Is There a Link between Suckling and Manipulation Behavior during Rearing in Pigs?
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Weight Gain and Results of Injury Evaluation
3.2. Development of Manipulative Behaviour during the Rearing Period
3.3. Dominance and Social Tension Index from the Suckling Period
3.4. Correlations between Traits
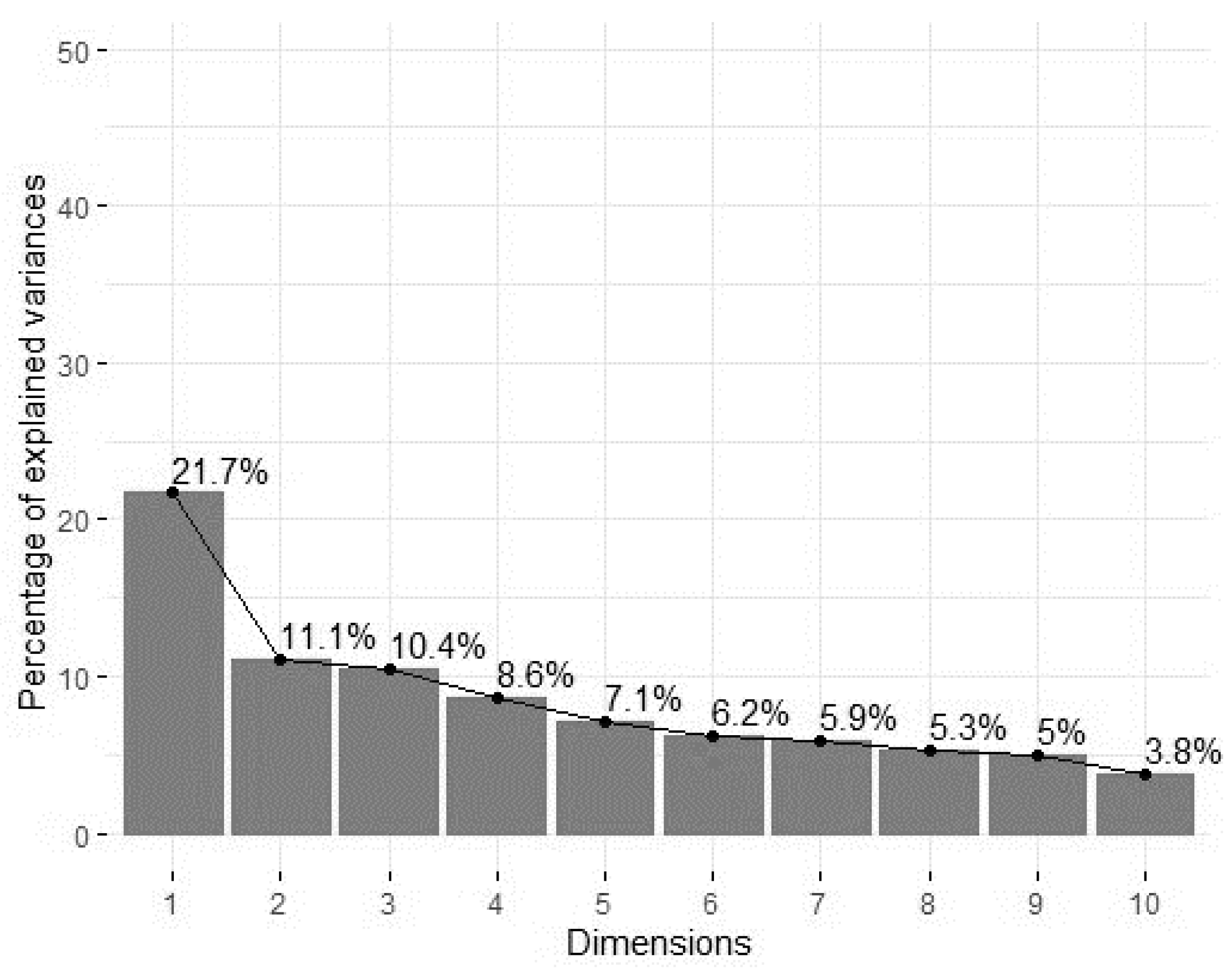
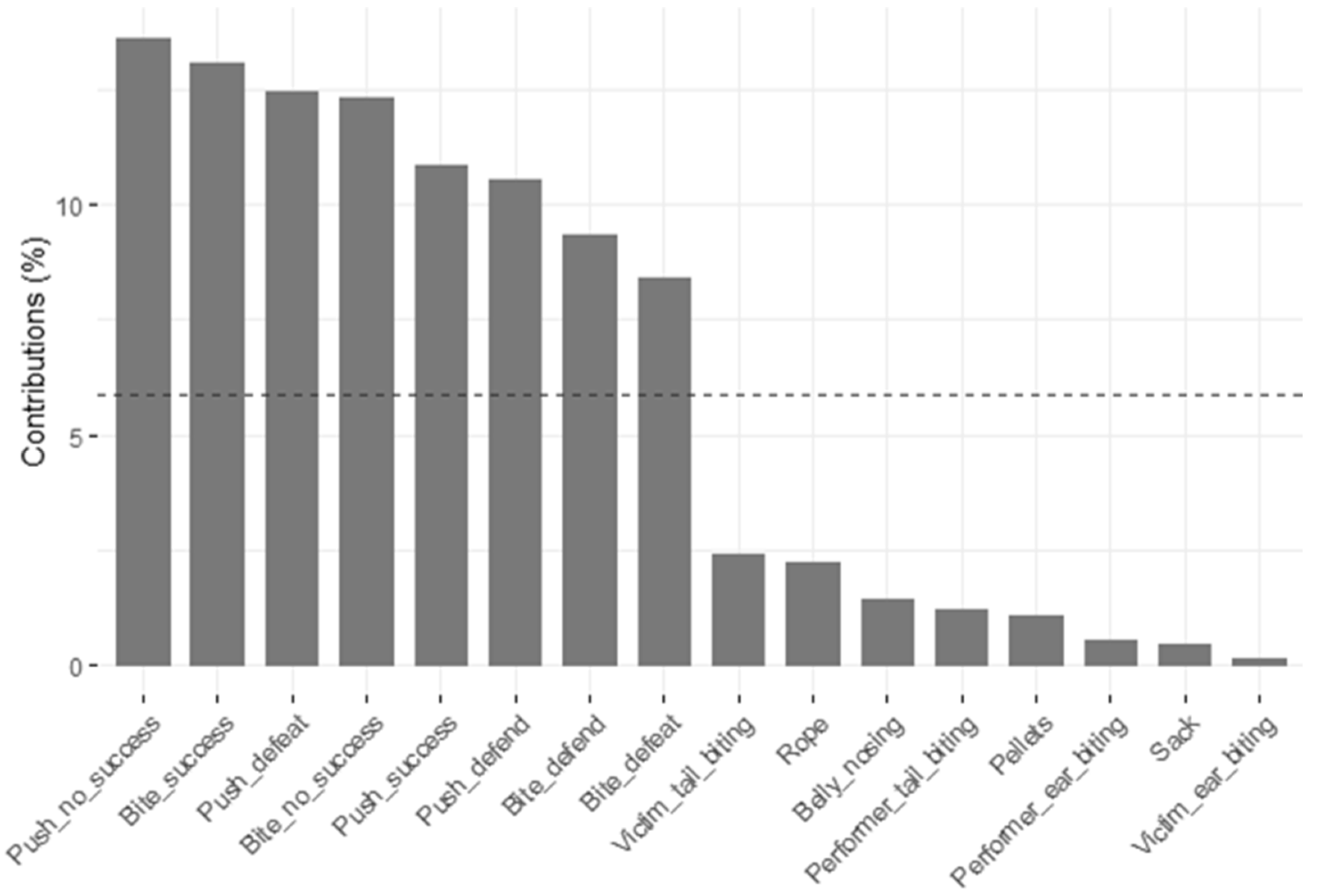
3.5. Principal Components and Clusters
4. Discussion
4.1. Manipulation Behaviors
4.2. Relationship between Suckling and Rearing Behaviors
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fraser, D. Attraction to blood as a factor in tail-biting by pigs. Appl. Anim. Behav. Sci. 1987, 17, 61–68. [Google Scholar] [CrossRef]
- Day, J.; Kyriazakis, I.; Lawrence, A. The effect of food deprivation on the expression of foraging and exploratory behaviour in the growing pig. Appl. Anim. Behav. Sci. 1995, 42, 193–206. [Google Scholar] [CrossRef]
- Camerlink, I.; Bijma, P.; Kemp, B.; Bolhuis, J.E. Relationship between growth rate and oral manipulation, social nosing, and aggression in finishing pigs. Appl. Anim. Behav. Sci. 2012, 142, 11–17. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). The Risks Associated with Tail Biting in Pigs and Possible Means to Reduce the Need for Tail Docking Considering the Different Housing and Husbandry Systems-Scientific Opinion of the Panel on Animal Health and Welfare. EFSA J. 2007, 5, 611. [Google Scholar] [CrossRef]
- Buijs, S.; Muns, R. A Review of the Effects of Non-Straw Enrichment on Tail Biting in Pigs. Animals 2019, 9, 824. [Google Scholar] [CrossRef]
- Kritas, S.K.; Morrison, R.B. Relationships between tail biting in pigs and disease lesions and condemnations at slaughter. Veter. Rec. 2007, 160, 149–152. [Google Scholar] [CrossRef]
- Taylor, N.R.; Main, D.C.; Mendl, M.; Edwards, S.A. Tail-biting: A new perspective. Veter. J. 2010, 186, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Van Putten, G. An Investigation into Tail-Biting among Fattening Pigs. Br. Veter. J. 1969, 125, 511–517. [Google Scholar] [CrossRef]
- D’Eath, R.B.; Arnott, G.; Turner, S.P.; Jensen, T.; Lahrmann, H.P.; Busch, M.E.; Niemi, J.K.; Lawrence, A.B.; Sandøe, P. Injurious tail biting in pigs: How can it be controlled in existing systems without tail docking? Animals 2014, 8, 1479–1497. [Google Scholar] [CrossRef] [PubMed]
- Zonderland, J.J.; Schepers, F.; Bracke, M.B.M.; Hartog, L.A.D.; Kemp, B.; Spoolder, H.A.M. Characteristics of biter and victim piglets apparent before a tail-biting outbreak. Animals 2011, 5, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Hoy, S.; Engel, D.; Jans-Wenstrup, I. Ethological investigations on the perpetrators and victims of tail biting in weaner pigs. Livest. Sci. 2020, 231, 103879. [Google Scholar] [CrossRef]
- Prunier, A.; Averos, X.; Dimitrov, I.; Edwards, S.A.; Hillmann, E.; Holinger, M.; Ilieski, V.; Leming, R.; Tallet, C.; Turner, S.P.; et al. Review: Early life predisposing factors for biting in pigs. Animals 2020, 14, 570–587. [Google Scholar] [CrossRef]
- Schrøder-Petersen, D.; Simonsen, H. Tail Biting in Pigs. Veter. J. 2001, 162, 196–210. [Google Scholar] [CrossRef]
- Widowski, T. Causes and Prevention of Tail Biting in Growing Pigs: A Review of Recent Research. In Proceedings of the 2nd London Swine Conference, London, ON, Canada, 11–12 April 2002; p. 47. [Google Scholar]
- Sonoda, L.T.; Fels, M.; Oczak, M.; Vranken, E.; Ismayilova, G.; Guarino, M.; Viazzi, S.; Bahr, C.; Berckmans, D.; Hartung, J. Tail biting in pigs-causes and management intervention strategies to reduce the behavioural disorder. A review. Berliner Münchener Tierärztliche Wochenschrift 2013, 126, 104–112. [Google Scholar]
