Thermal Tolerance and Physiological Changes in Mud Crab, Scylla paramamosain Crablet at Different Water Temperatures
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Production of Animals
2.2. Thermal Preference
2.3. Critical Thermal Minimum (CTmin) and Critical Thermal Maximum (CTmax)
2.4. Nursery Experiment
- SGRBW (% day−1) = ((ln final weight—ln initial weight)/days of culture) × 100
- SGRCW (% day−1) = (ln final carapace width—ln initial carapace width)/days of culture × 100
- Percentage of molting increment (%) = ((postmolt CW—premolt CW)/premolt CW) × 100
- Molting interval (days) = time period between two molt events
- Survival rate (%) = (final amount of sample/initial amount of sample) × 100
- Sex ratio (%) = (male or female/(male + female)) × 100
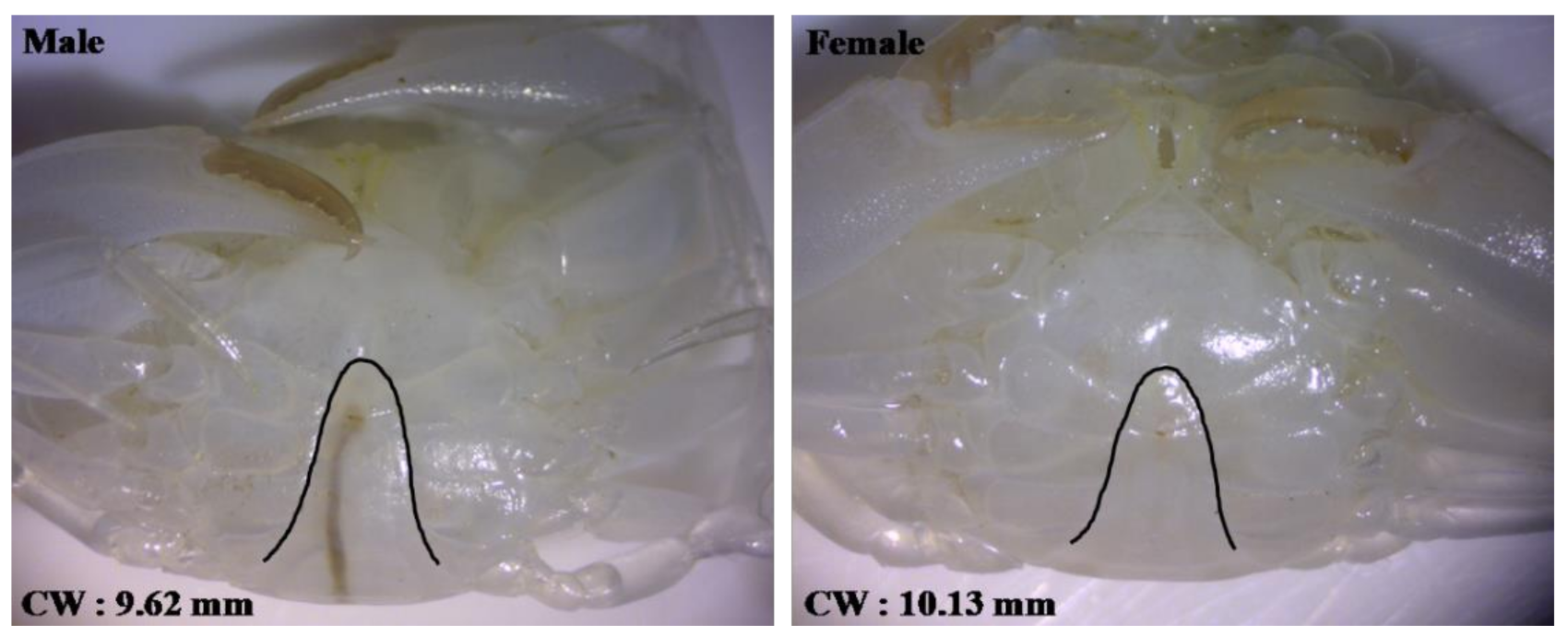
2.5. Sexing of S. paramamosain Crablet
2.6. Gill Histological Observation
2.7. Data Analysis
3. Results
3.1. Thermoregulatory Behavior
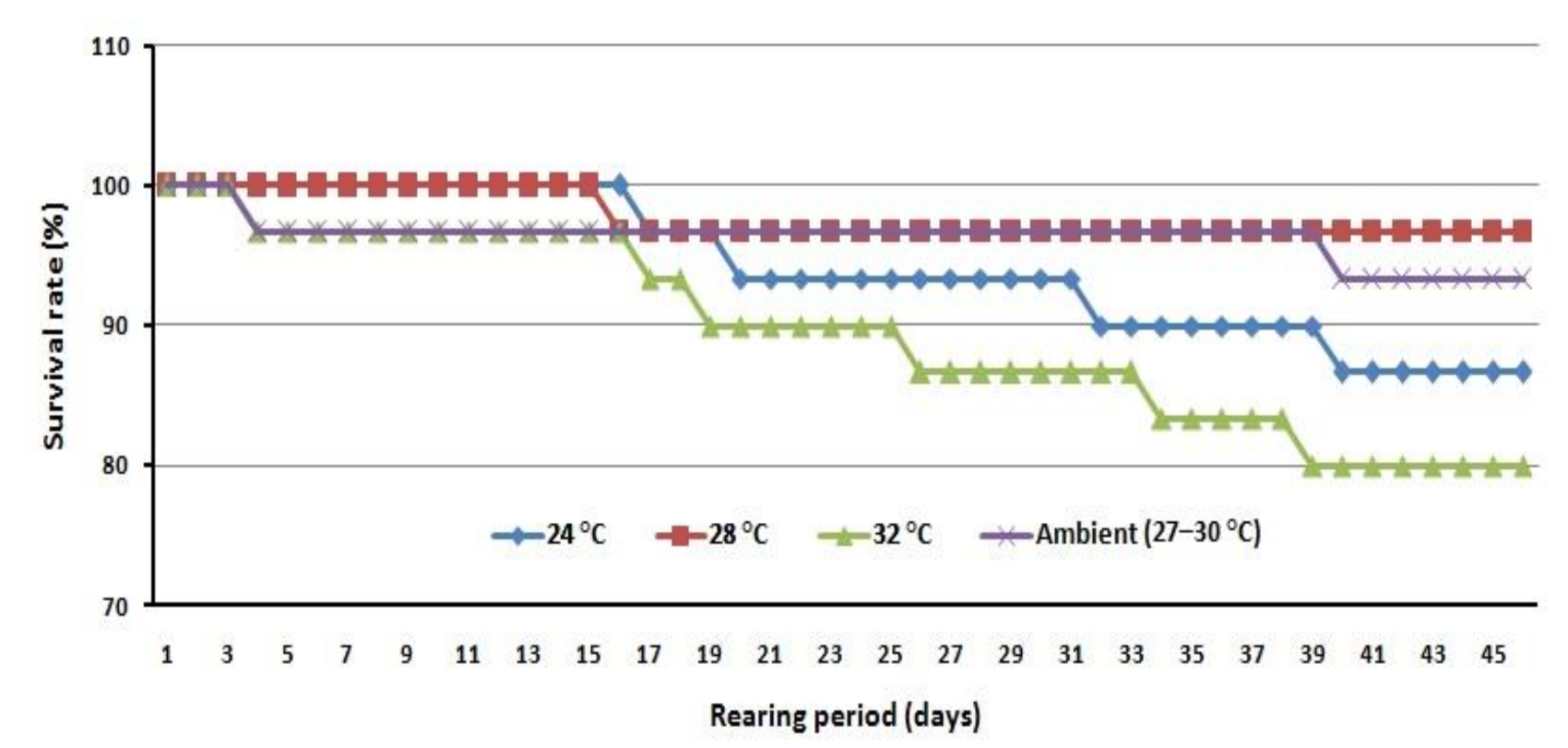
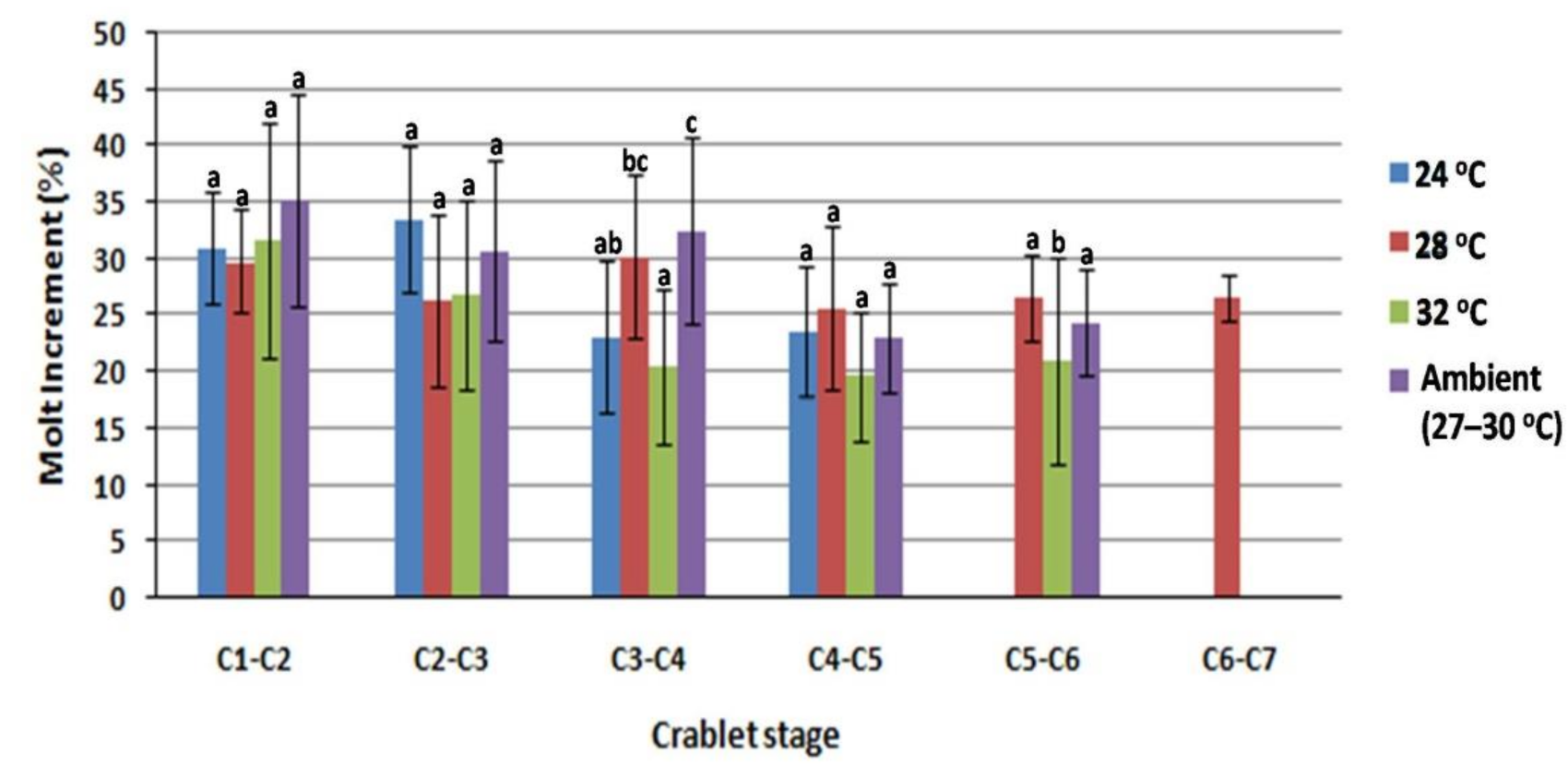
3.2. Effect of Water Temperature on Growth Performance, Molting Cycle, Survival Rate, and Sex Ratio
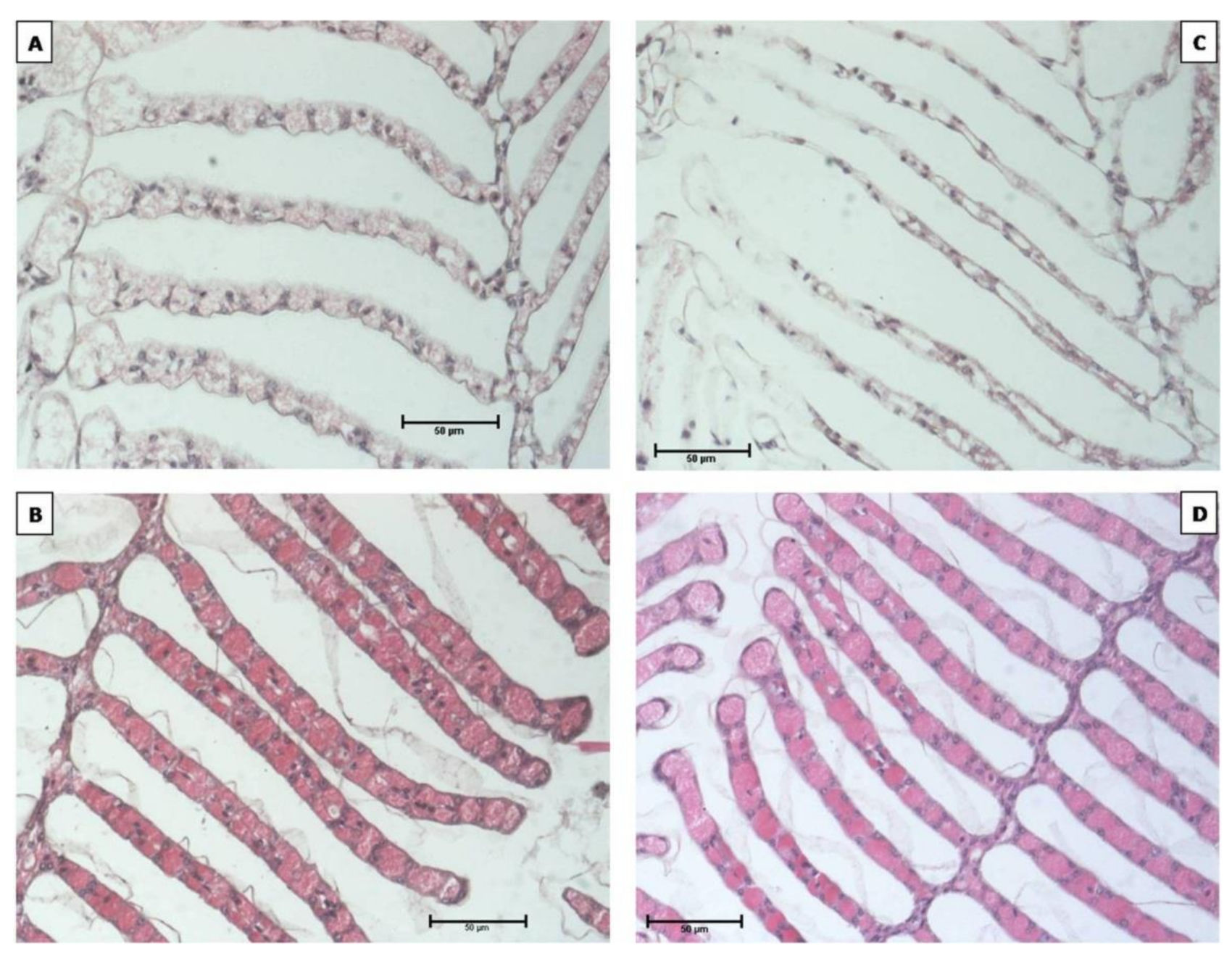
3.3. Gill Observation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Keenan, C.P.; Davie, P.J.F.; Mann, D.L. A revision of the genus Scylla De Haan, 1883 (Crustacea: Decapoda: Brachyura: Portunidae). Raffles Bull. Zool. 1998, 46, 217–245. [Google Scholar]
- Gong, J.; Yu, K.; Shu, L.; Ye, H.; Li, S.; Zeng, C. Evaluating the effects of temperature, salinity, starvation and autotomy on molting success, molting interval and expression of ecdysone receptor in early juvenile mud crabs, Scylla paramamosain. J. Exp. Mar. Biol. Ecol. 2015, 464, 11–17. [Google Scholar] [CrossRef]
- Ye, H.; Tao, Y.; Wang, G.; Lin, Q.; Chen, X.; Li, S. Experimental nursery culture of the mud crab Scylla paramamosain (Estampador) in China. Aquac. Int. 2010, 19, 313–321. [Google Scholar] [CrossRef]
- Alberts-Hubatsch, H.; Lee, S.Y.; Meynecke, J.-O.; Diele, K.; Nordhaus, I.; Wolff, M. Life-history, movement, and habitat use of Scylla serrata (Decapoda, Portunidae): Current knowledge and future challenges. Hydrobiologia 2016, 763, 5–21. [Google Scholar] [CrossRef]
