Whole Blood Transcriptome Profiling Reveals Positive Effects of Olive Leaves-Supplemented Diet on Cholesterol in Goats
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Plan, Animals and Diets
2.2. Milk and Blood Samples Collection
2.3. Blood Analysis
2.4. Cholesterol Evaluation in Milk
2.5. Library Preparation and RNA-Seq Analysis
2.6. Statistical Analysis
3. Results
3.1. Hematochemical Characterization
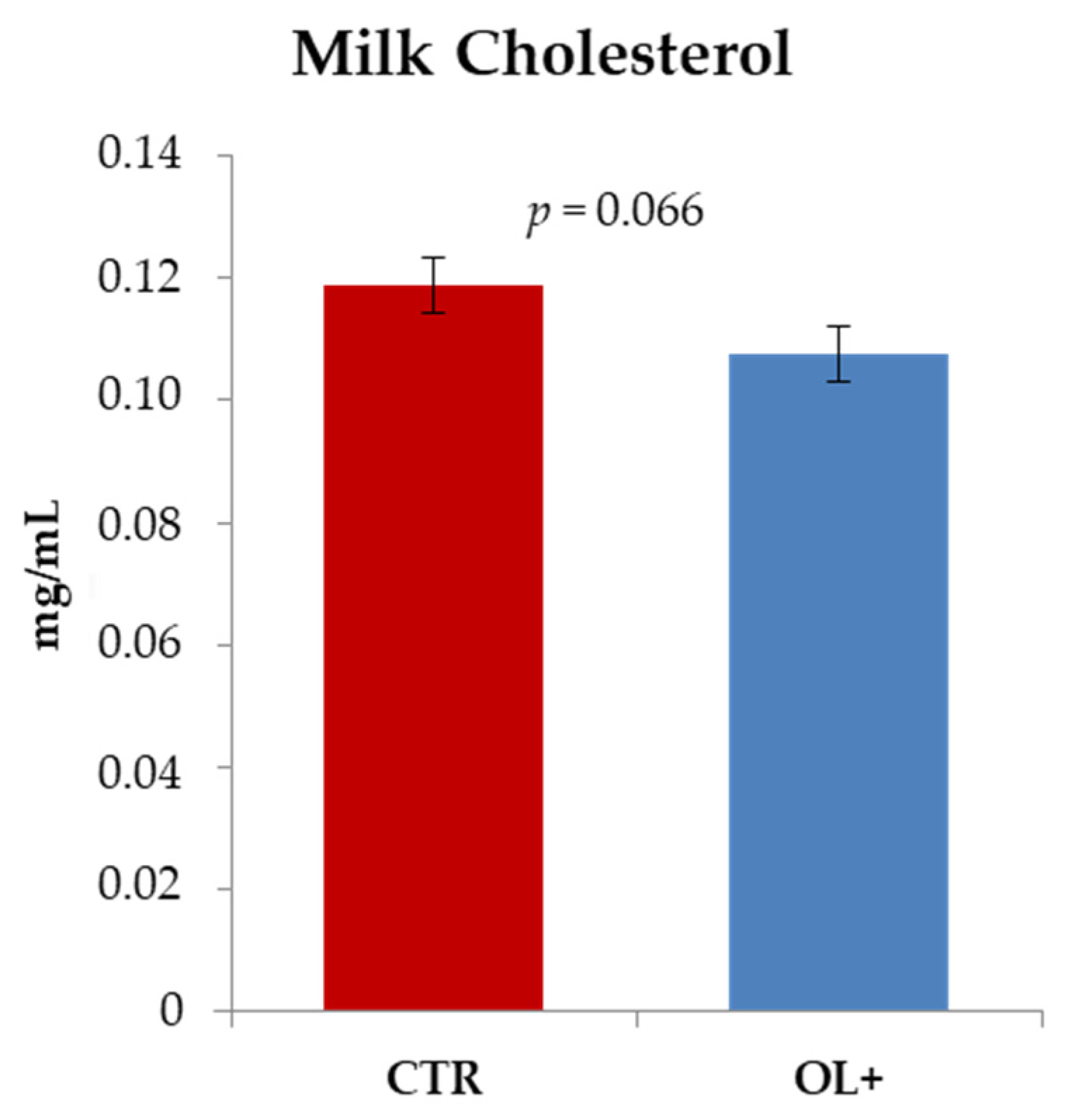
3.2. Cholesterol Amount in Goat Milk
3.3. Influence of Dietary OL Supplementation on Whole Blood Transcriptome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Olive Oil Council (IOOC). 2017. Available online: www.internationaloliveoil.org (accessed on 21 December 2020).