- Beattie, V.E.; Breuer, K.; O’Connell, N.E.; Sneddon, I.A.; Mercer, J.T.; Rance, K.A.; Sutcliffe, M.E.M.; Edwards, S.A. Factors identifying pigs predisposed to tail biting. Anim. Sci. 2005, 80, 307–312. [Google Scholar] [CrossRef]
- Van De Weerd, H.A.; Docking, C.M.; Day, J.E.L.; Edwards, S.A. The development of harmful social behaviour in pigs with intact tails and different enrichment backgrounds in two housing systems. Anim. Sci. 2005, 80, 289–298. [Google Scholar] [CrossRef]
- Ursinus, W.W.; Wijnen, H.J.; Bartels, A.C.; Dijvesteijn, N.; Van Reenen, C.G.; Bolhuis, J.E. Damaging biting behaviors in intensively kept rearing gilts: The effect of jute sacks and relations with production characteristics. J. Anim. Sci. 2014, 92, 5193–5202. [Google Scholar] [CrossRef] [PubMed]
- Breuer, K.; Sutcliffe, M.; Mercer, J.; Rance, K.; O’Connell, N.; Sneddon, I.; Edwards, S. Heritability of clinical tail-biting and its relation to performance traits. Livest. Prod. Sci. 2005, 93, 87–94. [Google Scholar] [CrossRef]
- Munsterhjelm, C.; Simola, O.; Keeling, L.; Valros, A.; Heinonen, M. Health parameters in tail biters and bitten pigs in a case–control study. Animals 2013, 7, 814–821. [Google Scholar] [CrossRef]
- Van de Weerd, H.A.; Docking, C.M.; Day, J.E.; Avery, P.J.; Edwards, S.A. A systematic approach towards developing environmental enrichment for pigs. Appl. Anim. Behav. Sci. 2003, 84, 101–118. [Google Scholar] [CrossRef]
- Bracke, M.B.; Zonderland, J.J.; Lenskens, P.; Schouten, W.G.; Vermeer, H.; Spoolder, H.A.; Hendriks, H.J.; Hopster, H. Formalised review of environmental enrichment for pigs in relation to political decision making. Appl. Anim. Behav. Sci. 2006, 98, 165–182. [Google Scholar] [CrossRef]
- Studnitz, M.; Jensen, M.B.; Pedersen, L.J. Why Do Pigs Root and in What Will They Root? A Review on the Exploratory Behaviour of Pigs in Relation to Environmental Enrichment. Appl. Anim. Behav. Sci. 2007, 107, 183–197. [Google Scholar] [CrossRef]
- Zonderland, J.J.; Wolthuis-Fillerup, M.; Van Reenen, C.G.; Bracke, M.B.; Kemp, B.; Hartog, L.A.D.; Spoolder, H.A. Prevention and treatment of tail biting in weaned piglets. Appl. Anim. Behav. Sci. 2008, 110, 269–281. [Google Scholar] [CrossRef]
- Abriel, M.; Jais, C. Influence of Housing Conditions on the Appearance of Cannibalism in Weaning Piglets. Landtechnik 2013, 68, 389–393. [Google Scholar]
- Abriel, M.; Jais, C.; Bernhardt, H. Influence of Pen Design and Space Allowance on Tail Biting in Weaning Piglets. Landtechnik 2014, 69, 308–314. [Google Scholar]
- Veit, C.; Traulsen, I.; Hasler, M.; Tölle, K.-H.; Burfeind, O.; Beilage, E.G.; Krieter, J. Influence of raw material on the occurrence of tail-biting in undocked pigs. Livest. Sci. 2016, 191, 125–131. [Google Scholar] [CrossRef]
- Jans-Wenstrup, I. Untersuchungen Zur Prävention der Caudophagie Bei Absetzferkeln Unter Besonderer Berücksichtigung Einer Pelletzulage. Ph.D. Thesis, Justus Liebig University, Gießen, Germany, 2018. [Google Scholar]
- Chou, J.-Y.; O’Driscoll, K.; D’Eath, R.B.; Sandercock, D.A.; Camerlink, I. Multi-Step Tail Biting Outbreak Intervention Protocols for Pigs Housed on Slatted Floors. Animals 2019, 9, 582. [Google Scholar] [CrossRef]
- Zonderland, J.J.; Kemp, B.; Bracke, M.B.M.; Hartog, L.A.D.; Spoolder, H.A.M. Individual piglets’ contribution to the development of tail biting. Animals 2011, 5, 601–607. [Google Scholar] [CrossRef]
- Camerlink, I.; Turner, S. The pig’s nose and its role in dominance relationships and harmful behaviour. Appl. Anim. Behav. Sci. 2013, 145, 84–91. [Google Scholar] [CrossRef]
- Brunberg, E.; Wallenbeck, A.; Keeling, L.J. Tail biting in fattening pigs: Associations between frequency of tail biting and other abnormal behaviours. Appl. Anim. Behav. Sci. 2011, 133, 18–25. [Google Scholar] [CrossRef]