- Hall, S.; Thatje, S. Temperature-driven biogeography of the deep-sea family Lithodidae (Crustacea: Decapoda: Anomura) in the Southern Ocean. Polar Biol. 2010, 34, 363–370. [Google Scholar] [CrossRef]
- Terblanche, J.S.; Hoffman, A.A.; Mitchell, K.A.; Rako, L.; le Roux, P.C.; Chown, L.S. Ecologically relevant measures of tolerance to potentially lethal temperatures. J. Exp. Biol. 2011, 214, 3713–3725. [Google Scholar] [CrossRef] [PubMed]
- Paschke, K.; Cumillaf, J.; Chimal, M.; Díaz, F.; Gebauer, P.; Rosas, C. Relationship between age and thermoregulatory behaviour of Lithodes santolla (Molina, 1782) (Decapoda, Lithodidae) juveniles. J. Exp. Mar. Biol. Ecol. 2013, 448, 141–145. [Google Scholar] [CrossRef]
- Cumillaf, J.P.; Blanc, J.; Paschke, K.; Gebauer, P.; Díaz, F.; Re, D.; Chimal, M.E.; Vásquez, J.; Rosas, C. Thermal biology of the sub-polar-temperate estuarine crab Hemigrapsus crenulatus (Crustacea: Decapoda: Varunidae). Biol. Open 2016, 5, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Abol-Munafi, A.B.; Azra, M.N. Climate change and portunid crabs sustainable aquaculture industry. J. Sustain. Sci. Manag. 2018, 13, 1–4. [Google Scholar]
- Azra, M.N.; Aaqillah-Amr, M.A.; Ikhwanuddin, M.; Ma, H.; Waiho, K.; Ostrensky, A.; Tavares, C.P.D.S.; Abol-Munafi, A.B. Effects of climate-induced water temperature changes on the life history of brachyuran crabs. Rev. Aquac. 2019, 12, 1211–1216. [Google Scholar] [CrossRef]
- Re, A.D.; Diaz, F.; Sierra, E.; Rodriguez, J.; Perez, E. Effect of salinity and temperature on thermal tolerance of brown shrimp Farfantepenaeus aztecus (Ives) (Crustacea, Penaeidae). J. Therm. Biol. 2005, 30, 618–622. [Google Scholar] [CrossRef]
- Hernández-Sandoval, P.; Díaz-Herrera, F.; Díaz-Gaxiola, J.M.; Martinez-Valenzuela, C.; García-Guerrero, M. Effect of temperature on growth, survival, thermal behavior, and critical thermal maximum in the juveniles of Macrobrachium occidentale (Holthuis, 1950) (Decapoda: Caridea: Palaemonidae) from Mexico. J. Crustac. Biol. 2018, 38, 483–488. [Google Scholar] [CrossRef]
- Huey, R.B. Physiological Consequences of Habitat Selection. Am. Nat. 1991, 137, S91–S115. [Google Scholar] [CrossRef]
- Tepler, S.; Mach, K.; Denny, M. Preference versus performance: Body temperature of the intertidal snail Chlorostoma fune-bralis. Biol. Bull. 2011, 220, 107–117. Available online: http://www.jstor.org/stable/23046933 (accessed on 29 March 2019). [CrossRef] [PubMed]
