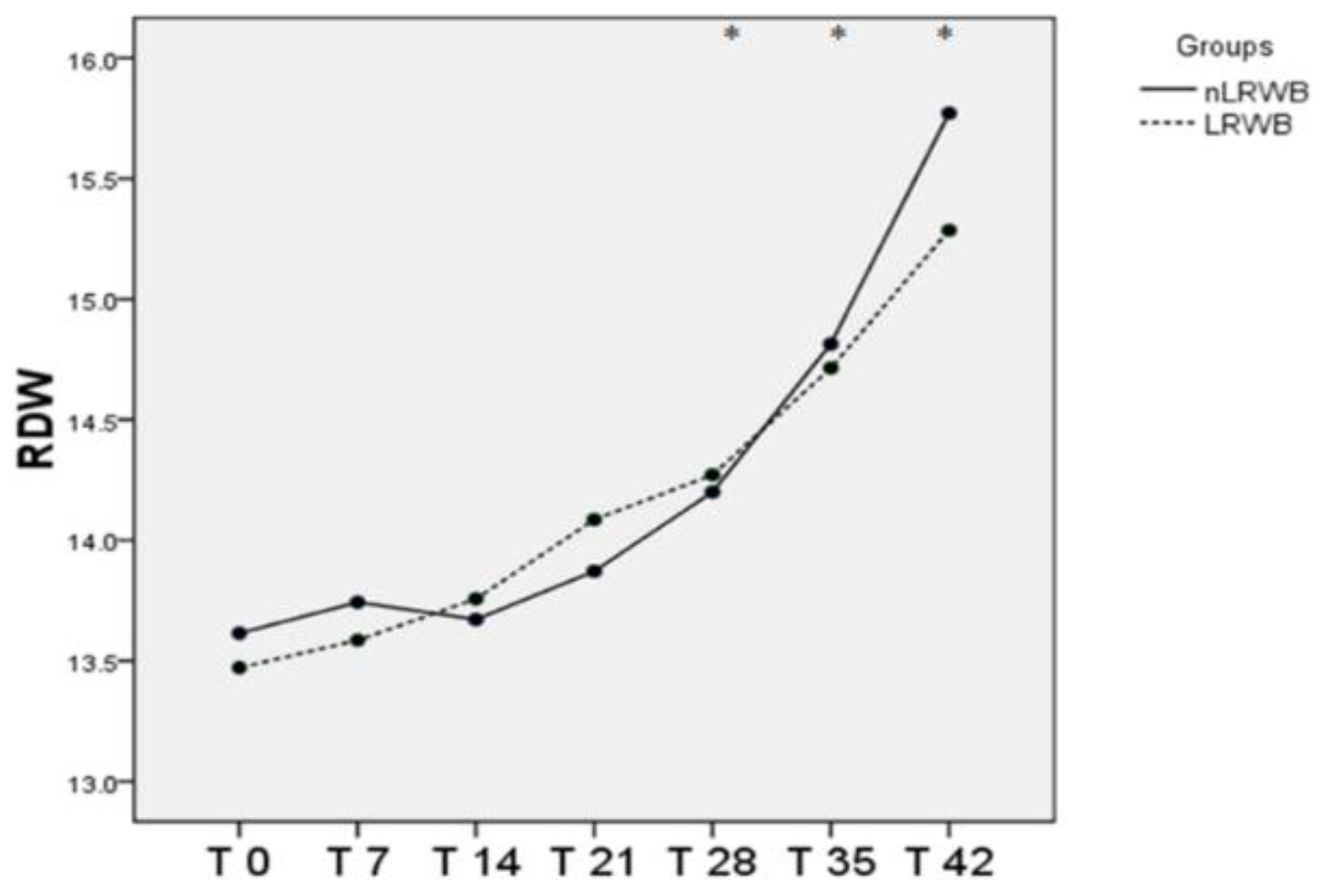
3.4. Blood Smear Evaluation and Morphological Index
3.5. Basal and Storage Hemolysis
3.6. Bacterial Culture
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Nogueira, D.; Rocha, S.; Abreu, E.; Costa, E.; Santos-Silva, A. Biochemical and Cellular Changes in Leukocyte-Depleted Red Blood Cells Stored for Transfusion. Transfus. Med. Hemother. 2015, 42, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.R.F.; Graça, R.M.C.; Cardoso, I.M.; Gopegui, R.R.; de Matos, A.J.F. In Vitro Hemolysis of Stored Units of Canine Packed Red Blood Cells. J. Vet. Emerg. Crit. Care 2018, 28, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Blood Transfusion Guide—EDQM Publications|EDQM—European Directorate for the Quality of Medicines. Available online: https://www.edqm.eu/en/blood-guide (accessed on 1 February 2021).