- Souilem, S.; El-Abbassi, A.; Kiai, H.; Hafidi, A.; Sayadi, S.; Galanakis, C.M. Olive oil production sector: Environmental effects and sustainability challenges. In Olive Mill Waste; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 1–28. [Google Scholar] [CrossRef]
- Federici, F.; Fava, F.; Kalogerakis, N.; Mantzavinos, D. Valorisation of agro-industrial by-products, effluents and waste: Concept, opportunities and the case of olive mill wastewaters. J. Chem. Technol. Biotechnol. 2009, 84, 895–900. [Google Scholar] [CrossRef]
- Ramachandran, S.; Singh, S.K.; Larroche, C.; Soccol, C.R.; Pandey, A. Oil cakes and their biotechnological applications–A review. Bioresour. Technol. 2007, 98, 2000–2009. [Google Scholar] [CrossRef] [PubMed]
- Shamsi, I.H.; Hussain, N.; Jiang, L. Agro-industrial by-products utilization in animal nutrition. In Technological Innovations in Major World Oil Crops; Springer: New York, NY, USA, 2012; Volume 2, pp. 209–220. [Google Scholar] [CrossRef]
- Mirzaei-Aghsaghali, A.; Maheri-Sis, N. Nutritive value of some agro-industrial by-products for ruminants-A review. World J. Zool. 2008, 3, 40–46. [Google Scholar]
- Ragland, D.; Thomas, C.R.; Harmon, B.G.; Miller, R.; Adeola, O. Nutritional evaluation of two agroindustrial by-products for ducks and pigs. J. Anim. Sci. 1998, 76, 2845–2852. [Google Scholar] [CrossRef]
- Chiofalo, B.; Di Rosa, A.R.; Lo Presti, V.; Chiofalo, V.; Liotta, L. Effect of supplementation of herd diet with olive cake on the composition profile of milk and on the composition, quality and sensory profile of cheeses made therefrom. Animals 2020, 10, 977. [Google Scholar] [CrossRef] [PubMed]
- Dal Bosco, A.; Mourvaki, E.; Cardinali, R.; Servili, M.; Sebastiani, B.; Ruggeri, S.; Mattioli, S.; Taticchi, A.; Esposto, S.; Castellini, C. Effect of dietary supplementation with olive pomaces on the performance and meat quality of growing rabbits. Meat Sci. 2012, 92, 783–788. [Google Scholar] [CrossRef]
- Luciano, G.; Pauselli, M.; Servili, M.; Mourvaki, E.; Serra, A.; Monahan, F.J.; Lanza, M.; Priolo, A.; Zinnai, A.; Mele, M. Dietary olive cake reduces the oxidation of lipids, including cholesterol, in lamb meat enriched in polyunsaturated fatty acids. Meat Sci. 2013, 93, 703–714. [Google Scholar] [CrossRef]
- Sahin, S.; Samli, R.; Tan, A.S.B.; Barba, F.J.; Chemat, F.; Cravotto, G.; Lorenzo, J.M. Solvent-free microwave-assisted extraction of polyphenols from olive tree leaves: Antioxidant and antimicrobial properties. Molecules 2017, 22, 1056. [Google Scholar] [CrossRef] [PubMed]
- Molina-Alcaide, E.; Yáñez-Ruiz, D.R. Potential use of olive by-products in ruminant feeding: A review. Anim. Feed Sci. Technol. 2008, 147, 247–264. [Google Scholar] [CrossRef]
- Innosa, D.; Bennato, F.; Ianni, A.; Martino, C.; Grotta, L.; Pomilio, F.; Martino, G. Influence of olive leaves feeding on chemical-nutritional quality of goat ricotta cheese. Eur. Food Res. Technol. 2020, 246, 923–930. [Google Scholar] [CrossRef]
- Bennato, F.; Innosa, D.; Ianni, A.; Martino, C.; Grotta, L.; Martino, G. Volatile profile in yogurt obtained from saanen goats fed with olive leaves. Molecules 2020, 25, 2311. [Google Scholar] [CrossRef] [PubMed]