- Ursinus, W.W.; Van Reenen, C.G.; Kemp, B.; Bolhuis, J.E. Tail biting behaviour and tail damage in pigs and the relationship with general behaviour: Predicting the inevitable? Appl. Anim. Behav. Sci. 2014, 156, 22–36. [Google Scholar] [CrossRef]
- Larsen, M.L.V.; Andersen, H.M.-L.; Pedersen, L.J. Changes in activity and object manipulation before tail damage in finisher pigs as an early detector of tail biting. Animals 2019, 13, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Schrøder-Petersen, D.L.; Heiskanen, T.; Ersbøll, A.K. Tail-in-mouth behaviour in slaughter pigs, in relation to internal factors such as: Age, size, gender, and motivational background. Acta Agric. Scand. Sect. A Anim. Sci. 2004, 54, 159–166. [Google Scholar] [CrossRef]
- Paoli, M.; Lahrmann, H.; Jensen, T.; D’Eath, R. Behavioural differences between weaner pigs with intact and docked tails. Anim. Welf. 2016, 25, 287–296. [Google Scholar] [CrossRef]
- Lahrmann, H.P.; Hansen, C.F.; D’Eath, R.; Busch, M.E.; Forkman, B. Tail posture predicts tail biting outbreaks at pen level in weaner pigs. Appl. Anim. Behav. Sci. 2018, 200, 29–35. [Google Scholar] [CrossRef]
- Lahrmann, H.P.; Hansen, C.F.; D’Eath, R.B.; Busch, M.E.; Nielsen, J.P.; Forkman, B. Early intervention with enrichment can prevent tail biting outbreaks in weaner pigs. Livest. Sci. 2018, 214, 272–277. [Google Scholar] [CrossRef]
- Larsen, M.L.V.; Andersen, H.M.-L.; Pedersen, L.J. Tail posture as a detector of tail damage and an early detector of tail biting in finishing pigs. Appl. Anim. Behav. Sci. 2018, 209, 30–35. [Google Scholar] [CrossRef]
- Wedin, M.; Baxter, E.M.; Jack, M.; Futro, A.; D’Eath, R.B. Early indicators of tail biting outbreaks in pigs. Appl. Anim. Behav. Sci. 2018, 208, 7–13. [Google Scholar] [CrossRef]
- Wallgren, T.; Larsen, A.; Gunnarsson, S. Tail Posture as an Indicator of Tail Biting in Undocked Finishing Pigs. Animals 2019, 9, 18. [Google Scholar] [CrossRef]
- Warns, F.K. Identification of Dominance in Suckling Piglets. Caudophagie bei Mastschweinen (Sus Scrofa) Während der Ferkelaufzucht: Früherkennung und Prävention durch Umweltanreicherung. Ph.D. Thesis, Georg August University, Goettingen, Germany, 2020; pp. 49–77. [Google Scholar]
- Welfare Quality. Welfare Quality ® Assessment Protocol for Pigs (Sows and Piglets, Growing and Finishing Pigs); Welfare Quality® Consortium: Lelystad, The Netherlands, 2009. [Google Scholar]
- Friedrich Loeffler Institute (FLI). Deutscher Schweine Bonitur Schlüssel; FLI: Greifswald, Germany, 2017. [Google Scholar]
- Bowen, D.W.; Brooks, R.J. Social organization of confined male collared lemmings (Dicrostonyx groenlandicus Traill). Anim. Behav. 1978, 26, 1126–1135. [Google Scholar] [CrossRef]
- Craig, J.; Biswas, D.; Guhl, A. Agonistic behaviour influenced by strangeness, crowding and heredity in female domestic fowl (Gallus gallus). Anim. Behav. 1969, 17, 498–506. [Google Scholar] [CrossRef]
- Hope, A.C.A. A Simplified Monte Carlo Significance Test Procedure. J. R. Stat. Soc. Ser. B. 1968, 30, 582–598. [Google Scholar] [CrossRef]
- Chambers, J.M.; Freeny, A.E.; Heiberger, R.M. Analysis of Variance; Designed Experiments. Stat. Models S 2017, 5, 145–193. [Google Scholar] [CrossRef]
- Yandell, B. Practical Data Analysis for Designed Experiments; Routledge: Abingdon, UK, 2017. [Google Scholar]
- Best, D.J.; Roberts, D.E. Algorithm AS 89: The Upper Tail Probabilities of Spearman’s Rho. J. R. Stat. Soc. Ser. C 1975, 24, 377–379. [Google Scholar] [CrossRef]
- Wickham, H. Getting started with qplot. In ggplot2; Springer: Berlin/Heidelberg, Germany, 2009; pp. 9–26. [Google Scholar]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses. R Package Version 1.5. 2017. Available online: https://cran.r-project.org/web/packages/factoextra/factoextra.pdf (accessed on 19 April 2021).