- Díaz, F.; Sierra, E.; Re, A.D.; Rodríguez, L. Behavioural thermoregulation and critical thermal limits of Macrobrachium acanthurus (Wiegman). J. Therm. Biol. 2002, 27, 423–428. [Google Scholar] [CrossRef]
- Beitinger, T.L.; Bennett, W.A.; McCauley, R.W. Temperature Tolerances of North American Freshwater Fishes Exposed to Dynamic Changes in Temperature. Environ. Biol. Fishes 2000, 58, 237–275. [Google Scholar] [CrossRef]
- Lutterschmidt, W.I.; Hutchison, V.H. The critical thermal maximum: Data to support the onset of spasms as the definitive end point. Can. J. Zool. 1997, 75, 1553–1560. [Google Scholar] [CrossRef]
- Serezli, R.; Atalar, M.S.; Hamzacebi, S.; Kurtoglu, I.Z.; Yandi, I. To what extent does temperature affect sex ratio in red cherry shrimp, Neocaridina davidi? the scenario global warming to offspring sex ratio. Fresenius Environ. Bull. 2017, 26, 7575–7579. [Google Scholar]
- Tessema, M.; Müller-Belecke, A.; Hörstgen-Schwark, G. Effect of rearing temperatures on the sex ratios of Oreochromis niloticus populations. Aquaculture 2006, 258, 270–277. [Google Scholar] [CrossRef]
- Azaza, M.; Dhraïef, M.; Kraïem, M. Effects of water temperature on growth and sex ratio of juvenile Nile tilapia Oreochromis niloticus (Linnaeus) reared in geothermal waters in southern Tunisia. J. Therm. Biol. 2008, 33, 98–105. [Google Scholar] [CrossRef]
- Khater, E.-S.G.; Ali, S.A.; Mohamed, W.E. Effect of Water Temperature on Masculinization and Growth of Nile Tilapia Fish. J. Aquac. Res. Dev. 2017, 8, 1–5. [Google Scholar] [CrossRef]
- Arfah, H.; Mariam, S. Alimuddin Effect of Temperature on Reproduction and Sex Ratio of Guppy (Poecilia reticulata Peters). J. Akuakultur Indones. 2007, 4, 1–4. [Google Scholar] [CrossRef]
- Selim, K.M.; Shinomiya, A.; Otake, H.; Hamaguchi, S.; Sakaizumi, M. Effects of high temperature on sex differentiation and germ cell population in medaka, Oryzias latipes. Aquaculture 2009, 289, 340–349. [Google Scholar] [CrossRef]
- Mylonas, C.; Anezaki, L.; Divanach, P.; Zanuy, S.; Piferrer, F.; Ron, B.; Peduel, A.; Ben Atia, I.; Gorshkov, S.; Tandler, A. Influence of rearing temperature at two periods during early life on growth and sex differentiation of two strains of European sea bass. Fish Physiol. Biochem. 2003, 28, 167–168. [Google Scholar] [CrossRef]