- Obrador, R.; Musulin, S.; Hansen, B. Red Blood Cell Storage Lesion. J. Vet. Emerg. Crit. Care 2015, 25, 187–199. [Google Scholar] [CrossRef]
- Lacerda, L.A.; Hlavac, N.R.C.; Terra, S.R.; Back, F.P.; Jane Wardrop, K.; González, F.H.D. Effects of Four Additive Solutions on Canine Leukoreduced Red Cell Concentrate Quality during Storage. Vet. Clin. Pathol. 2014, 43, 362–370. [Google Scholar] [CrossRef]
- Antwi-Baffour, S.; Adjei, J.K.; Tsyawo, F.; Kyeremeh, R.; Botchway, F.A.; Seidu, M.A. A Study of the Change in Sodium and Potassium Ion Concentrations in Stored Donor Blood and Their Effect on Electrolyte Balance of Recipients. Biomed Res. Int. 2019, 2019, 8162975. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, R.; Katharia, R. Oxidative Injury as Contributory Factor for Red Cells Storage Lesion during Twenty Eight Days of Storage. Blood Transfus. 2012, 10, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Opoku-Okrah, C.; Acquah, B.K.S.; Dogbe, E.E. Changes in Potassium and Sodium Concentrations in Stored Blood. Pan. Afr. Med. J. 2015, 20, 236. [Google Scholar] [CrossRef]
- Kim, Y.; Abplanalp, W.A.; Jung, A.D.; Schuster, R.M.; Lentsch, A.B.; Gulbins, E.; Caldwell, C.C.; Pritts, T.A. Endocytosis of Red Blood Cell Microparticles by Pulmonary Endothelial Cells Is Mediated by Rab5. Shock 2018, 49, 288–294. [Google Scholar] [CrossRef]
- Kim, Y.; Xia, B.T.; Jung, A.D.; Chang, A.L.; Abplanalp, W.A.; Caldwell, C.C.; Goodman, M.D.; Pritts, T.A. Microparticles from Stored Red Blood Cells Promote a Hypercoagulable State in a Murine Model of Transfusion. Surgery 2018, 163, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Purcell, S.L.; Claus, M.; Hosgood, G.; Smart, L. Effect of Leukoreduction on Concentrations of Interleukin-8, Interleukin-1β, and Tumor Necrosis Factor-α in Canine Packed Red Blood Cells during Storage. Am. J. Vet. Res. 2015, 76, 969–974. [Google Scholar] [CrossRef]
- Corsi, R.; McMichael, M.A.; Smith, S.A.; O’Brien, M.; Herring, J.; Ngwenyama, T.R.; Galligan, A.; Beloshapka, A.N.; Deng, P.; Swanson, K.S. Cytokine Concentration in Stored Canine Erythrocyte Concentrates. J. Vet. Emerg. Crit. Care 2014, 24, 259–263. [Google Scholar] [CrossRef]
- Graf, C.; Raila, J.; Schweigert, F.J.; Kohn, B. Effect of Leukoreduction Treatment on Vascular Endothelial Growth Factor Concentration in Stored Canine Blood Transfusion Products. Am. J. Vet. Res. 2012, 73, 2001–2006. [Google Scholar] [CrossRef]
- Smith, S.A.; Ngwenyama, T.R.; O’Brien, M.; Herring, J.M.; Corsi, R.; Galligan, A.; Beloshapka, A.N.; Deng, P.; Swanson, K.S.; McMichael, M. Procoagulant Phospholipid Concentration in Canine Erythrocyte Concentrates Stored with or without Prestorage Leukoreduction. Am. J. Vet. Res. 2015, 76, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Refaai, M.A.; Conley, G.W.; Hudson, C.A.; Spinelli, S.L.; Phipps, R.P.; Morrell, C.N.; Blumberg, N.; McRae, H.L. Evaluation of the Procoagulant Properties of a Newly Developed Platelet Modified Lysate Product. Transfusion 2020, 60, 1579–1589. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Sun, J.; Solomon, S.B.; Klein, H.G.; Natanson, C. Transfusion of Older Stored Blood and Risk of Death: A Meta-Analysis. Transfusion 2012, 52, 1184–1195. [Google Scholar] [CrossRef] [PubMed]
- Solomon, S.B.; Wang, D.; Sun, J.; Kanias, T.; Feng, J.; Helms, C.C.; Solomon, M.A.; Alimchandani, M.; Quezado, M.; Gladwin, M.T.; et al. Mortality Increases after Massive Exchange Transfusion with Older Stored Blood in Canines with Experimental Pneumonia. Blood 2013, 121, 1663–1672. [Google Scholar] [CrossRef]
- Hann, L.; Brown, D.C.; King, L.G.; Callan, M.B. Effect of Duration of Packed Red Blood Cell Storage on Morbidity and Mortality in Dogs after Transfusion: 3,095 Cases (2001–2010). J. Vet. Intern. Med. 2014, 28, 1830–1837. [Google Scholar] [CrossRef]
- Weinberg, J.A.; McGwin, G.; Vandromme, M.J.; Marques, M.B.; Melton, S.M.; Reiff, D.A.; Kerby, J.D.; Rue, L.W. Duration of Red Cell Storage Influences Mortality after Trauma. J. Trauma 2010, 69, 1427–1431. [Google Scholar] [CrossRef]