- Innosa, D.; Ianni, A.; Faccia, M.; Martino, C.; Grotta, L.; Saletti, M.A.; Pomilio, F.; Martino, G. Physical, nutritional, and sensory properties of cheese obtained from goats fed a dietary supplementation with olive leaves. Animals 2020, 10, 2238. [Google Scholar] [CrossRef] [PubMed]
- Iannaccone, M.; Elgendy, R.; Giantin, M.; Martino, C.; Giansante, D.; Ianni, A.; Dacasto, M.; Martino, G. RNA sequencing-based whole-transcriptome analysis of friesian cattle fed with grape pomace-supplemented diet. Animals 2018, 8, 188. [Google Scholar] [CrossRef]
- Iannaccone, M.; Ianni, A.; Elgendy, R.; Martino, C.; Giantin, M.; Cerretani, L.; Dacasto, M.; Martino, G. Iodine supplemented diet positively affect immune response and dairy product quality in fresian cow. Animals 2019, 9, 866. [Google Scholar] [CrossRef] [PubMed]
- Iannaccone, M.; Ianni, A.; Contaldi, F.; Esposito, S.; Martino, C.; Bennato, F.; De Angelis, E.; Grotta, L.; Pomilio, F.; Giansante, D.; et al. Whole blood transcriptome analysis in ewes fed with hemp seed supplemented diet. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- European Union. Directive 2010/63/EU of the European Parliament; European Union: Strasbourg, France, 2010. [Google Scholar]
- European Economic Community. European Economic Community. Directive 86/609/EEC; European Economic Community: Bruxelles, Belgium, 1986. [Google Scholar]
- Oh, H.I.; Shin, T.S.; Chang, E.J. Determination of cholesterol in milk and dairy products by high-performance liquid chromatography. Asian-Australas J. Anim. Sci. 2001, 14, 1465–1469. [Google Scholar] [CrossRef]
- Fast QC. A Quality Control Tool for High Throughput Sequence Data. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc (accessed on 12 October 2020).
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Capra Hircus. Available online: http://uswest.ensembl.org/Capra_hircus/ (accessed on 15 August 2020).
- Shi, W.; Liao, Y. Rsubread/Subread Users Guide; Olivia Newton-John Cancer Research Institute: Melbourne, Australia, 2011. [Google Scholar]
- Pauletto, M.; Elgendy, R.; Ianni, A.; Marone, E.; Giantin, M.; Grotta, L.; Ramazzotti, S.; Bennato, F.; Dacasto, M.; Martino, G. Nutrigenomic effects of long-term grape pomace supplementation in dairy cows. Animals 2020, 10, 714. [Google Scholar] [CrossRef]
- Liew, C.C.; Ma, J.; Tang, H.C.; Zheng, R.; Dempsey, A.A. The peripheral blood transcriptome dynamically reflects system wide biology: A potential diagnostic tool. J. Lab. Clin. Med. 2006, 147, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Olmez, E.; Vural, K.; Gok, S.; Ozturk, Z.; Kayalar, H.; Ayhan, S.; Var, A. Olive leaf extract improves the atherogenic lipid profile in rats Fed a high cholesterol diet. Phytother. Res. 2015, 29, 1652–1657. [Google Scholar] [CrossRef]
- Iannaccone, M.; Ianni, A.; Ramazzotti, S.; Grotta, L.; Marone, E.; Cichelli, A.; Martino, G. Whole blood transcriptome analysis reveals positive effects of dried olive pomace-supplemented diet on inflammation and cholesterol in laying hens. Animals 2019, 9, 427. [Google Scholar] [CrossRef] [PubMed]
- Obeidat, B.S. The effects of feeding olive cake and Saccharomyces cerevisiae supplementation on performance, nutrient digestibility and blood metabolites of Awassi lambs. Anim. Feed Sci. Technol. 2017, 231, 131–137. [Google Scholar] [CrossRef]