- Rigaud, T.; Antoine, D.; Marcade’, I.; Juchault, P. The effect of temperature on sex ratio in the isopod Porcellionides pruinosus: Environmental sex determination or a by-product of cytoplasmic sex determination? Evol. Ecol. 1997, 11, 205–215. [Google Scholar] [CrossRef]
- Voordouw, M.; Anholt, B.R. Environmental sex determination in a splash pool copepod. Biol. J. Linn. Soc. 2002, 76, 511–520. [Google Scholar] [CrossRef][Green Version]
- Ospina-Álvarez, N.; Piferrer, F. Temperature-Dependent Sex Determination in Fish Revisited: Prevalence, a Single Sex Ratio Response Pattern, and Possible Effects of Climate Change. PLoS ONE 2008, 3, e2837. [Google Scholar] [CrossRef]
- Ruscoe, I.M.; Shelley, C.C.; Williams, G.R. The combined effects of temperature and salinity on growth and survival of juvenile mud crabs (Scylla serrata Forskål). Aquaculture 2004, 238, 239–247. [Google Scholar] [CrossRef]
- Anh, N.T.N.; Ut, V.N.; Wille, M.; Van Hoa, N.; Sorgeloos, P. Effect of different forms of Artemia biomass as a food source on survival, molting and growth rate of mud crab (Scylla paramamosain). Aquac. Nutr. 2010, 17, e549–e558. [Google Scholar] [CrossRef]
- Yuan, Q.; Wang, Q.; Zhang, T.; Li, Z.; Liu, J. Effects of water temperature on growth, feeding and molting of juvenile Chinese mitten crab Eriocheir sinensis. Aquaculture 2017, 468, 169–174. [Google Scholar] [CrossRef]
- Heasman, M.P. Aspects of the general biology and fishery of the mud crab Scylla serrata (forskal) in Moreton Bay, Queensland. Ph.D. Thesis, University of Queensland Library, Queensland, Australia, 1980. [Google Scholar]
- Syafaat, M.N. Effect of Water Temperature on Growth Performance, Moulting Cycle, Survival Rate and Sex Ratio of Mud Crab, Scylla Paramamosain during Nursery Phase. Master’s Thesis, Universiti Malaysia, Terengganu, Malaysia, 2019. [Google Scholar]
- Azra, M.N.; Chen, J.-C.; Ikhwanuddin, M.; Abol-Munafi, A.B. Thermal tolerance and locomotor activity of blue swimmer crab Portunus pelagicus instar reared at different temperatures. J. Therm. Biol. 2018, 74, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Pörtner, H.-O.; Farrell, A.P. ECOLOGY: Physiology and Climate Change. Science 2008, 322, 690–692. [Google Scholar] [CrossRef] [PubMed]