- D’Eath, R.B. Socialising piglets before weaning improves social hierarchy formation when pigs are mixed post-weaning. Appl. Anim. Behav. Sci. 2005, 93, 199–211. [Google Scholar] [CrossRef]
- Meese, G.; Ewbank, R. The establishment and nature of the dominance hierarchy in the domesticated pig. Anim. Behav. 1973, 21, 326–334. [Google Scholar] [CrossRef]
- Ewbank, R.; Bryant, M. Aggressive behaviour amongst groups of domesticated pigs kept at various stocking rates. Anim. Behav. 1972, 20, 21–28. [Google Scholar] [CrossRef]
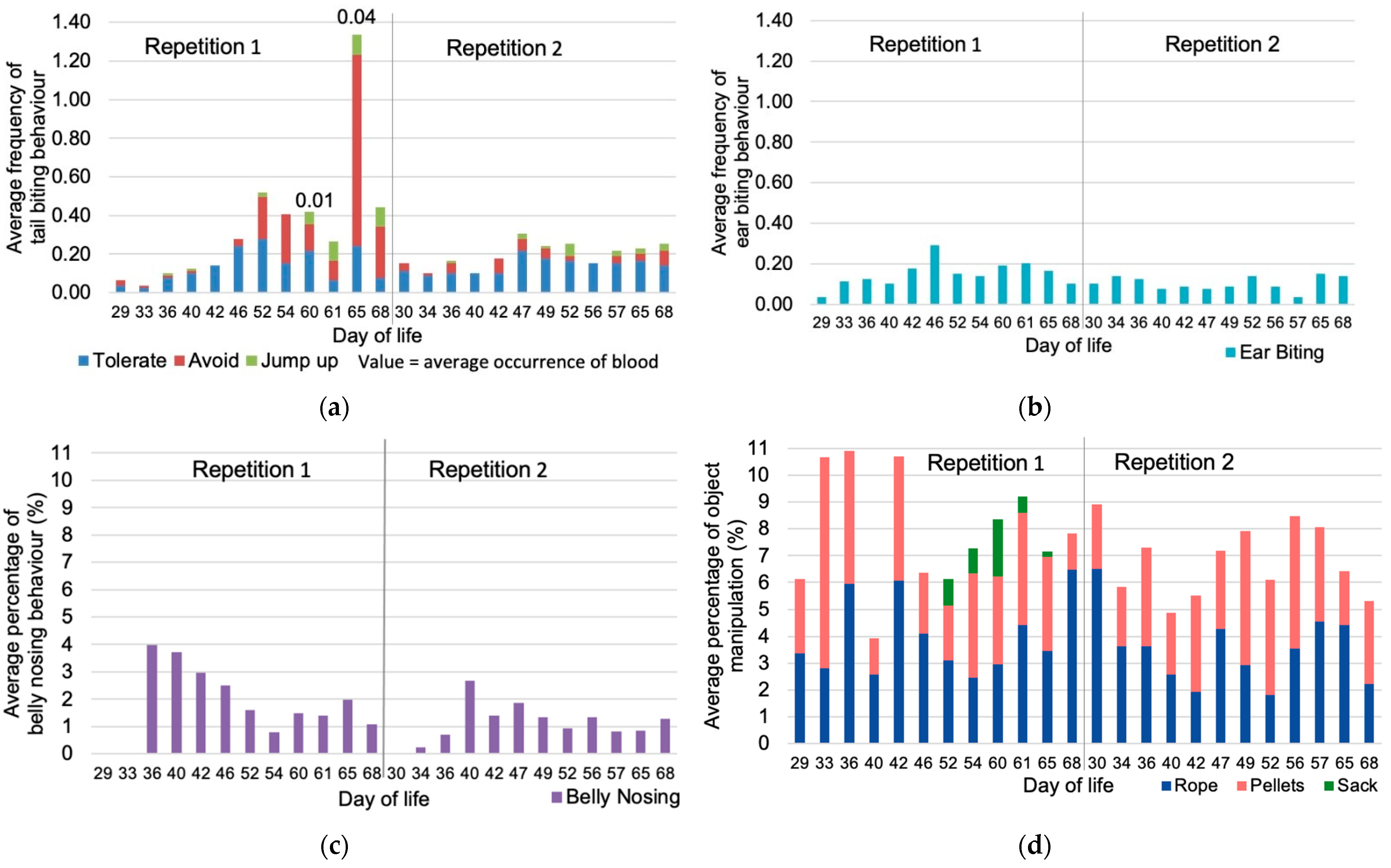

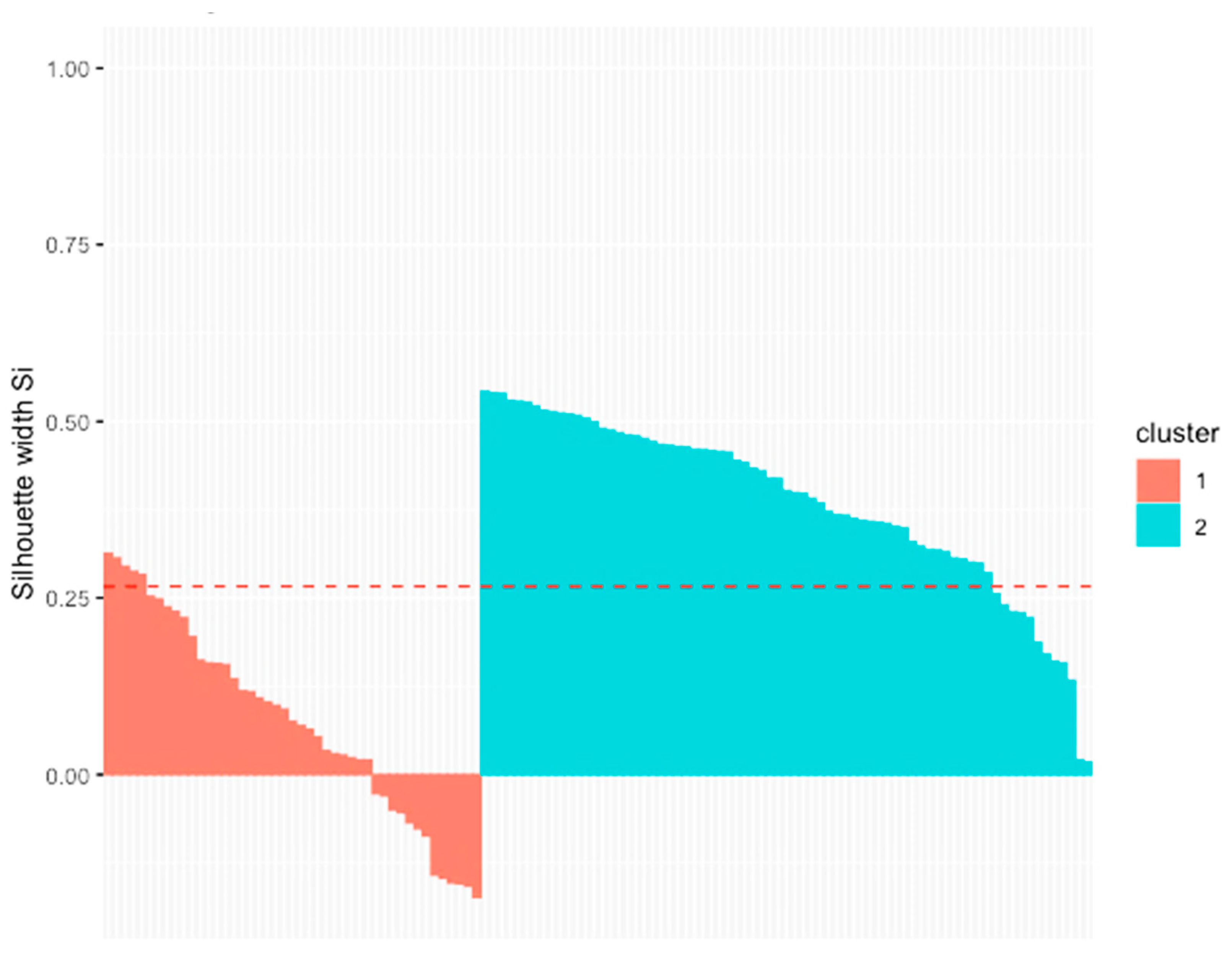
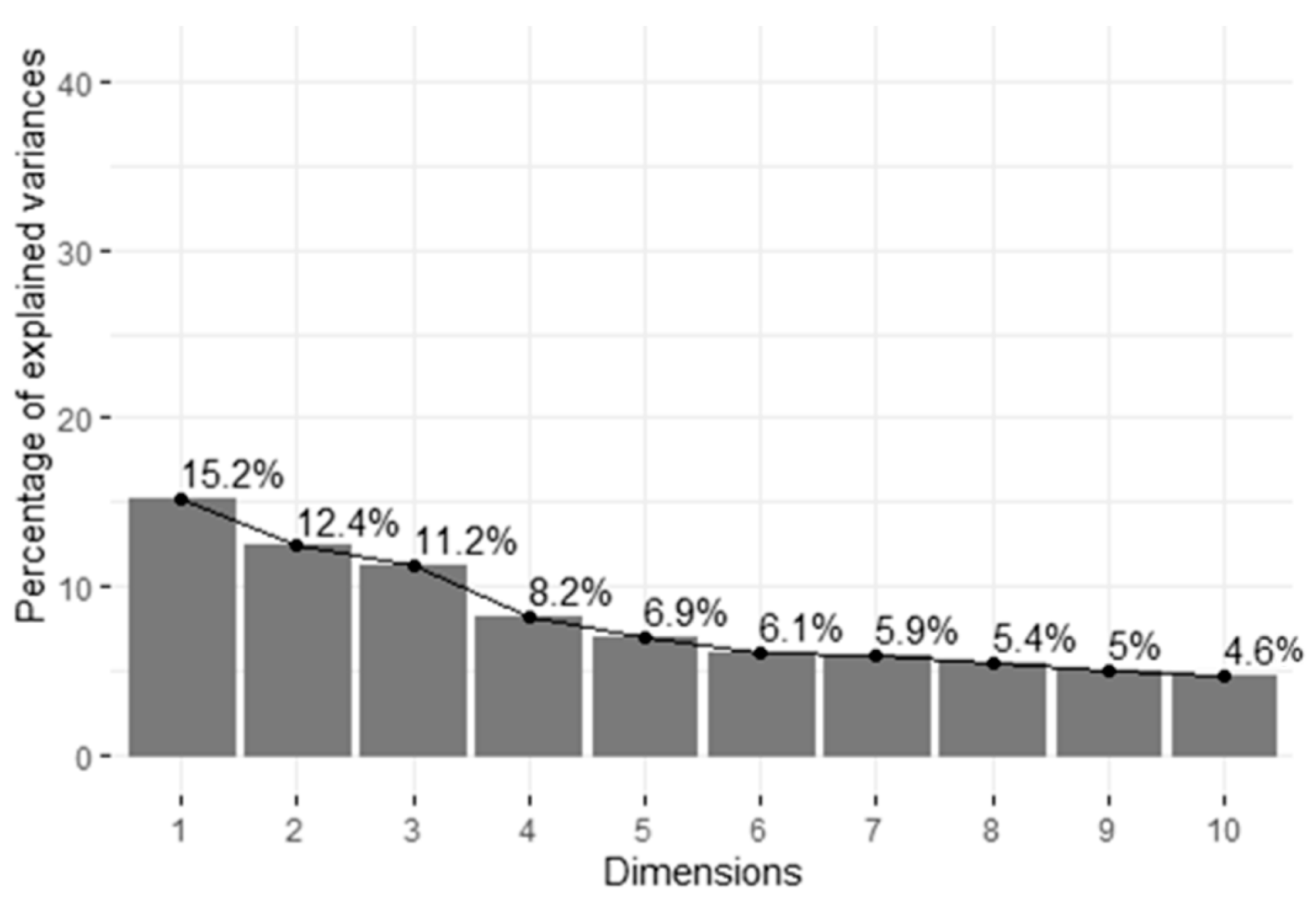
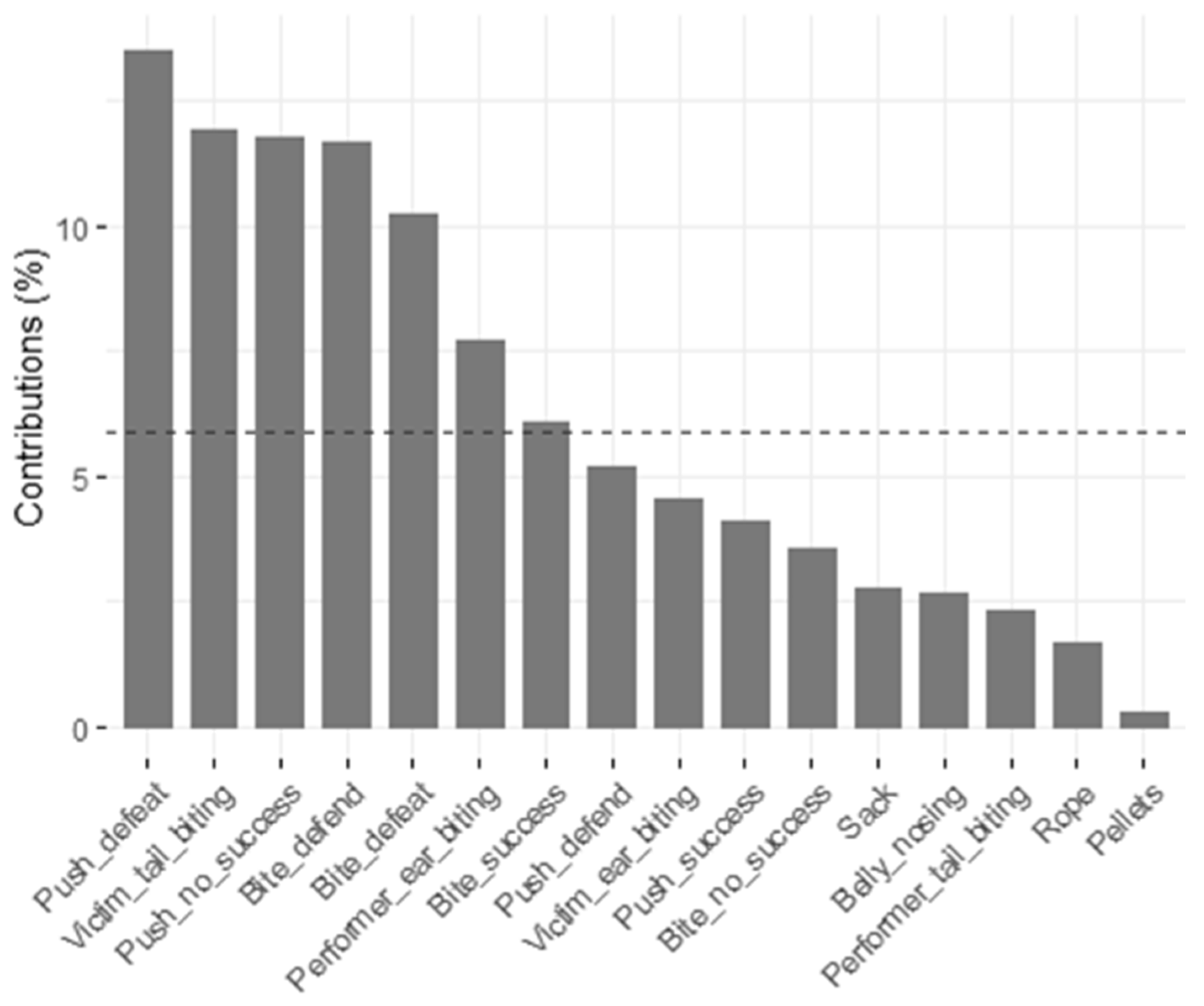
| Repetition | Pen | Weaned Pigs (N) | Average Weaning Weight (kg) | Dams (N) | Sex Ratio Male:Female | Average Weight at the End of Rearing (kg) |
|---|---|---|---|---|---|---|
| 1 | 1 | 9 | 6.12 ± 0.21 | 4 | 4:5 | 19.79 ± 2.43 |
| 2 | 10 | 6.74 ± 0.26 | 5 | 6:4 | 22.03 ± 2.62 | |
| 3 | 10 | 7.30 ± 0.22 | 5 | 5:5 | 20.97 ± 2.50 | |
| 4 | 10 | 7.80 ± 0.17 | 5 | 5:5 | 23.06 ± 2.34 | |
| 5 | 10 | 8.53 ± 0.23 | 5 | 5:5 | 25.34 ± 2.63 | |
| 6 | 10 | 9.38 ± 0.31 | 4 | 5:5 | 25.72 ± 2.55 | |
| 2 | 1 | 9 | 6.60 ± 0.23 | 5 | 4:5 | 21.20 ± 3.13 |
| 2 | 10 | 7.33 ± 0.28 | 5 | 5:5 | 21.23 ± 3.46 | |
| 3 | 10 | 7.96 ± 0.15 | 5 | 5:5 | 22.53 ± 2.86 | |
| 4 | 10 | 8.40 ± 0.20 | 5 | 5:5 | 22.51 ± 2.01 | |
| 5 | 10 | 9.08 ± 0.27 | 5 | 5:5 | 23.66 ± 3.00 | |
| 6 | 10 | 9.58 ± 0.24 | 4 | 5:5 | 25.10 ± 1.84 |
| Parameter | Score 0 | Score 1 | Score 2 |
|---|---|---|---|
| Lameness | No lameness | Lameness | - |
| Injury of the body sides | No injury | One side injured | Both sides injured |
| Injury of the carpal joints | No injury | One side injured | Both sides injured |
| Injury of the ears | No injury | One side injured | Both sides injured |
| Parameter | Score 0 | Score 1 | Score 2 |
|---|---|---|---|
| Tail length | Original length | Partial loss | Total loss (stump max. 1 cm) |
| Hair coat | Covered | Not covered | - |
| Cleanliness | Clean | Dirty | - |
| Skin perforation | No visible perforation | Superficial punctual perforation | Deeper skin perforation |
| Blood | No blood | Fresh blood | - |
| Necrosis | No necrosis | Necrosis, tissue change | - |
| Behavior | Description | Observation Method |
|---|---|---|
| Animal directed behavior | ||