- Andreasen, J.J.; Dethlefsen, C.; Modrau, I.S.; Baech, J.; Schonheyder, H.C.; Moeller, J.K.; Johnsen, S.P.; North-West Denmark Transfusion Study Group. Storage Time of Allogeneic Red Blood Cells Is Associated with Risk of Severe Postoperative Infection after Coronary Artery Bypass Grafting. Eur. J. Cardiothorac. Surg. 2011, 39, 329–334. [Google Scholar] [CrossRef]
- Yoshida, T.; Prudent, M.; D’alessandro, A. Red Blood Cell Storage Lesion: Causes and Potential Clinical Consequences. Blood Transfus. 2019, 17, 27–52. [Google Scholar] [CrossRef]
- Koch, C.G.; Li, L.; Sessler, D.I.; Figueroa, P.; Hoeltge, G.A.; Mihaljevic, T.; Blackstone, E.H. Duration of Red-Cell Storage and Complications after Cardiac Surgery. N. Engl. J. Med. 2008, 358, 1229–1239. [Google Scholar] [CrossRef]
- Spinella, P.C.; Carroll, C.L.; Staff, I.; Gross, R.; Mc Quay, J.; Keibel, L.; Wade, C.E.; Holcomb, J.B. Duration of Red Blood Cell Storage Is Associated with Increased Incidence of Deep Vein Thrombosis and in Hospital Mortality in Patients with Traumatic Injuries. Crit. Care 2009, 13, R151. [Google Scholar] [CrossRef]
- Klein, H.G. The Red Cell Storage Lesion(s): Of Dogs and Men. Blood Transfus. 2017, 15, 107–111. [Google Scholar] [CrossRef]
- Kisielewicz, C.; Self, I.A. Canine and Feline Blood Transfusions: Controversies and Recent Advances in Administration Practices. Vet. Anaesth. Analg. 2014, 41, 233–242. [Google Scholar] [CrossRef]
- Locke, R.; Paul, D.; Touch, S.; Mackley, A.; Maduskuie, V.; Fawcett, P. Cytokine Load in Prestorage Leukoreduced PRBC Transfusions in Premature Infants. J. Perinatol. 2005, 25, 526–530. [Google Scholar] [CrossRef]
- Tinmouth, A.; Fergusson, D.; Yee, I.C.; Hébert, P.C.; ABLE Investigators; Canadian Critical Care Trials Group. Clinical Consequences of Red Cell Storage in the Critically Ill. Transfusion 2006, 46, 2014–2027. [Google Scholar] [CrossRef] [PubMed]
- Luk, C.S.; Gray-Statchuk, L.A.; Cepinkas, G.; Chin-Yee, I.H. WBC Reduction Reduces Storage-Associated RBC Adhesion to Human Vascular Endothelial Cells under Conditions of Continuous Flow in Vitro. Transfusion 2003, 43, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Ergul Ekiz, E.; Arslan, M.; Akyazi, I.; Eraslan, E. The Effects of Prestorage Leukoreduction and Storage Duration on the in Vitro Quality of Canine Packed Red Blood Cells. Turk. J. Vet. Anim. Sci. 2012, 36, 711–717. [Google Scholar] [CrossRef]
- Stefanetti, V.; Miglio, A.; Cappelli, K.; Capomaccio, S.; Sgariglia, E.; Marenzoni, M.L.; Antognoni, M.T.; Coletti, M.; Mangili, V.; Passamonti, F. Detection of Bacterial Contamination and DNA Quantification in Stored Blood Units in 2 Veterinary Hospital Blood Banks. Vet. Clin. Pathol. 2016, 45, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Miglio, A.; Stefanetti, V.; Antognoni, M.T.; Cappelli, K.; Capomaccio, S.; Coletti, M.; Passamonti, F. Stored Canine Whole Blood Units: What Is the Real Risk of Bacterial Contamination? J. Vet. Intern. Med. 2016, 30, 1830–1837. [Google Scholar] [CrossRef]
- Mangiaterra, S.; Rossi, G.; Antognoni, M.T.; Cerquetella, M.; Marchegiani, A.; Miglio, A.; Gavazza, A. Canine Blood Group Prevalence and Geographical Distribution around the World: An Updated Systematic Review. Animals 2021, 11, 342. [Google Scholar] [CrossRef] [PubMed]
- Marenzoni, M.L.; Antognoni, M.T.; Baldelli, F.; Miglio, A.; Stefanetti, V.; Desario, C.; Di Summa, A.; Buonavoglia, C.; Decaro, N. Detection of Parvovirus and Herpesvirus DNA in the Blood of Feline and Canine Blood Donors. Vet. Microbiol. 2018, 224, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Miglio, A.; Gavazza, A.; Siepi, D.; Bagaglia, F.; Misia, A.; Antognoni, M.T. Hematological and Biochemical Reference Intervals for 5 Adult Hunting Dog Breeds Using a Blood Donor Database. Animals 2020, 10, 1212. [Google Scholar] [CrossRef]
- Antognoni, M.T.; Veronesi, F.; Morganti, G.; Mangili, V.; Fruganti, G.; Miglio, A. Natural Infection of Anaplasma Platys in Dogs from Umbria Region (Central Italy). Vet. Ital. 2014, 50, 49–56. [Google Scholar] [CrossRef]