- Portner, H.O.; Dupont, S.; Melzner, F.; Storch, D.; Thomdyke, M. Studies of metabolic rate and other characters across life stages. In Guide to Best Practices of Ocean Acidification Research and Data Reporting; Riebesell, U., Fabry, V., Gattuso, J.P., Eds.; Publications Office of the European Union: Luxemburg, 2010; pp. 167–180. Available online: http://www.eurosfaire.prd.fr/7pc/doc/1303284415_kina24328enc_002.pdf (accessed on 29 March 2019).
- Mann, D.; Paterson, B. Status of Crab Seed Production and Grow-Out in Queensland. In Mud Crab Aquaculture in Australia and Southest Asia, Proceedings of the ACIAR Crab Aquaculture Scoping Study and Workshop, Bribie Island, Australia, 28–29 April 2003; Allan, G., Fielder, D., Eds.; Australian Centre for International Agricultural Research: Canberra, Australia, 2004; pp. 36–41. Available online: https://core.ac.uk/download/pdf/6627662.pdf (accessed on 12 November 2013).
- Hartnoll, R.G. Growth in Crustacea—Twenty years on. Hydrobiology 2001, 449, 111–122. [Google Scholar] [CrossRef]
- A Kuhn, A.; Darnell, M.Z. Elevated temperature induces a decrease in intermolt period and growth per molt in the lesser blue crab Callinectes similis Williams, 1966 (Decapoda: Brachyura: Portunidae). J. Crustac. Biol. 2019, 39, 22–27. [Google Scholar] [CrossRef]
- Cadman, L.R.; Weinstein, M.P. Effects of temperature and salinity on the growth of laboratory-reared juvenile blue crabs Callinectes sapidus Rathbun. J. Exp. Mar. Biol. Ecol. 1988, 121, 193–207. [Google Scholar] [CrossRef]
- Nurdiani, R.; Zeng, C. Effects of temperature and salinity on the survival and development of mud crab, Scylla serrata (Forsskål), larvae. Aquac. Res. 2007, 38, 1529–1538. [Google Scholar] [CrossRef]
- Baylon, J.C. Effects of Salinity and Temperature on Survival and Development of Larvae and Juveniles of the Mud Crab, Scylla serrata (Crustacea: Decapoda: Portunidae). J. World Aquac. Soc. 2010, 41, 858–873. [Google Scholar] [CrossRef]
- Shelley, C.; Lovatelli, A. Mud Crab Aquaculture—A Practical Manual; FAO Fisheries and Aquaculture Technical Paper No.567; FAO: Rome, Italy, 2011; p. 78. Available online: http://www.fao.org/3/ba0110e/ba0110e00.htm (accessed on 15 March 2013).