| Tail biting | Visible tail biting: biting on the tail of a pig (“victim”) by another pig (“performer”); visible tail-in-mouth behavior: light chewing on the tail of a victim by a performer; new action counted after an interruption of at least 3 s with no contact between the snout of the performer and victim’s tail | Continuously, individual performer and victim, reaction of victim |
| → Reaction of the victim: | ||
| Tolerate | No visible reaction as a consequence of tail biting | |
| Avoid | Pulling in the tail, move away (slides forward while lying) or walking away calmly | |
| Jump up | Sudden rising of the animal from a lying or resting position; sudden leap forward when the animal was already standing | |
| Blood | Visible fresh blood on the victim’s tail immediately after a bite; recorded only on first appearance, not on follow-up bites on the same pig when fresh blood was already visible | |
| Ear biting | Visible ear biting or chewing on the ear of a pig (“victim”) by another pig (“performer”); new action counted after an interruption of at least 3 s with no contact between the snout of the performer and victim’s ear | Continuously, individual performer and victim |
| Belly nosing | Rhythmically raising and lowering the snout of a pig (“performer”) with pressure in the area of the abdominal cavity of another pig while it was lying; at least 2 s of continuous movement | Scan sampling, performer |
| Object orientated behavior | ||
| Cotton rope and metal chain with plastic piece (“rope”) | Visible “take in the mouth”, chewing, holding or pulling on one of the components; targeted searching, sniffing or moving one of the components with the snout; visible movement of the components | Scan sampling, performer |
| Piglet bowl with pellet mix (“pellets”) | Lowered head in or over the piglet bowl; visible manipulation of the bowl, rooting under the outer edge of the bowl, chewing of bowl components or pellets | Scan sampling, performer |
| Jute sack (“sack”) | Visible “take in the mouth”, chewing, holding or pulling on the jute sack; targeted searching, sniffing or moving the sack with the snout; visible movement of the sack | Scan sampling, performer |
| Parameter | Repetition 1 | Repetition 2 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day of Life | Day of Life | |||||||||||
| 35 | 42 | 49 | 56 | 63 | 70 | 35 | 42 | 49 | 56 | 63 | 70 | |
| General health condition | ||||||||||||