- Tommaso, M.D.; Miglio, A.; Crisi, P.E.; Boari, A.; Rocconi, F.; Antognoni, M.T.; Luciani, A. Frequency of Blood Types A, B and AB in a Population of Non-Pedigree Domestic Cats from Central Italy. Animals 2020, 10, 1937. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, D.M.W. Veterinary Hematology: A Diagnostic Guide and Color Atlas John W. Harvey Saunders/Elsevier, St. Louis, MO, ISBN: 978-1-4377-0173-9, Softcover, 384 Pages, 2012, $79.95 (USD). Vet. Clin. Pathol. 2012, 41, 607. [Google Scholar] [CrossRef]
- Harvey, J.W. Veterinary Hematology: A Diagnostic Guide and Color Atlas; Elsevier Health Sciences: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Harboe, M. A Method for Determination of Hemoglobin in Plasma by Near-Ultraviolet Spectrophotometry. Scand. J. Clin. Lab. Investig. 1959, 11, 66–70. [Google Scholar] [CrossRef]
- Han, V.; Serrano, K.; Devine, D.V. A Comparative Study of Common Techniques Used to Measure Haemolysis in Stored Red Cell Concentrates. Vox Sang. 2010, 98, 116–123. [Google Scholar] [CrossRef]
- Sonker, A.; Dubey, A.; Chaudhary, R. Evaluation of a Red Cell Leukofilter Performance and Effect of Buffy Coat Removal on Filtration Efficiency and Post Filtration Storage. Indian J. Hematol. Blood Transfus. 2014, 30, 321–327. [Google Scholar] [CrossRef][Green Version]
- Blajchman, M.; Bardossy, L.; Carmen, R.; Goldman, M.; Heddle, N.; Singal, D. An Animal Model of Allogeneic Donor Platelet Refractoriness: The Effect of the Time of Leukodepletion. Blood 1992, 79, 1371–1375. [Google Scholar] [CrossRef]
- Ramos, R.R.; Curtis, B.R.; Duffy, B.F.; Chaplin, H. Low Retention of White Cell Fragments by Polyester Fiber White Cell-Reduction Platelet Filters. Transfusion 1994, 34, 31–34. [Google Scholar] [CrossRef]
- Stack, G.; Snyder, E.L. Cytokine Generation in Stored Platelet Concentrates. Transfusion 1994, 34, 20–25. [Google Scholar] [CrossRef]
- Heddle, N.M.; Klama, L.; Singer, J.; Richards, C.; Fedak, P.; Walker, I.; Kelton, J.G. The Role of the Plasma from Platelet Concentrates in Transfusion Reactions. N. Engl. J. Med. 1994, 331, 625–628. [Google Scholar] [CrossRef]
- Brecher, M.E.; Pineda, A.A.; Torloni, A.S.; Harbaugh, C.A.; Emery, R.L.; Moore, S.B.; Carmen, R.; Nelson, E. Prestorage Leukocyte Depletion: Effect on Leukocyte and Platelet Metabolites, Erythrocyte Lysis, Metabolism, and in Vivo Survival. Semin. Hematol. 1991, 28, 3–9. [Google Scholar] [PubMed]
- 21 CFR 640—Additional Standards for Human Blood and Blood Products—Content Details—CFR-2016-Title21-Vol7-Part640. Available online: https://www.govinfo.gov%2Fapp%2Fdetails%2FCFR-2016-title21-vol7%2FCFR-2016-title21-vol7-part640 (accessed on 9 February 2021).
- Stefani, A.; Capello, K.; Carminato, A.; Wurzburger, W.; Furlanello, T.; Bertazzo, V.; Marsilio, E.; Albertin, E.; Pietra, G.L.; Bozzato, E.; et al. Effects of Leukoreduction on Storage Lesions in Whole Blood and Blood Components of Dogs. J. Vet. Intern. Med. 2021. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, L.; Wardrop, K.J.; Sellon, R.K.; Meyers, K.M. Use of a Prestorage Leukoreduction Filter Effectively Removes Leukocytes from Canine Whole Blood While Preserving Red Blood Cell Viability. J. Vet. Intern. Med. 2000, 14, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, I.; Al Marwani, A.; Mamdouh Nasr, K.; Abdulla Kano, N.; Hadwan, T. Time Dependent Assessment of Morphological Changes: Leukodepleted Packed Red Blood Cells Stored in SAGM. Biomed Res. Int. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Sollberger, T.; Walter, R.; Brand, B.; Contesse, J.; Meredith, D.O.; Reinhart, W.H. Influence of Prestorage Leucocyte Depletion and Storage Time on Rheologic Properties of Erythrocyte Concentrates. Vox Sang. 2002, 82, 191–197. [Google Scholar] [CrossRef]
- Carson, J.L.; Guyatt, G.; Heddle, N.M.; Grossman, B.J.; Cohn, C.S.; Fung, M.K.; Gernsheimer, T.; Holcomb, J.B.; Kaplan, L.J.; Katz, L.M.; et al. Clinical Practice Guidelines From the AABB: Red Blood Cell Transfusion Thresholds and Storage. JAMA 2016, 316, 2025. [Google Scholar] [CrossRef]
- CFR—Code of Federal Regulations Title 21. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?cfrpart=640 (accessed on 20 July 2020).