- Ikhwanuddin, M.; Azra, M.N.; Talpur, M.A.D.; Abol-Munafi, A.B.; Shabdin, M.L. Optimal water temperature and salinity for production of blue swimming crab, Portunus pelagicus 1st day juvenile crab. AACL Bioflux 2012, 5, 4–8. [Google Scholar]
- Mann, D.L.; Asakawa, T.; Pizzutto, M.; Keenan, C.P. Investigation of an Artemia-based diet for larvae of the mud crab Scylla serrata. Asian Fish. Sci. 2001, 14, 175–184. [Google Scholar]
- Azra, M.N.; Ikhwanuddin, M. Larval culture and rearing techniques of commercially important crab, Portunus pelagicus (Linnaeus, 1758): Present status and future prospects. Songklanakarin J. Sci. Technol. 2015, 37, 135–145. [Google Scholar]
- Paital, B.; Chainy, G. Effects of temperature on complexes I and II mediated respiration, ROS generation and oxidative stress status in isolated gill mitochondria of the mud crab Scylla serrata. J. Therm. Biol. 2014, 41, 104–111. [Google Scholar] [CrossRef]
- Matozzo, V.; Gallo, C.; Marin, M.G. Effects of temperature on cellular and biochemical parameters in the crab Carcinus aestuarii (Crustacea, Decapoda). Mar. Environ. Res. 2011, 71, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Anger, K. The biology of decapod crustacean larvae. In Crustacean Issues; Vonk, R., Ed.; CRC Press/Balkema: Lisse, The Netherlands, 2001; Volume 14, 417p. [Google Scholar]
- Defur, P.L. Systemic Respiratory Adaptations to Air Exposure in Intertidal Decapod Crustaceans. Am. Zool. 1988, 28, 115–124. [Google Scholar] [CrossRef]
- Barra, J.-A.; Pequeux, A.; Humbert, W. A morphological study on gills of a crab acclimated to fresh water. Tissue Cell 1983, 15, 583–596. [Google Scholar] [CrossRef]
- Nascimento, A.A.; Araújo, F.G.; Gomes, I.D.; Mendes, R.M.M.; Sales, A. Fish Gills Alterations as Potential Biomarkers of Environmental Quality in a Eutrophized Tropical River in South-Eastern Brazil. Anat. Histol. Embryol. 2012, 41, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Venezia, L.D.; Zago, C.; Siebers, D.; Menetto, A. Structural elements of the gills of the shore crab Carcinus mediterraneus. Helgol. Mar. Res. 1992, 46, 425–434. [Google Scholar] [CrossRef][Green Version]
- Shi, X.; Waiho, K.; Li, X.; Ikhwanuddin, M.; Miao, G.; Lin, F.; Zhang, Y.; Li, S.; Zheng, H.; Liu, W.; et al. Female-specific SNP markers provide insights into a WZ/ZZ sex determination system for mud crabs Scylla paramamosain, S. tranquebarica and S. serrata with a rapid method for genetic sex identification. BMC Genomics 2018, 19, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hong, L. Studies of Androgenic Gland in the Mud Crab S. Paramamosain. Ph.D. Thesis, The Chinese University of Hong Kong, Hong Kong, China, 2004. Available online: https://search.proquest.com/docview/305051091?pq-origsite=gscholar (accessed on 21 December 2016).