| Lameness | 2% a | 0%a | 0% a | 0% a | 0% a | 0% a | 0% a | 0% a | 0% a | 0% a | 0% a | 0% a |
| Body side(s) injured | 0% a | 0% a | 0% a | 24% b,c | 56% d,e | 71% d | 37% c,e | 0% a | 5% a | 15% b | 38% c,e | 59% d,e |
| Carpal joint(s) injured | 61% a | 34% a | 51% a,b | 39% a,b | 0% c | 5% c,d | 49% a,b | 31% b | 37% a,b | 61% a | 2% c,d | 10% d |
| Ear(s) injured | 15% a,b | 5% b,c | 12% a,b | 2% c | 19% a | 22% a | 80% d | 20% a | 8% a,b,c | 20% a | 3% b,c | 17% a |
| Tail lesions | ||||||||||||
| Fresh blood | 2% a | 0% a | 0% a | 8% b,c | 22% c,d | 36% d | 0%a | 0%a | 0% a | 0%a | 7% b | 15% b,c |
| Not covered with hair | 3% a | 0% a | 5% a | 22% b,c | 41% c | 51% c | 0% a | 0% a | 0% a | 0% a | 10% b | 2% a |
| Visible and deeper skin perforation | 5% a,e | 3% a | 10% a,b | 17% b,c,e | 47% d | 64% d | 5% a | 12% a,b | 0% a | 15% b,c,e | 25% c | 47% d |
| Partial loss | 0% a | 0% a | 0% a | 12% b | 24% b,c | 29% c | 2% a | 0% a | 0% a | 0% a | 5% a | 15% b,c |
| Necrosis, tissue change | 2% a,b | 0% a | 2% a,b | 7% b,c | 19% c | 14% c | 0% a | 0% a | 0% a | 0% a | 15% c | 2% a,b |
| Covered with dirt | 10% a | 25% b,c | 27% b,c | 37% b,d | 44% b | 44% b | 31% b,c,d | 25% b,c | 41% b | 19% a,c,d | 29% b,c | 14% a,c |
| Parameter | Performer Tail Biting (n/h) | Victim Tail Biting (n/h) | Performer Ear Biting (n/h) | Victim Ear Biting (n/h) | Belly Nosing (%) | Rope (%) | Pellets (%) | Sack (%) |
|---|---|---|---|---|---|---|---|---|
| Mean ± SD | 0.27 ± 0.48 | 0.27 ± 0.22 | 0.13 ± 0.11 | 0.13 ± 0.11 | 0.30 ± 0.31 | 3.75 ± 1.38 | 3.41 ± 1.45 | 0.20 ± 0.51 |
| Analysis of variance (p value) | ||||||||
| Repetition | 0.083 | <0.001 | 0.026 | 0.020 | 0.005 | 0.122 | 0.383 | <0.001 |
| Sow | 0.187 | 0.626 | 0.528 | 0.286 | <0.001 | 0.002 | 0.050 | <0.001 |
| Sex | 0.381 | 0.928 | 0.575 | 0.056 | 0.278 | 0.034 | 0.472 | 0.658 |
| Pen | 0.953 | 0.008 | 0.102 | 0.125 | 0.002 | 0.006 | 0.755 | <0.001 |
| Dominance Index | Social Tension Index | ||||||
|---|---|---|---|---|---|---|---|
| Repetition | Pen | Mean ± SD | min | max | Mean ± SD | min | max |
| 1 | 1 | 0.08 ± 0.36 | −0.58 | 0.49 | −3.33 ± 13.24 | −25 | 22 |
| 1 | 2 | 0.05 ± 0.24 | −0.31 | 0.45 | −6.10 ± 10.56 | −27 | 5 |
| 1 | 3 | −0.03 ± 0.33 | −0.60 | 0.35 | −2.20 ± 9.60 | −15 | 13 |
| 1 | 4 | 0.09 ± 0.29 | −0.56 | 0.48 | −3.60 ± 9.85 | −19 | 11 |
| 1 | 5 | 0.20 ± 0.33 | −0.12 | 0.86 | 1.50 ± 6.98 | −8 | 18 |