- Circular of Information for the Use of Human Blood and Blood Components. Available online: https://www.aabb.org/docs/default-source/default-document-library/resources/circular-of-information-10-17.pdf (accessed on 1 February 2021).
- Jaeger, B.; Reems, M. Visual Inspection of Stored Canine Blood for Hemolysis Compared with Measured Plasma-Free Hemoglobin to Assess Suitability for Transfusion. Can. Vet. J. 2018, 59, 1171–1174. [Google Scholar] [PubMed]
- Shastry, S.; Shivhare, A.; Murugesan, M.; Baliga, P.B. Red Cell Storage Lesion and the Effect of Buffy-Coat Reduction on the Biochemical Parameters. Transfus. Apher. Sci. 2019, 58, 179–182. [Google Scholar] [CrossRef]
- Antonelou, M.H.; Tzounakas, V.L.; Velentzas, A.D.; Stamoulis, K.E.; Kriebardis, A.G.; Papassideri, I.S. Effects of Pre-Storage Leukoreduction on Stored Red Blood Cells Signaling: A Time-Course Evaluation from Shape to Proteome. J. Proteom. 2012, 76, 220–238. [Google Scholar] [CrossRef]
- Hess, J.R.; Sparrow, R.L.; van der Meer, P.F.; Acker, J.P.; Cardigan, R.A.; Devine, D.V. Red Blood Cell Hemolysis during Blood Bank Storage: Using National Quality Management Data to Answer Basic Scientific Questions. Transfusion 2009, 49, 2599–2603. [Google Scholar] [CrossRef]
- Tzounakas, V.L.; Georgatzakou, H.T.; Kriebardis, A.G.; Voulgaridou, A.I.; Stamoulis, K.E.; Foudoulaki-Paparizos, L.E.; Antonelou, M.H.; Papassideri, I.S. Donor Variation Effect on Red Blood Cell Storage Lesion: A Multivariable, yet Consistent, Story. Transfusion 2016, 56, 1274–1286. [Google Scholar] [CrossRef]
- Tzounakas, V.L.; Kriebardis, A.G.; Papassideri, I.S.; Antonelou, M.H. Donor-Variation Effect on Red Blood Cell Storage Lesion: A Close Relationship Emerges. Proteom. Clin. Appl. 2016, 10, 791–804. [Google Scholar] [CrossRef] [PubMed]
- Koseoglu, M.; Hur, A.; Atay, A.; Cuhadar, S. Effects of Hemolysis Interferences on Routine Biochemistry Parameters. Biochem. Med. 2011, 21, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Ghezelbash, B.; Azarkeivan, A.; Pourfathollah, A.A.; Deyhim, M.; Hajati, E.; Goodarzi, A. Comparative Evaluation of Biochemical and Hematological Parameters of Pre-Storage Leukoreduction during RBC Storage. Int. J. Hematol. Oncol. Stem. Cell Res. 2018, 12, 35–42. [Google Scholar] [PubMed]
- Rapoport, I.; Rapoport, T.A.; Rapoport, S.M. Analysis of PH-Induced Changes of the Glycolysis of Human Erythrocytes. Acta Biol. Med. Ger. 1978, 37, 393–401. [Google Scholar]