- Rebolledo, A.P.; Collin, R. Thermal tolerance of the zoea I stage of four Neotropical crab species (Crustacea: Decapoda). Zoologia 2018, 35, 1–5. [Google Scholar] [CrossRef]
- Ikhwanuddin, M.; Abol-Munafi, A.B.; Azra, M.N. Data on the molting duration and time of hardening of instar crab at different culture temperatures. Data Brief 2019, 25, 104196. [Google Scholar] [CrossRef]
- McArley, T.J.; Hickey, A.J.R.; Herbert, N.A. Chronic warm exposure impairs growth performance and reduces thermal safety margins in the common triplefin fish (Forsterygion lapillum). J. Exp. Biol. 2017, 220, 3527–3535. [Google Scholar] [CrossRef] [PubMed]
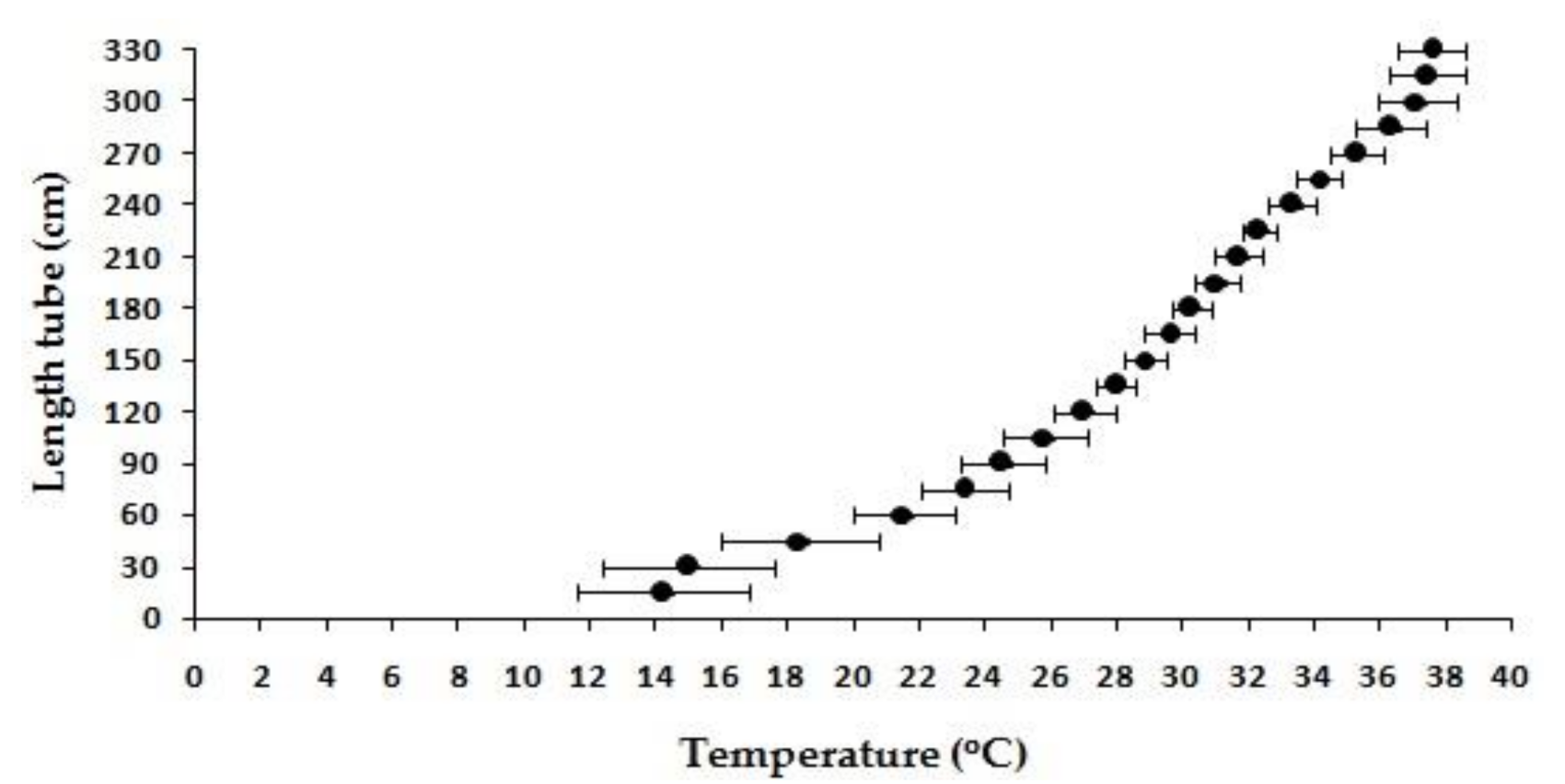
| Experiment | CTmin (°C) | CTmax (°C) ** | Preferred Temperature (°C) | * Thermal Tolerance Interval (TTI) |
|---|---|---|---|---|
| 1 | 17 | 40 | 27 | 23 |
| 2 | 18 | 40 | 31 | 22 |
| 3 | 17 | 40 | 31.5 | 23 |
| Average (±SD) | 17.33 ± 0.58 | 40 ± 0.00 | 29.83 ± 2.47 | 22.67 ± 0.58 |
| Parameter | Treatment * | |||
|---|---|---|---|---|
| A (24 °C) ± SD | B (28 °C) ± SD | C (32 °C) ± SD | D (Ambient Temp.) ± SD | |
| Experimental duration (days) | 45 | 45 | 45 | 45 |
| Initial animal sample (ind.) | 30 | 30 | 30 | 30 |
| Final animal sample (ind.) | 26 | 29 | 24 | 28 |
| Mean of initial carapace width (mm) | 1.62 ± 0.05 | 1.62 ± 0.05 | 1.62 ± 0.05 | 1.62 ± 0.05 |
| Mean of final carapace width (mm) | 8.81 ± 1.29 a | 12.30 ± 1.52 b | 9.59 ± 0.68 ab | 11.73 ± 1.65 ab |
| Mean of final carapace length (mm) | 6.27 ± 0.93 a | 8.38 ± 0.89 a | 6.76 ± 0.53 a | 8.26 ± 1.06 a |
| Mean of initial body weight (g) | 0.0035 ± 0.00 | 0.0035 ± 0.00 | 0.0035 ± 0.00 | 0.0035 ± 0.00 |
| Mean of final body weight (g) | 0.11 ± 0.05 a | 0.29 ± 0.09 a | 0.12 ± 0.03 a | 0.27 ± 0.09 a |
| Specific growth rate (SGRBW) (%) | 7.28 ± 1.31 a | 9.69 ± 0.75 b | 7.83 ± 0.56 ab | 9.48 ± 1.02 ab |
| Specific growth rate (SGRCW) (%) | 3.74 ± 0.34 a | 4.50 ± 0.28 b | 3.95 ± 0.16 a | 4.38 ± 0.35 b |
| Composition of C4 (%) | 30.55 ± 4.81 | - | - | - |
| Composition of C5 (%) | 69.44 ± 4.81 | 3.33 ± 5.77 | 6.66 ± 11.54 | 8.93 ± 7.79 |
| Composition of C6 (%) | - | 74.40 ± 21.15 | 93.33 ± 11.54 | 91.07 ± 7.79 |
| Composition of C7 (%) | - | 22.27 ± 15.38 | - | - |
| Survival Rate (%) | 87 ± 5.77 ab | 97 ± 5.77 a | 80 ± 5.00 b | 93 ± 5.77 ab |
| Sex ratio (%) | ||||
| Male | 80.09 ± 18.86 a | 44.81 ± 10.50 ab | 41.94 ± 19.44 b | 76.30 ± 5.13 ab |
| Female | 19.91 ± 18.86 a | 55.18 ± 10.50 ab | 58.05 ± 19.44 b | 23.70 ± 5.13 ab |
| Parameter | Molting Interval (MI) (days) | |||
|---|---|---|---|---|
| 24 °C | 28 °C | 32 °C | 27–30 °C | |
| M * to C1 | 1.46 ± 0.72 a | 1.80 ± 0.74 a | 1.63 ± 0.56 a | 1.33 ± 0.51 a |
| C1 to C2 | 7.63 ± 1.25 a | 3.92 ± 1.86 ab | 2.96 ± 1.22 b | 4.67 ± 0.50 ab |
| C2 to C3 | 8.78 ± 1.76 a | 5.68 ± 1.37 b | 5.77 ± 0.87 b | 5.88 ± 0.99 b |
| C3 to C4 | 10.83 ± 3.60 a | 6.62 ± 0.99 b | 7.37 ± 1.78 ab | 7.34 ± 1.03 ab |