- Innosa, D.; Ianni, A.; Palazzo, F.; Martino, F.; Bennato, F.; Grotta, L.; Martino, G. High temperature and heating effect on the oxidative stability of dietary cholesterol in different real food systems arising from eggs. Eur. Food Res. Technol. 2019, 245, 1533–1538. [Google Scholar] [CrossRef]
- Brown, A.J.; Jessup, W. Oxysterols: Sources, cellular storage and metabolism, and new insights into their roles in cholesterol homeostasis. Mol. Asp. Med. 2009, 30, 111–122. [Google Scholar] [CrossRef]
- Gómez-Cortés, P.; Viturro, E.; Juárez, M.; De La Fuente, M.A. Alternative to decrease cholesterol in sheep milk cheeses. Food Chem. 2015, 188, 325–327. [Google Scholar] [CrossRef]
- Symeou, S.; Tsiafoulis, C.G.; Gerothanassis, I.P.; Miltiadou, D.; Tzamaloukas, O. Nuclear magnetic resonance screening of changes in fatty acid and cholesterol content of ovine milk induced by ensiled olive cake inclusion in Chios sheep diets. Small Rum. Res. 2019, 177, 111–116. [Google Scholar] [CrossRef]
- Prochnow, C.; Bransteitter, R.; Klein, M.G.; Goodman, M.F.; Chen, X.S. The APOBEC-2 crystal structure and functional implications for the deaminase AID. Nature 2007, 445, 447–451. [Google Scholar] [CrossRef]
- Smith, H.C.; Bennett, R.P.; Kizilyer, A.; McDougall, W.M.; Prohaska, K.M. Functions and regulation of the APOBEC family of proteins. In Seminars in Cell & Developmental Biology; Academic Press: Cambridge, MA, USA, 2012; Volume 23, pp. 258–268. [Google Scholar] [CrossRef]
- Davidson, N.O.; Shelness, G.S. Apolipoprotein B: mRNA editing, lipoprotein assembly, and presecretory degradation. Ann. Rev. Nutr. 2000, 20, 169–193. [Google Scholar] [CrossRef] [PubMed]
- Barter, P.J.; Ballantyne, C.M.; Carmena, R.; Cabezas, M.C.; Chapman, M.J.; Couture, P.; De Graaf, J.; Durrington, P.N.; Faergeman, O.; Frohlich, J.; et al. Apo B versus cholesterol in estimating cardiovascular risk and in guiding therapy: Report of the thirty-person/ten-country panel. J. Int. Med. 2006, 259, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Hong, S.H.; Chan, B.H.J.; Rudolph, F.B.; Clark, S.C.; Chan, L. APOBEC-2, a cardiac-and skeletal muscle-specific member of the cytidine deaminase supergene family. Biochem. Biophys. Res. Comm. 1999, 260, 398–404. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ianni, A.; Bennato, F.; Martino, C.; Colapietro, M.; Martino, G. Whole Blood Transcriptome Profiling Reveals Positive Effects of Olive Leaves-Supplemented Diet on Cholesterol in Goats. Animals 2021, 11, 1150. https://doi.org/10.3390/ani11041150
Ianni A, Bennato F, Martino C, Colapietro M, Martino G. Whole Blood Transcriptome Profiling Reveals Positive Effects of Olive Leaves-Supplemented Diet on Cholesterol in Goats. Animals. 2021; 11(4):1150. https://doi.org/10.3390/ani11041150
Chicago/Turabian StyleIanni, Andrea, Francesca Bennato, Camillo Martino, Martina Colapietro, and Giuseppe Martino. 2021. "Whole Blood Transcriptome Profiling Reveals Positive Effects of Olive Leaves-Supplemented Diet on Cholesterol in Goats" Animals 11, no. 4: 1150. https://doi.org/10.3390/ani11041150
APA StyleIanni, A., Bennato, F., Martino, C., Colapietro, M., & Martino, G. (2021). Whole Blood Transcriptome Profiling Reveals Positive Effects of Olive Leaves-Supplemented Diet on Cholesterol in Goats. Animals, 11(4), 1150. https://doi.org/10.3390/ani11041150