- Li, Y.; Xiong, Y.; Wang, R.; Tang, F.; Wang, X. Blood Banking-Induced Alteration of Red Blood Cell Oxygen Release Ability. Blood Transfus. 2016, 14, 238–244. [Google Scholar] [CrossRef]
- Wilson, C.R.; Pashmakova, M.B.; Heinz, J.A.; Johnson, M.C.; Minard, H.M.; Bishop, M.A.; Barr, J.W. Biochemical Evaluation of Storage Lesion in Canine Packed Erythrocytes. J. Small Anim. Pract. 2017, 58, 678–684. [Google Scholar] [CrossRef]
- Castranova, V.; Weise, M.J.; Hoffman, J.F. Anion Transport in Dog, Cat, and Human Red Cells. Effects of Varying Cell Volume and Donnan Ratio. J. Gen. Physiol. 1979, 74, 319–334. [Google Scholar] [CrossRef]
- Hall, T.L.; Barnes, A.; Miller, J.R.; Bethencourt, D.M.; Nestor, L. Neonatal Mortality Following Transfusion of Red Cells with High Plasma Potassium Levels. Transfusion 1993, 33, 606–609. [Google Scholar] [CrossRef]
- Fujise, H.; Higa, K.; Nakayama, T.; Wada, K.; Ochiai, H.; Tanabe, Y. Incidence of Dogs Possessing Red Blood Cells with High K in Japan and East Asia. J. Vet. Med. Sci. 1997, 59, 495–497. [Google Scholar] [CrossRef]
- Kamel, N.; Goubran, F.; Ramsis, N.; Ahmed, A.S. Effects of storage time and leucocyte burden of packed and buffy-coat depleted red blood cell units on red cell storage lesion. Blood Transfus. 2010, 8, 260–266. [Google Scholar] [CrossRef] [PubMed]
- AlMoshary, M.; Mussaed, E.A.; Arab-Din, M. Biochemical Profile Changes in Stored Donor Blood for Transfusion. Pak. J. Med. Sci. 2019, 35, 1697–1700. [Google Scholar] [CrossRef]
- Gillissen, G. Demonstration of LDH activity as an expression of damage in leukocytes. Z. Med. Mikrobiol. Immunol. 1966, 152, 134–139. [Google Scholar] [CrossRef]
- De Simone, C.; Ferrari, M.; Sorice, F. LDH Isoenzyme Distribution in Human Eosinophils. Int. Arch. Allergy Appl. Immunol. 1983, 71, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Plum, J.R.; De Smedt, M.; Sabbe, L.J.; De Roose, J.E. LDH Analysis of Human Thymocytes and Thymocyte Subsets. J. Immunol. 1984, 132, 730–734. [Google Scholar]
- Högman, C.F.; Hedlund, K.; Akerblom, O.; Venge, P. Red Blood Cell Preservation in Protein-Poor Media: I. Leukocyte Enzymes as a Cause of Hemolysis. Transfusion 1978, 18, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Hertfelder, H.J.; Süwer, V.; Popov-Cenić, S.; Tschesche, H.; Hanfland, P. Leukocyte Proteinase Release during Storage of Red Cell Concentrates. Eur. J. Clin. Chem. Clin. Biochem. 1994, 32, 441–447. [Google Scholar] [CrossRef][Green Version]