| 1 | 6 | 0.22 ± 0.21 | −0.11 | 0.58 | 0.90 ± 8.23 | −9 | 18 |
| 2 | 1 | −0.08 ± 0.38 | −0.51 | 0.64 | −0.11 ± 7.75 | −12 | 11 |
| 2 | 2 | −0.07 ± 0.32 | −0.67 | 0.37 | −4.10 ± 6.89 | −18 | 3 |
| 2 | 3 | 0.01 ± 0.18 | −0.33 | 0.29 | 3.10 ± 6.76 | −8 | 13 |
| 2 | 4 | 0.06 ± 0.31 | −0.47 | 0.55 | −1.70 ± 8.30 | −12 | 14 |
| 2 | 5 | 0.05 ± 0.35 | −0.60 | 0.67 | 0.80 ± 7.35 | −6 | 18 |
| 2 | 6 | 0.03 ± 0.30 | −0.39 | 0.63 | −4.60 ± 8.63 | −17 | 8 |
| Trait | Rearing Weight | DI 1 | STI 2 | Tail Biting Performer | Tail Biting Receiver | Ear Biting Performer | Ear Biting Receiver | Belly Nosing | Rope | Pellets | Sack |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Weaning weight | 0.517 *** | 0.113 | 0.089 | −0.199 * | −0.098 | −0.130 | −0.089 | 0.049 | −0.132 | 0.001 | −0.030 |
| Rearing weight | 0.198 * | −0.020 | −0.119 | −0.030 | 0.017 | −0.065 | 0.003 | −0.060 | −0.072 | 0.011 | |
| DI 1 | −0.466 *** | −0.256 ** | 0.073 | 0.165 | 0.068 | 0.000 | 0.054 | 0.046 | −0.012 | ||
| STI 2 | 0.148 | −0.075 | −0.069 | −0.042 | −0.067 | −0.007 | 0.100 | 0.070 | |||
| Tail biting performer | −0.106 | 0.108 | 0.037 | −0.010 | 0.037 | −0.168 | 0.059 | ||||
| Tail biting receiver | 0.043 | −0.071 | −0.027 | 0.036 | 0.042 | 0.276 ** | |||||
| Ear biting performer | 0.123 | 0.091 | −0.023 | 0.054 | −0.146 | ||||||
| Ear biting receiver | 0.117 | 0.123 | −0.029 | −0.099 | |||||||
| Belly nosing | 0.086 | 0.056 | −0.059 | ||||||||
| Rope | 0.053 | 0.244 ** | |||||||||
| Pellets | −0.029 | ||||||||||
| Blood | 0.077 | 0.048 | 0.069 | −0.135 | 0.379 *** | −0.034 | 0.042 | 0.102 | 0.024 | −0.026 | 0.062 |
| Hair coat | 0.110 | 0.134 | 0.041 | −0.088 | 0.265 ** | 0.033 | 0.156 | 0.243 ** | 0.019 | 0.043 | 0.435 *** |
| Skin lesion | 0.042 | 0.124 | 0.034 | −0.073 | 0.348 *** | 0.141 | 0.064 | 0.050 | −0.162 | 0.068 | 0.051 |
| Partial loss | −0.074 | −0.108 | 0.013 | −0.015 | 0.313 *** | −0.162 | −0.177 | −0.087 | −0.010 | −0.097 | 0.555 *** |
| Necrosis | 0.126 | 0.201 * | 0.048 | 0.071 | −0.051 | 0.008 | 0.134 | 0.104 | −0.201 * | −0.098 | −0.036 |
| Dirtiness | 0.075 | 0.109 | 0.043 | 0.083 | 0.017 | −0.012 | 0.159 | −0.052 | −0.084 | −0.087 | 0.062 |
| Injured body side(s) | 0.306 *** | 0.080 | −0.076 | 0.012 | 0.140 | −0.029 | 0.062 | 0.203 * | 0.121 | −0.124 | 0.156 |
| Injured carpal joint(s) | −0.113 | −0.043 | 0.168 | −0.011 | −0.078 | 0.095 | −0.020 | −0.045 | −0.085 | 0.129 | −0.133 |