| C4 to C5 | 13.00 ± 1.52 a | 8.47 ± 1.64 b | 10.50 ± 1.29 b | 9.33 ± 2.18 b |
| C5 to C6 | - | 10.66 ± 2.03 a | 7.66 ± 0.92 b | 11.95 ± 2.14 a |
| C6 to C7 | - | 9.25 ± 0.66 | - | - |
| Mean duration from M to C5 stage (days) | 38.58 ± 2.90 a | 27.03 ± 0.89 b | 29.68 ± 0.91 ab | 28.18 ± 3.40 ab |
| Parameter | Treatment | |||
|---|---|---|---|---|
| 24 °C | 28 °C | 32 °C | 27–30 °C | |
| Carapace Width: | ||||
| M | 1.62 ± 0.05 | 1.62 ± 0.05 | 1.62 ± 0.05 | 1.62 ± 0.05 |
| C1 | 3.35 ± 0.13 a | 3.40 ± 0.48 a | 3.46 ± 0.18 a | 3.41 ± 0.10 a |
| C2 | 4.43 ± 0.17 a | 4.50 ± 0.14 ab | 4.50 ± 0.19 ab | 4.80 ± 0.34 b |
| C3 | 5.89 ± 0.41 a | 5.62 ± 0.42 a | 5.73 ± 0.24 a | 6.00 ± 0.25 a |
| C4 | 7.47 ± 0.60 ab | 7.35 ± 0.56 ab | 7.07 ± 0.56 a | 7.83 ± 0.61 b |
| C5 | 9.48 ± 0.70 a | 9.12 ± 0.68 a | 8.22 ± 0.60 b | 9.56 ± 0.73 a |
| C6 | - | 11.85 ± 0.85 a | 9.63 ± 0.37 a | 12.08 ± 1.07 a |
| C7 | - | 14.30 ± 0.72 | - | - |
| Carapace Length: | ||||
| C1 | 3.02 ± 0.10 a | 3.00 ± 0.18 a | 3.17 ± 0.16 a | 3.03 ± 0.10 a |
| C2 | 3.64 ± 0.12 a | 3.53 ± 0.18 a | 3.62 ± 0.20 a | 3.71 ± 0.32 a |
| C3 | 4.43 ± 0.20 a | 4.33 ± 0.30 a | 4.43 ± 0.21 a | 4.55 ± 0.30 a |
| C4 | 5.33 ± 0.40 ab | 5.39 ± 0.34 ab | 5.09 ± 0.46 a | 5.69 ± 0.40 b |
| C5 | 6.75 ± 0.50 a | 6.46 ± 0.43 a | 5.85 ± 0.42 b | 6.78 ± 0.46 a |
| C6 | - | 8.16 ± 0.51 ab | 6.78 ± 0.23 a | 8.49 ± 0.69 a |
| C7 | - | 9.31 ± 0.41 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Syafaat, M.N.; Azra, M.N.; Mohamad, F.; Che-Ismail, C.Z.; Amin-Safwan, A.; Asmat-Ullah, M.; Syahnon, M.; Ghazali, A.; Abol-Munafi, A.B.; Ma, H.; et al. Thermal Tolerance and Physiological Changes in Mud Crab, Scylla paramamosain Crablet at Different Water Temperatures. Animals 2021, 11, 1146. https://doi.org/10.3390/ani11041146
Syafaat MN, Azra MN, Mohamad F, Che-Ismail CZ, Amin-Safwan A, Asmat-Ullah M, Syahnon M, Ghazali A, Abol-Munafi AB, Ma H, et al. Thermal Tolerance and Physiological Changes in Mud Crab, Scylla paramamosain Crablet at Different Water Temperatures. Animals. 2021; 11(4):1146. https://doi.org/10.3390/ani11041146
Chicago/Turabian StyleSyafaat, Muhammad Nur, Mohamad Nor Azra, Faridah Mohamad, Che Zulkifli Che-Ismail, Adnan Amin-Safwan, Mohammad Asmat-Ullah, Mohammad Syahnon, Azmie Ghazali, Ambok Bolong Abol-Munafi, Hongyu Ma, and et al. 2021. "Thermal Tolerance and Physiological Changes in Mud Crab, Scylla paramamosain Crablet at Different Water Temperatures" Animals 11, no. 4: 1146. https://doi.org/10.3390/ani11041146
APA StyleSyafaat, M. N., Azra, M. N., Mohamad, F., Che-Ismail, C. Z., Amin-Safwan, A., Asmat-Ullah, M., Syahnon, M., Ghazali, A., Abol-Munafi, A. B., Ma, H., & Ikhwanuddin, M. (2021). Thermal Tolerance and Physiological Changes in Mud Crab, Scylla paramamosain Crablet at Different Water Temperatures. Animals, 11(4), 1146. https://doi.org/10.3390/ani11041146