- Mohandas, N.; Chasis, J.A. Red Blood Cell Deformability, Membrane Material Properties and Shape: Regulation by Transmembrane, Skeletal and Cytosolic Proteins and Lipids. Semin. Hematol. 1993, 30, 171–192. [Google Scholar] [PubMed]




| Parameters | Groups | Day of Donation | p for Repeated Measurements | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| T0 Day 0 | T1 Day 7 | T2 Day 14 | T3 Day 21 | T4 Day 28 | T5 Day 35 | T6 Day 42 | Among Times | Among Groups | ||
| RBC (×1012/L) | nLRWB | 5.23 ± 0.422 | 5.05 ± 0.543 | 5.04 ± 0.552 | 5.15 ± 0.652 | 5.09 ± 0.656 | 5.11 ± 0.664 | 5.17 ± 0.676 | 0.85 | 0.97 |
| LRWB | 5.18 ± 0.38 | 5.23 ± 0.36 | 5.16 ± 0.43 | 5.27 ± 0.426 | 5.19 ± 0.445 | 5.19 ± 0.474 | 5.32 ± 0.524 | |||
| HCT (%) | nLRWB | 34.5 ± 4.1 * | 30.9 ± 4.3 | 30.5 ±4.2 * | 31.4 ± 4.7 | 31.7 ± 5.1 | 32.4 ± 5.2 | 33.6 ± 5.3 * | <0.01 (p = 0.004) | 0.96 |
| LRWB | 34.0 ± 3.8 * | 31.6 ± 2.8 | 30.8 ± 3.3 * | 31.6 ± 3.2 | 31.4 ± 3.5 | 31.8 ± 3.7 | 32.9 ± 3.8 * | |||
| Hb (g/dL) | nLRWB | 12.5 ± 1.8 | 12.3 ± 2.1 | 12.3 ± 2.1 | 12.2 ± 2.2 | 12.2 ± 2.1 | 12.3 ± 2.1 | 12.3 ± 2.2 | 0.29 | 0.87 |
| LRWB | 12.3 ± 1.7 | 12.5 ± 1.4 | 12.5 ± 1.5 | 12.5 ± 1.4 | 12.6 ± 1.4 | 12.5 ± 1.4 | 12.5 ± 1.5 | |||
| MCV (fL) | nLRWB | 65.7 ± 4.3 * | 61.1 ± 4.2 * | 60.4 ± 4.5 * | 60.9 ± 4.4 * | 62.17 ± 4.9 * | 63.3 ± 4.7 * | 64.8 ± 5.0 * | <0.001 | 0.06 |
| LRWB | 65.59 ± 4.31 * | 60.41 ± 4.38 * | 59.70 ± 4.60 * | 59.97 ± 4.57 * | 60.57 ± 4.88 * | 61.33 ± 4.88 * | 61.94 ± 5.23 * | |||
| RDW (%) | nLRWB | 13.6 ± 0.7 | 13.74 ± 0.7 | 13.67 ± 0.7 | 13.87 ± 0.7 | 14.20 ± 1.2 * | 14.81 ± 1.5 * | 15.77 ± 1.8 * | 0.02 | 0.89 |
| LRWB | 13.47 ± 0.76 | 13.58 ± 0.7 | 13.76 ± 0.8 | 14.09 ± 0.9 | 14.27 ± 1.1 * | 14.71 ± 1.3 * | 15.29 ± 1.8 * | |||
| WBC (×109/L) | nLRWB | 8.87 ± 1.31 | 9.25 ± 1.77 | 9.29 ± 1.60 | 9.14 ± 1.79 | 9.22 ± 1.77 | 9.12 ± 1.90 | 8.57 ± 1.80 | 0.07 | <0.001 |
| LRWB | 0.027 ± 0.06 | 0.002 ± 0.00 | 0.00 ± 0.01 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.01 | |||
| PLT (×109/L) | nLRWB | 227.7 ± 97.9 | 209.0 ± 95.1 | 175.7 ± 90.2 | 186.4 ± 83.5 | 176.2 ± 80.3 | 165.5 ± 76.9 | 191.2 ± 77.9 | 0.08 | <0.001 |
| LRWB | 11.5 ± 19.6 | 4.1 ± 6.3 | 5.0 ± 8.4 | 6.8 ± 8.6 | 9.8 ± 9.8 | 12.2 ± 10.7 | 16.7 ± 11.5 | |||