| Injured ear(s) | 0.130 | 0.088 | −0.031 | −0.147 | 0.147 | −0.091 | −0.060 | −0.001 | −0.126 | −0.139 | 0.154 |
| Behavior | PC 1 | PC 2 | PC 3 | PC 4 | PC 5 |
|---|---|---|---|---|---|
| Eigen value | 3.117 | 1.774 | 1.447 | 1.326 | 1.129 |
| Variance explained (%) | 19.484 | 11.088 | 9.041 | 8.286 | 7.054 |
| Variance explained (%, cumulative) | 19.484 | 30.572 | 39.613 | 47.899 | 54.953 |
| Suckling behavior 1 | |||||
| Push—success | 0.638 | −0.365 | −0.267 | −0.042 | 0.194 |
| Push—no success | 0.654 | −0.509 | 0.042 | 0.020 | 0.006 |
| Bite—success | 0.591 | 0.494 | 0.157 | −0.147 | −0.266 |
| Bite—no success | 0.565 | 0.510 | 0.067 | −0.072 | −0.282 |
| Push—defeat | 0.716 | −0.378 | −0.076 | −0.033 | −0.010 |
| Bite—defeat | 0.431 | 0.348 | 0.056 | −0.008 | 0.380 |
| Push—defend | 0.733 | −0.149 | −0.066 | 0.017 | 0.062 |
| Bite—defend | 0.528 | 0.507 | −0.083 | 0.228 | 0.043 |
| Rearing behavior | |||||
| Belly nosing | 0.168 | −0.185 | 0.586 | −0.077 | 0.056 |
| Rope | 0.065 | −0.195 | 0.493 | 0.586 | 0.143 |
| Pellets | −0.175 | −0.158 | 0.000 | −0.161 | 0.352 |
| Sack | −0.043 | 0.039 | −0.187 | 0.750 | 0.106 |
| Tail biting performer | −0.061 | 0.059 | 0.394 | 0.377 | −0.364 |
| Tail biting receiver | 0.035 | 0.366 | −0.264 | 0.258 | 0.497 |
| Ear biting performer | −0.135 | 0.313 | 0.277 | −0.291 | 0.423 |
| Ear biting receiver | 0.106 | −0.008 | 0.632 | −0.110 | 0.272 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Warns, F.K.; Gültas, M.; van Asten, A.L.; Scholz, T.; Gerken, M. Is There a Link between Suckling and Manipulation Behavior during Rearing in Pigs? Animals 2021, 11, 1175. https://doi.org/10.3390/ani11041175
Warns FK, Gültas M, van Asten AL, Scholz T, Gerken M. Is There a Link between Suckling and Manipulation Behavior during Rearing in Pigs? Animals. 2021; 11(4):1175. https://doi.org/10.3390/ani11041175
Chicago/Turabian StyleWarns, Friederike K., Mehmet Gültas, Astrid L. van Asten, Tobias Scholz, and Martina Gerken. 2021. "Is There a Link between Suckling and Manipulation Behavior during Rearing in Pigs?" Animals 11, no. 4: 1175. https://doi.org/10.3390/ani11041175
APA StyleWarns, F. K., Gültas, M., van Asten, A. L., Scholz, T., & Gerken, M. (2021). Is There a Link between Suckling and Manipulation Behavior during Rearing in Pigs? Animals, 11(4), 1175. https://doi.org/10.3390/ani11041175