| Parameters | Type of Units | Day of Donation | p for Repeated Measurements | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| T0 Day of Donation | T1 Day 7 | T2 Day 14 | T3 Day 21 | T4 Day 28 | T5 Day 35 | T6 Day 42 | Among Different Times | Between Groups (nLRWB vs. LRWB) | ||
| Na+ (mEq/L) | nLRWB | 154.0 ± 1.00 * | 159.20 ± 0.84 | 158.40 ± 7.70 | 164.80 ± 5.45 | 163.00 ± 2.35 | 166.00 ± 3.16 * | 169.20 ± 3.56 * | <0.001 | 0.37 |
| LRWB | 153.2 ± 2.4 * | 158.2 ± 1.3 | 156.6 ± 6.1 | 162.0 ± 4.1 | 159.8 ± 2.7 | 161.4 ± 3.2 * | 165.6 ± 3.4 * | |||
| K+ (mEq/L) | nLRWB | 2.5 ± 0.2 * | 3.9 ± 0.3 * | 4.1 ± 0.5 * | 4.3 ± 0.4 * | 4.6 ± 0.4 * | 4.8 ± 0.4 * | 5.0 ± 0.5 * | <0.001 | 0.21 |
| LRWB | 2.5 ± 0.2 * | 3.8 ± 0.2 * | 4.0 ± 0.3 * | 4.2 ± 0.3 | 4.2 ± 0.4 | 4.2 ± 0.4 * | 4.3 ± 0.4 * | |||
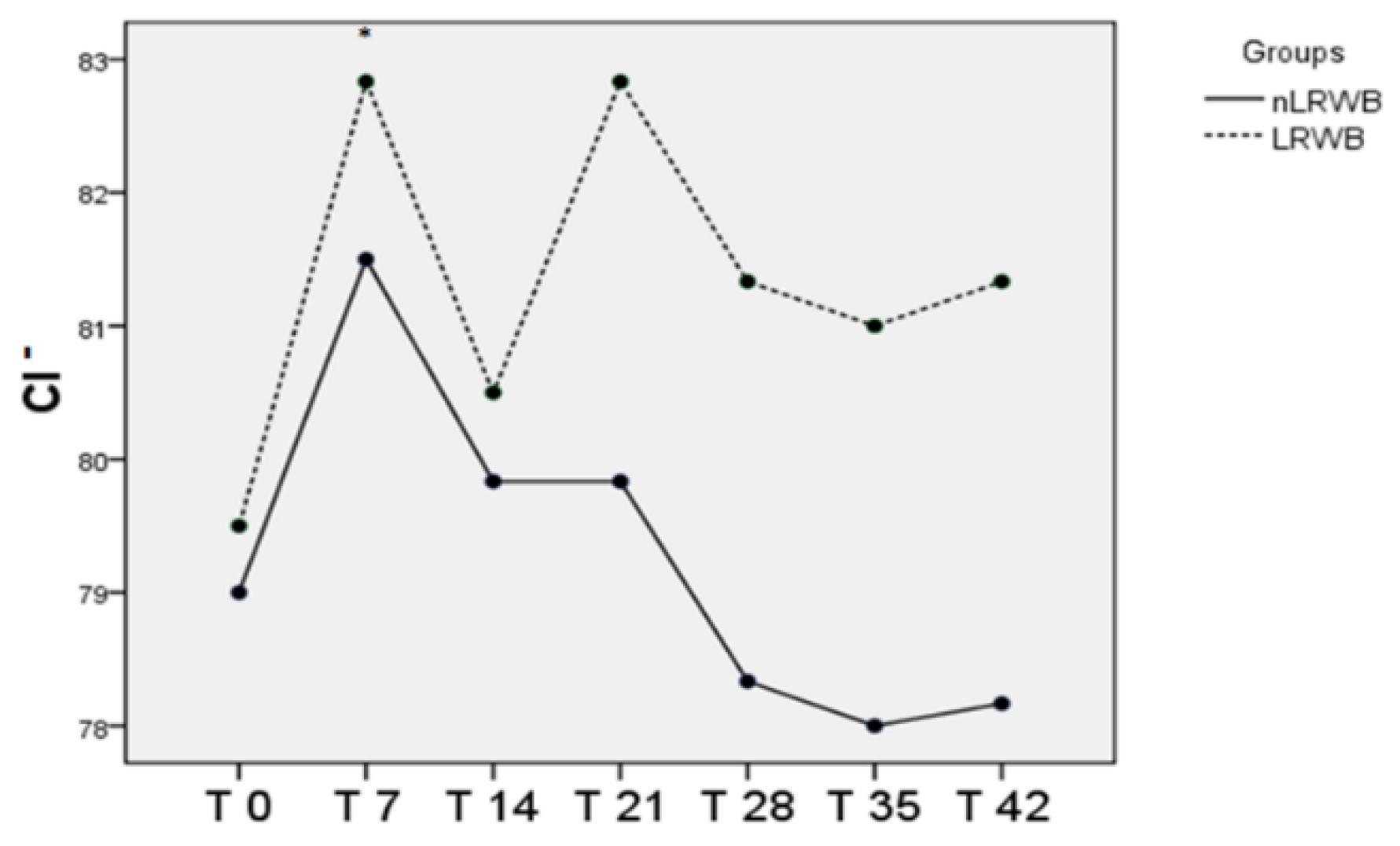
| Cl− (mEq/L) | nLRWB | 78.0 ± 4.1 | 80.8 ± 3.9 * | 79.6 ± 2.5 | 79.2 ± 3.2 | 77.4 ± 2.7 | 77.2 ± 3.2 | 77.6 ± 3.1 | <0.01 | 0.75 |
| LRWB | 79.4 ± 5.1 | 81.8 ± 4.6 * | 80.4 ± 2.8 | 82.2 ± 5.4 | 80.4 ± 3.5 | 80.0 ± 4.3 | 80.6 ± 4.0 | |||
| LDH (U/L) | nLRWB | 237.4 ± 255.6 | 217.2 ± 76.6 | 201.8 ± 67.2 | 258.2 ± 62.8 | 374.0 ± 153.2 * | 612.4 ± 173.5 * | 808.6 ± 108.7 * | <0.001 | <0.001 |
| LRWB | 27.0 ± 15.3 | 63.4 ± 17.8 | 61.2 ± 35.0 | 69.4 ± 26.7 | 111.6 ± 30.9 * | 129.4 ± 39.5 * | 162.2 ± 61.3 * | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antognoni, M.T.; Marenzoni, M.L.; Misia, A.L.; Avellini, L.; Chiaradia, E.; Gavazza, A.; Miglio, A. Effect of Leukoreduction on Hematobiochemical Parameters and Storage Hemolysis in Canine Whole Blood Units. Animals 2021, 11, 925. https://doi.org/10.3390/ani11040925
Antognoni MT, Marenzoni ML, Misia AL, Avellini L, Chiaradia E, Gavazza A, Miglio A. Effect of Leukoreduction on Hematobiochemical Parameters and Storage Hemolysis in Canine Whole Blood Units. Animals. 2021; 11(4):925. https://doi.org/10.3390/ani11040925
Chicago/Turabian StyleAntognoni, Maria Teresa, Maria Luisa Marenzoni, Ambra Lisa Misia, Luca Avellini, Elisabetta Chiaradia, Alessandra Gavazza, and Arianna Miglio. 2021. "Effect of Leukoreduction on Hematobiochemical Parameters and Storage Hemolysis in Canine Whole Blood Units" Animals 11, no. 4: 925. https://doi.org/10.3390/ani11040925
APA StyleAntognoni, M. T., Marenzoni, M. L., Misia, A. L., Avellini, L., Chiaradia, E., Gavazza, A., & Miglio, A. (2021). Effect of Leukoreduction on Hematobiochemical Parameters and Storage Hemolysis in Canine Whole Blood Units. Animals, 11(4), 925. https://doi.org/10.3390/ani11040925







